Abstract
NO3− uptake by plant roots is rapidly inhibited by exposure to NH4+. The rapidity of the effect has led to the presumption that the inhibition results from the direct effects of NH4+ at the plasma membrane. The mechanism of this inhibition, however, has been in contention. In the present study we used the radiotracer 13N to determine the relative effects of short-term exposures to NH4+ on the 13NO3− influx, efflux, and partitioning of absorbed 13N in barley (Hordeum vulgare) roots. Plants were grown without NO3− or NO2− (uninduced for NO3− uptake), or with 0.1, 1.0, 10 mm NO3−, or 0.1 mm NO2− (to generate plant roots induced for NO3− uptake). Exposure to 1 mm NH4+ strongly reduced influx; the effect was most pronounced in plants induced for NO3− uptake when NO3− absorption was measured at low external NO3−. At higher [NO3−] and in uninduced plants the inhibitory effect was much diminished, indicating that NH4+ inhibition of influx was mediated via effects on the inducible high-affinity transport system rather than on the constitutive high-affinity transport system or the low-affinity transport system. Exposure to NH4+ also caused increased NO3− efflux; the largest effect was at low external [NO3−] in uninduced plants. In absolute terms, the reduction of influx made the dominant contribution to the observed reduction of net uptake of NO3−. Differences in response between plants induced with NO3− and those induced with NO2− indicate that NO2− may not be an appropriate analog for NO3− under all conditions.
The inhibitory effects exerted by the NH4+ ion upon NO3− uptake by the roots of higher plants have been studied extensively (Weissman, 1950; Lycklama, 1963; Fried et al., 1965; Minotti et al., 1969; Jackson et al., 1976; Rao and Rains, 1976; Doddema and Telkamp, 1979; MacKown et al., 1982a; Deane-Drummond and Glass, 1983; Rufty et al., 1983; Breteler and Siegerist, 1984; Glass et al., 1985; Ingemarsson et al., 1987; Oscarson et al., 1987; Lee and Drew, 1989; Warner and Huffaker, 1989; de la Haba et al., 1990; Ayling, 1993; Aslam et al., 1994, 1997; Chaillou et al., 1994). It is evident that there are short-term effects of NH4+ on NO3− uptake that are presumed to result from the direct effects of NH4+ on the plasma membrane; these short-term effects are apparent within minutes of exposure to NH4+. Moreover, longer-term effects due to NH4+ and/or assimilation products of NH4+ are thought to operate at the transcriptional level (Glass and Siddiqi, 1995; Krapp et al., 1998; Zhuo et al., 1999).
Despite the efforts of many investigators, a lack of consensus persists concerning the mechanism(s) responsible for the short-term inhibition of NO3− uptake by NH4+; specifically, whether the NH4+ effect is achieved by the direct inhibition of influx or by stimulating efflux. Although early reports suggested that NH4+ enhanced NO3− efflux (Jackson et al., 1976; Doddema and Telkamp, 1979; MacKown et al., 1982a; Deane-Drummond and Glass, 1983; Deane-Drummond, 1985, 1986), later studies using 13NO3− clearly documented an inhibition of influx (Glass et al., 1985; Lee and Clarkson, 1986; Ingemarsson et al., 1987; Oscarson et al., 1987; Lee and Drew, 1989; Ayling, 1993; King et al., 1993).
The debate has recently been revived by Aslam and coworkers (1994, 1997), who concluded that the main effect of NH4+ on net NO3− uptake was through stimulation of NO3− efflux; they discounted the significance of influx inhibition. A resolution of this controversy has proved difficult because the experiments have used different species or cultivars; more importantly, different techniques were used to determine NO3− fluxes. Furthermore, in none of the above studies were both influx and efflux of NO3− measured directly and simultaneously; rather, conclusions were based upon measurements of net flux or influx or, where efflux was determined, upon the use of NO3− analogs such as ClO3− (Deane-Drummond and Glass, 1983; Deane-Drummond, 1985, 1986) and NO2− (Aslam et al., 1994). Glass et al. (1985) and Siddiqi et al. (1992) have questioned whether analogs of this sort are appropriate. In addition, Glass et al. (1985) and Ingemarsson et al. (1987) have argued that design features of experiments may have caused perturbations from a steady state, which might explain the sometimes large increases in NO3− efflux that were observed in some of the above studies.
We have designed the present study to address such issues. To eliminate problems associated with the choice of plant material, we used the same barley cultivar (CM-72) used by Aslam and coworkers (1994, 1997), whose studies led to the conclusion that the short-term inhibition of NO3− uptake by NH4+ resulted exclusively from the stimulation of efflux. In this study we have determined the influx and efflux of NO3− by using 13NO3− under both steady-state and perturbation protocols, by directly measuring influx and efflux, and by estimating influx calculated from CAE. To investigate possible differential effects of NH4+ on the CHATS, IHATS, and LATS for NO3− transport, we measured NO3− uptake at [NO3−] that characterize these transport systems and we used plants induced or uninduced for NO3− transport. In addition, the experiments were designed to compare the effect of NH4+ on NO3− influx and efflux in plants induced for NO3− uptake by prior exposure to either NO3− or NO2−.
MATERIALS AND METHODS
Plant Growth Conditions
Barley (Hordeum vulgare L. cv CM-72) seeds were surface-sterilized in 1% NaOCl for 10 min, rinsed with deionized water, and germinated in sterilized moist sand in the dark as described by Siddiqi et al. (1989). Seeds were placed on plastic mesh fitted into Plexiglas (Atohaas Americas, Philadelphia, PA) disks, with 40 to 50 seeds per disk for influx experiments and 15 to 20 seeds per disk for efflux experiments (Siddiqi et al., 1989, 1991). After 3 d of germination in the dark, seedlings were transferred to 8-L hydroponic Plexiglas tanks located in walk-in controlled-environment growth chambers. The seedlings grew in hydroponic tanks for 4 d, after which we performed labeling experiments as described below. Growth chambers were maintained at 20°C ± 2°C, 70% RH, and set to a 16-h light/8-h dark photoperiod. Fluorescent lamps (model 1500, F96T12/CW/VHO, 215 W, Philips, Eindhoven, The Netherlands) provided a photon flux of approximately 300 μmol m−2 s−1, measured at plant level (LI-189 light meter and LI-190SA quantum sensor, LI-COR).
Nutrient Solutions
After the 3-d germination treatment in sand, seedlings were cultivated for 4 d in hydroponic media in 8-L Plexiglas tanks. We used deionized distilled water and reagent-grade chemicals in the preparation of all nutrient solutions. Modified, one-quarter-strength Johnson's nutrient solution (2 mm KH2PO4, 2 mm K2SO4, 1 mm MgSO4, 4 mm Ca2+ provided as CaSO4 and/or Ca[NO3]2, and the micronutrients 50 μm Cl, 25 μm B, 20 μm Fe as Fe-EDTA, 2 μm Mn, 2 μm Zn, and 0.5 μm Cu) was used in all experiments (Siddiqi et al., 1989). NO3− was provided (in the form of CA[NO3]2) at 0.1, 1.0, or 10 mm starting 24 h before initiating the experiments. When experiments used NO2− to induce NO3− transport, it was provided as NaNO2 (at 0.1 mm). During labeling experiments NH4+ was added as (NH4)2SO4 at 1 mm. Electric circulating pumps (model IC-2, Brinkmann) continuously mixed the nutrient solutions in tanks. Continuous infusion of nutrient stock solution via peristaltic pumps (Technicon Proportioning Pump II, Technicon Instrument Co., Tarrytown, NY) allowed steady-state control of nutrient concentrations in the tanks. We checked the solutions daily for [K+] using a spectrophotometer (model 443, Instrumentation Laboratory, Lexington, MA). Powdered CaCO3 maintained the solution pH at 6.5 ± 0.3. We monitored the pH daily with a microprocessor-based pocket-size pH meter (pH Testr2 model 59000-20, Cole Parmer, Chicago, IL). The [NO3−]o was measured spectrophotometrically by the method of Cawse (1967).
Influx Analysis
The radiotracer 13N (with a half-life of 9.98 min) was produced by the Tri-University Meson Facility cyclotron at the University of British Columbia (Vancouver, Canada) by proton irradiation of water, producing mostly 13NO3− with high radiochemical purity (Kronzucker et al., 1995b). The irradiated solutions were supplied in sealed 20-mL glass vials with a starting activity of 700 to 740 MBq. At this activity level, sufficient counts were present in eluates and plant samples even after loading periods of up to 60 min and a total elution period of 22 min in efflux experiments (see below). Procedures for the removal of radiocontaminants were carried out as described in detail elsewhere (Kronzucker et al., 1995a, 1995b). A volume of 100 mL of purified 13NO3−-containing “stock” solution was prepared in a fume hood and transferred into the controlled-environment chambers where the experiments were performed. All uptake solutions were premixed and contained in individual 500-mL plastic vessels behind lead shielding.
The chemical composition of the uptake, prewash, and desorption solutions was identical to the growth solution in the hydroponic tanks (see above) and contained 0.1, 1.0 or 10 mm NO3−. When NH4+ was present in uptake solutions it was provided at a concentration of 1 mm. In experiments where NO2− was used to induce NO3− transport (King et al., 1992; Aslam et al., 1997), NO2− was not present during loading with 13NO3−, but only during the induction period (24 h); it was replaced by NO3− during 13NO3− loading and flux measurement. Uninduced plants received no N during growth but were exposed to 0.1 mm NO3− for flux determinations.
To minimize plant perturbation during experiments, a syringe was used to add tracer to the individual uptake vessels. At the start of the influx experiments, barley seedlings were transferred from the hydroponic growth tanks to prewash solutions in 1-L vessels for 5 min prior to addition of radioisotope to the uptake solutions. This protocol minimized plant perturbation and allowed the roots to equilibrate to the exact solution temperature and composition used during flux analysis. The roots were then exposed to tracer for 5 min. Immediately after loading with isotope, roots were dipped into nonlabeled solutions for 5 s to minimize the carryover of label by the root surface to the desorption solution. Roots were then desorbed for 2 min in unlabeled solution, which was otherwise chemically identical to the influx solution, to remove the 13NO3− contained in the cell-wall free space. The duration of these steps was based on the half-lives of exchange of NO3− for the root surface, the free space, and the cytoplasm as determined by efflux analysis (see below; Siddiqi et al., 1991; Kronzucker et al., 1995a, 1995b, 1995e).
We chose exposure times of 5 min for 13NO3− influx, because we expected the contribution of tracer efflux from the cytoplasm to be negligible during this time (Lee and Clarkson, 1986; Siddiqi et al., 1991; Kronzucker et al., 1995a, 1995b, 1995d, and 1995e). After desorption, seedling roots were excised from the shoots; the roots were spun in a low-speed centrifuge for 30 s to remove any surface liquid; and the fresh weights of the roots and shoots were measured. The plant organs were then introduced into 20-mL scintillation vials, and a γ-counter (Minaxi δ, Auto-γ 5000, Packard Instruments, Meriden, CT) determined the radioactivities of the roots and shoots, measuring the 511-keV positron-electron annihilation radiation generated by the recombination of ambient electrons and β+ particles emitted from 13N. Using the specific activity (13N/[13N + 14N] cpm μmol−1) of the loading solution and the total root fresh weight of each seedling, we calculated the NO3− fluxes and expressed them in micromoles per gram root fresh weight per hour.
In addition to direct influx determinations by 13N count accumulation over the 5-min loading periods (designated as Φoc*), influx was also determined by CAE (Φoc) and net flux was determined by 14N depletion over a period of up to 2 h (Φnet*). We repeated all experiments at least three times. Each experimental treatment consisted of three to four replicates (n ≥ 9).
CAE
The protocol for CAE was essentially as described elsewhere (Lee and Clarkson, 1986; Siddiqi et al., 1991; Kronzucker et al., 1995a, 1995b, 1995e). Roots of intact barley seedlings were immersed for 60 min in 120-mL darkened plastic beakers containing the 13NO3−-labeled solution. NO3− concentrations were 0.1, 1.0, or 10 mm. NH4+ was added at a 1 mm concentration unless otherwise indicated for the duration of loading and elution; or it was added only at a specified time during the elution of tracer from the cytoplasm (Figs. 2 and 3) to study the immediate effect of NH4+ upon NO3− efflux. Pretreatment of uninduced and NO2−-induced plants took place as described above. Conditions closely approximating a steady state with respect to all other nutrients were maintained throughout growth by completely replacing solutions in the 8-L tanks every day.
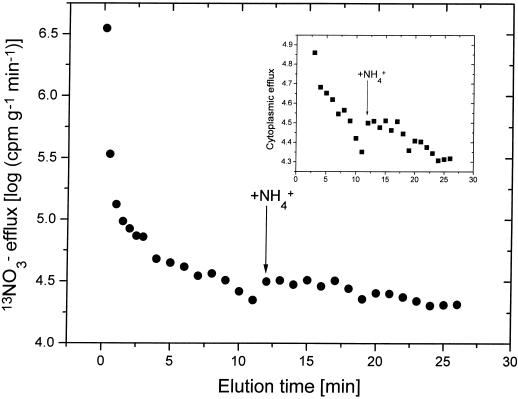
Figure 2.
13NO3−-efflux plot for intact seedlings of cv CM-72 maintained at 0.1 mm [NO3−]o with a one-time addition (and continued presence) of 1.0 mm NH4+ during cytoplasmic efflux (at 12 min during tracer elution). Inset shows magnified cytoplasmic exchange.
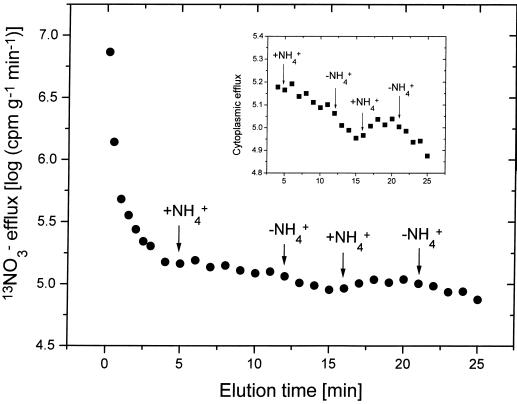
Figure 3.
13NO3−-efflux plot for intact seedlings of cv CM-72 maintained at 0.1 mm [NO3−]o with two-time addition and subsequent withdrawal of 1.0 mm NH4+ during cytoplasmic efflux (additions at 5 and 16 min; withdrawals at 12 and 21 min during tracer elution). Inset shows magnified cytoplasmic exchange.
We maintained steady-state conditions during loading and elution. We chose the duration of the loading period on the basis of the half-lives of exchange for the cytoplasmic compartment for NO3− in barley (compare below with Siddiqi et al., 1991). Therefore, 60 min of exposure to tracer ensured that cytoplasmic specific activity was ≥95% of that in the loading solution (Kronzucker et al., 1995e). After loading with 13N, seedlings were transferred to efflux funnels (Siddiqi et al., 1991; Kronzucker et al., 1995b) and the roots were eluted with 20-mL aliquots of nonradioactive solution after varying time intervals. Using an elution protocol lasting 22 min, these time intervals ranged from 5 s to 2 min, as described by Kronzucker et al. (1995b), except when we monitored the response of the NO3− efflux to the NH4+ addition during elution (Figs. 2 and 3); we used 1-min intervals in those cases to ensure appropriate time resolution.
Eluates from a total of 25 time intervals were collected, and the γ-counter (see above) determined the radioactivities of 20-mL subsamples from each eluate. After each final elution, we excised the seedling roots from the shoots, spun the roots in a low-speed centrifuge for 30 s to remove surface liquid, and determined the fresh weights of the roots and shoots. We then introduced the plant organs into 20-mL scintillation vials and determined the radioactivities of the roots and shoots as described previously for the influx experiments. We repeated the experiments three times with two replicates per experiment. We pooled the data from several experiments (n ≥ 6) to calculate means and se. Symbols and calculation of fluxes in CAE were as follows: Φco, efflux from the cytoplasmic compartment at time 0 divided by the specific activity of 13N in the loading solution; Φnet, net flux, obtained from the accumulation of 13N in the plants at the end of the loading period (60 min); Φoc, unidirectional influx, calculated from Φnet + Φco; Φxylem, flux of 13N to the shoot at the end of the elution period; and Φred/vac, combined flux to reduced N and the vacuole, resulting in Φnet − Φxylem. Calculations of half-lives of exchange and cytosolic concentrations were done as described in detail elsewhere (Lee and Clarkson, 1986; Siddiqi et al., 1991; Kronzucker et al., 1995a, 1995b, 1995c, 1995e).
RESULTS AND DISCUSSION
Effect of NH4+ on Half-Lives of Cellular NO3− Exchange
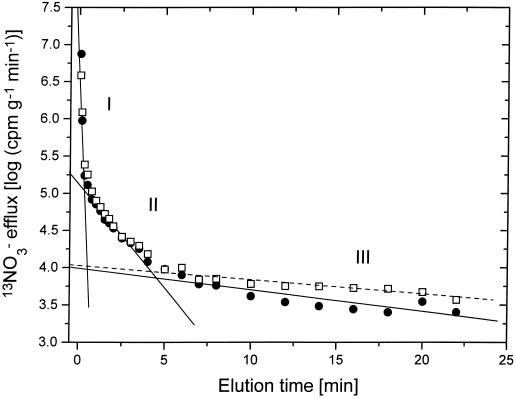
As in our previous studies with barley, rice, and spruce (Siddiqi et al., 1991; Kronzucker et al., 1995a, 1995b, 1997; H.J. Kronzucker, A.D.M. Glass, and M.Y. Siddiqi, unpublished results), CAE revealed NO3− exchange with three subcellular compartments (Fig. 1). These corresponded to a surface film, a binding component in the cell wall, and the cytoplasm, an interpretation substantiated by previously reported compartment identity tests for the CAE technique with 13N (Siddiqi et al., 1991; Kronzucker et al., 1995a, 1995b, 1995e). Half-lives of exchange for the surface film and the cell wall free space were very similar (approximately 2 and 30 s, respectively) to those reported in our previous studies (Siddiqi et al., 1991; Kronzucker et al., 1995a, 1995b) and did not change significantly as a function of [NO3−]o. The half-lives for cytoplasmic NO3− exchange without NH4+ additions are shown in Table I. Cytoplasmic half-life values of NO3− exchange ranged from 7.75 to 12.94 min, with slightly shorter half-lives at higher [NO3−]o and longer half-lives in NO2−-induced plants (Lee and Clarkson, 1986; Siddiqi et al., 1991; Devienne et al., 1994; Kronzucker et al., 1995a, 1995b, 1995c, 1995e, 1997).
Figure 1.
Representative semilogarithmic plots for the rate of release of 13NO3− versus time of elution for roots of intact cv CM-72 seedlings maintained under steady-state conditions of 0.1 mm [NO3−]o without NH4+ (•) and following the addition of 1 mm NH4+ (□). Plots include linear regression lines for the three phases of efflux: I, surface film; II, cell wall; and III, cytoplasm. Regression lines are dashed for the plus-NH4+ treatment and solid for the control (phases I and II overlapped). For derivation of compartmental parameters, see text.
Table I.
Half-lives of NO3− exchange for the cytoplasmic compartment and cytoplasmic [NO3−] ([NO3−]cyt) in roots of intact cv CM-72 plants, determined by compartmental analysis
| Treatment |
t1/2,
Cytoplasm
|
[NO3−]cyt
|
||
|---|---|---|---|---|
| − | + | − | + | |
| min | mm | |||
| 0.1 mm NO3− | 10.94 ± 0.57 | 14.78 ± 1.04 | 41 ± 2.89 | 38.75 ± 3.06 |
| 1.0 mm NO3− | 8.52 ± 0.4 | 13.32 ± 0.99 | 51.69 ± 1.49 | 59.4 ± 2.8 |
| 10 mm NO3− | 7.75 ± 0.37 | 6.75 ± 0.24 | 76.18 ± 1.03 | 67.27 ± 9.34 |
| Uninduced | 9.17 ± 0.98 | 8.55 ± 0.49 | 2.05 ± 0.45 | 2.19 ± 0.31 |
| NO2− induced | 12.94 ± 0.53 | 17.59 ± 0.98 | 48.8 ± 3.1 | 50.6 ± 2.71 |
Plants were exposed to and labeled at the indicated concentrations of NO3− (steady state). Uninduced plants were grown in N-free solution but were exposed to 0.1 mm NO3− during labeling and elution. NO2−-induced plants were exposed to 0.1 mm NO2− for 24 h prior to labeling and elution at 0.1 mm NO3−. NH4+ was present at 1.0 mm during labeling and elution in + treatments. Data are ±se (n ≥ 6).
In general, half-life values for the cytoplasm were longer in the cv CM-72 used in the present study compared with the Klondike variety of barley used in previous studies (Siddiqi et al., 1991; Devienne et al., 1994; Kronzucker et al., 1995a, 1995b), indicating a larger relative accumulation capacity for NO3− (at comparable fluxes) in the former variety. The addition of NH4+ significantly affected cytoplasmic exchange kinetics in NO2−-induced plants as well as in plants grown at 0.1 and at 1 mm [NO3−]o: Half-lives increased in all of these cases (Fig. 1), whereas they remained unaffected in uninduced plants and at 10 mm [NO3−]o (Table I). These differences in half-life values are direct reflections of differences in flux partitioning, as discussed below.
Effect of NH4+ on NO3− Uptake and Subcellular N-Flux Partitioning
The addition of NH4+ led to a reduction of net NO3− uptake, as estimated by compartmental analysis (Table II) or as ascertained by direct methods (Table III). Moreover, there was a close correspondence between the values for the percentage of inhibition determined by CAE (Table II) and those determined independently by 14N depletion (Table III), although the absolute flux values tended to be higher in CAE determinations. The inhibitory effect of externally applied NH4+ is in agreement with other studies (see the introduction), although genetic variability and even a stimulation of NO3− uptake by NH4+ in isolated cases (Bloom and Finazzo, 1986) have been reported in the responses, which ranged from strong to low levels of inhibition (Schrader et al., 1972; Pan et al., 1985). In the present study the inhibition of net NO3− uptake by 1.0 mm NH4+ depended upon [NO3−] and the N species used to induce NO3− uptake prior to exposure to NH4+. In plants that were uninduced for NO3− uptake, NH4+ reduced net NO3− uptake by approximately 25% when measured in solutions containing 0.1 mm [NO3−]o. In plants induced for NO3− uptake, the corresponding values for inhibition were 35% and 25% when net uptake was measured in solutions containing 0.1 and 1.0 mm [NO3−]o, respectively, whereas at 10 mm [NO3−]o, 1 mm external NH4+ had no significant effect. The inhibition of net NO3− uptake was significantly less (approximately 14%–17%) when plants were induced with NO2− and NO3− uptake was measured using 0.1 mm [NO3−]o. The shortcomings of measuring net fluxes by depletion have been discussed previously (Kronzucker et al., 1995d, 1996). In particular, unless short-term (5–10 min) estimates are used, it is possible that plant acclimation will occur in response to declining [NO3−]o during the measurement.
Table II.
Component fluxes of NO3− as determined by compartmental analysis
| Treatment | Φoc
|
Φco
|
Φnet
|
Φred/vac
|
Φ*xylem
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| − | + | − | + | − | + | − | + | − | + | |
| μmol g−1 h−1 | ||||||||||
| 0.1 mm NO3− | 8.61 ± 0.77 | 5.77 ± 0.59 | 0.57 ± 0.02 | 0.84 ± 0.13 | 7.57 ± 0.76 | 4.93 ± 0.7 | 5.3 ± 0.6 | 3.49 ± 0.62 | 2.27 ± 0.25 | 1.44 ± 0.1 |
| 1.0 mm NO3− | 12.93 ± 0.88 | 10.02 ± 0.13 | 1.6 ± 0.56 | 1.46 ± 0.36 | 11.33 ± 1.44 | 8.55 ± 1.06 | 6.85 ± 1.22 | 5.86 ± 1.08 | 4.47 ± 0.22 | 2.69 ± 0.02 |
| 10 mm NO3− | 20.94 ± 0.73 | 21.58 ± 0.57 | 10.56 ± 3.97 | 12.14 ± 0.98 | 10.38 ± 4.7 | 9.44 ± 1.54 | 6.68 ± 2.08 | 5.95 ± 1.76 | 3.7 ± 2.62 | 3.49 ± 2.17 |
| Uninduced | 0.53 ± 0.12 | 0.48 ± 0.04 | 0.07 ± 0.01 | 0.13 ± 0.01 | 0.46 ± 0.1 | 0.34 ± 0.02 | 0.36 ± 0.07 | 0.28 ± 0.03 | 0.096 ± 0.03 | 0.055 ± 0.005 |
| NO2− induced | 8.1 ± 0.69 | 7.16 ± 0.74 | 0.29 ± 0.02 | 0.47 ± 0.09 | 7.8 ± 0.75 | 6.69 ± 0.49 | 6.67 ± 0.62 | 5.46 ± 0.49 | 1.13 ± 0.22 | 1.23 ± 0.09 |
Barley plants were exposed to and labeled at the indicated concentrations of NO3− for 24 h prior to experiments. Uninduced plants were grown in N-free solution but exposed to 0.1 mm NO3− during labeling and elution. “NO2−-induced” plants were exposed to 0.1 mm NO2− for 24 h prior to labeling and elution at 0.1 mm NO3−. NH4+ was present at 1.0 mm during labeling and elution in + treatments. Data are ±se (n ≥ 6).
Table III.
Estimates of NO3− influx and net flux into roots of intact cv CM-72 plants by methods independent of compartmental analysis
| Treatment | Φoc
|
Φnet
|
||
|---|---|---|---|---|
| − | + | − | + | |
| μmol g−1 h−1 | ||||
| 0.1 mm NO3− | 5.16 ± 0.45 | 3.26 ± 0.47 | 3.97 ± 0.7 | 2.56 ± 0.61 |
| 1 mm NO3− | 6.91 ± 0.34 | 5.37 ± 0.38 | 8.57 ± 0.16 | 6.34 ± 0.27 |
| 10 mm NO3− | 10.52 ± 0.4 | 10.04 ± 0.93 | N/D | N/D |
| Uninduced | 0.42 ± 0.02 | 0.24 ± 0.02 | N/D | N/D |
| NO2− induced | 4.49 ± 0.16 | 3.87 ± 0.08 | 5.55 ± 0.13 | 4.59 ± 0.16 |
Plants were exposed to, and fluxes were measured at, the indicated concentrations of NO3− (steady state). Uninduced plants were grown in N-free solution prior to flux measurement at 0.1 mm NO3−. NO2−-induced plants were exposed to 0.1 mm NO2− for 24 h prior to flux measurement at 0.1 mm NO3−. NH4+ was present at 1.0 mm during uptake in + treatments. Data are ±se (n ≥ 9).
NH4+ had a distinct and more potent effect on NO3− uptake in the high-affinity transport range (i.e. on the IHATS), which is evident below 1 mm [NO3−]o, than in the range of the LATS, which operates at [NO3−]o ≥ 1 mm (Siddiqi et al., 1989; King et al., 1993; Kronzucker et al., 1995d). This provides additional support for the argument that high- and low-affinity transport systems are biochemically distinct modes of transport (for review, see Glass and Siddiqi, 1995). NO2−-induced plants were substantially less sensitive to NH4+ inhibition of NO3− uptake than NO3−-induced plants. Aslam and coworkers (1994, 1997) used plants induced by and “labeled” with NO2− as model systems for NO3−-induced plants. Although NO2− and NO3− have been shown to act competitively at the level of uptake (Aslam et al., 1992; Siddiqi et al., 1992), the present findings suggest that NO2− may not serve as a satisfactory quantitative analog for NO3−. This is consistent with the finding by Siddiqi et al. (1992) that NO2− was not capable of inducing NO3− reductase activity in barley. Aslam et al. (1987, 1993, 1997), however, reached the opposite conclusion.
The effect of 1 mm NH4+ on 13NO3− influx followed the same pattern (with one exception) as that observed for net uptake, i.e. the extent of inhibition declined with increasing [NO3−]o, with 33% inhibition at 0.1 mm, 23% at 1.0 mm, and no effect at 10 mm. Inhibition was much smaller in NO2−-induced plants (11.6%). This was true when influx was determined by CAE and count accumulation after a 5-min exposure to tracer (Tables II and III). The one exception was that influx in uninduced plants (determined by CAE) was unaffected by NH4+, in contrast to the situation for net uptake by uninduced plants. We interpret this result to indicate that, like LATS, the NO3− influx via CHATS (King et al., 1992; Kronzucker et al., 1995d) is unaffected by NH4+. That exposure to NH4+ diminished net uptake in uninduced plants indicates an effect on efflux of NO3− in uninduced plants.
We measured CAE on uninduced plants by exposing roots to 0.1 mm [NO3−]o for the duration of the efflux analyses (the labeling and elution procedures lasted 82 min); by this time, the physiological induction of the IHATS and NO3− reductase would be relatively small (Friemann et al., 1992; Glass and Siddiqi, 1995; Kronzucker et al., 1995a, 1995b). In contrast to its lack of effect on constitutive influx, NH4+ stimulated NO3− efflux by as much as 86% (Table II) in uninduced plants. Although not measured directly, efflux was probably even larger during the shorter 5-min exposures to NH4+ (see discussion of short-term efflux enhancements below), which may explain the apparent depression of influx that we saw in the short-term determinations after the addition of NH4+ (Table III), but not in the CAE determinations (Table II).
Efflux stimulation was also substantial in NO2−-induced plants (approximately 62%), whereas in plants grown at 0.1 mm [NO3−]o, efflux was enhanced by less than 50% and no efflux stimulation at all was seen in plants grown at 1.0 and 10 mm [NO3−]o (Table II; Fig. 1). NO3− efflux (expressed as a percentage of influx) also increased as [NO3−]o was raised from 0.1 to 10 mm even in the absence of NH4+ (Table II), as previously reported (Siddiqi et al., 1991; Kronzucker et al., 1995b; Volk, 1997). Our results confirm a distinct difference between inducible high-affinity transport and low-affinity transport; they also confirm the fact that NO2− induction of NO3− transport cannot be used as a quantitative model for NO3− induction and provision under steady-state conditions. The latter point is particularly important, because experiments on plants pretreated in this way have led to the conclusion that the NH4+ inhibition of net NO3− uptake results exclusively from the effects on NO3− efflux and that influx is unaffected (Aslam et al., 1994). Furthermore, the relative effect of NH4+ was high in the present study (>50% stimulation) because efflux is typically low under control conditions, and the absolute contribution to reduced net uptake was still small in comparison with the contribution arising from reduced influx.
Our experiments show that in NO2−-induced plants, the combined flux of 13NO3− and 13N-assimilation products to the shoot (Φ*xylem) was unaffected by the addition of NH4+, whereas it was reduced in all other treatments (Table II). Also, Φ*xylem in NO2−-induced plants was substantially lower than in NO3−-induced plants, and approximated that of plants induced at 0.1 mm [NO3−]o after the application of 1 mm NH4+. Because significant suppression of NO3− reductase activity by NH4+ is well documented (MacKown et al., 1982a, 1982b; Breteler and Siegerist, 1984; Pan et al., 1985; de la Haba et al., 1990; Aslam et al., 1997; however, see Oaks et al., 1979), Φ*xylem under these conditions will be mostly in the form of 13NO3− after NH4+ addition. Therefore, the lack of an effect of NH4+ on Φ*xylem in NO2−-induced plants (measured at 0.1 mm [NO3−]o) appears to support the biochemically based conclusion arrived at by Siddiqi et al. (1992) and King et al. (1993) that NO3− reductase activity is not induced to any significant extent by NO2− in barley roots, in contrast to its full induction by NO3−. Claims to the contrary have been made by Aslam et al. (1987, 1993; see Glass and Siddiqi, 1995).
Our CAE analyses do not allow a separation of the reduced and unreduced components of the xylary 13N-translocation term, nor do they permit separation of the components of Φred/vac, namely the biochemical N flux to reduced N, and the N flux to the vacuole (Lee and Clarkson, 1986; Siddiqi et al., 1991; Kronzucker et al., 1995a, 1995b). However, given that the cytoplasmic [NO3−] values in NO2−- and NO3−-induced plants were indistinguishable after 13N loading (Table I), and thus similar values of Φ*xylem for NO3− are to be expected, we must conclude that NO2− induction leads to a relatively greater stimulation of the NO3− flux to the vacuole (in both the control and NH4+-treated plants). The reduction in Φred/vac after NH4+ addition to NO2−-induced plants (Table II) appears to be a direct result of the inhibition of influx and the stimulation of efflux rather than the effect upon NO3− reductase activity.
The clear differences in flux partitioning between NO3−- and NO2−-induced plants revealed by CAE lead us to caution against the use of NO2− as an “analog” for NO3−. Similar concerns regarding the use of 36ClO3− as an analog for NO3− were expressed in earlier work (Deane-Drummond and Glass, 1983; Dean-Drummond, 1985, 1986), although the basis of the failure to faithfully trace NO3− in the case of ClO3− was more straightforward (Glass et al., 1985; Lee and Drew, 1989; Siddiqi et al., 1992; Aslam et al., 1994; Glass and Siddiqi, 1995). Doddema and Telkamp (1979) also observed a significant rise in NO3− efflux upon the addition of NH4+; however, this response was transient and restricted to the perturbational condition (see below). Our present analyses demonstrate that an enhancement of the efflux component makes only a small contribution to the reduction of net uptake, whereas the principal effect of NH4+ on NO3− uptake comes through the inhibition of influx (except in uninduced plants and in plants pretreated with NO2−, where the efflux contribution is magnified).
Kinetics of the Response
It has been shown previously that the inhibition of NO3− influx by NH4+ is an immediate phenomenon, detectable even within 15 s (Glass et al., 1985; Ingemarsson et al., 1987; Lee and Drew, 1989; Ayling, 1993; Aslam et al., 1994), and that it is reversible with relaxation times of only 2 to 3 min (Lee and Drew, 1989). In the present study we tested the immediacy and reversibility of the stimulation of NO3− efflux by NH4+ (in plants grown with 0.1 mm [NO3−]o); we added NH4+ under perturbational conditions only during the elution of the cytoplasmic compartment in 13NO3− efflux experiments. Figures 2 and 3 show that the effect of NH4+ on NO3− efflux was evident immediately after its addition to the elution solutions (Fig. 2). Upon withdrawal of NH4+ from these solutions, NO3− efflux rapidly returned to normal (Fig. 3); repeated additions/withdrawals confirmed that the process was fully reversible. Notwithstanding this clear response, the absolute contribution to the inhibition of NO3− net uptake was small compared with the large reduction of influx. Moreover, in a separate study on rice (H.J. Kronzucker, A.D.M. Glass, and M.Y. Siddiqi, unpublished results), we found that in long-term studies under steady-state conditions in which both NO3− and NH4+ are provided, NO3− efflux was reduced rather than enhanced. Under these conditions efflux as a percentage of influx was very similar to that seen when only NO3− was provided; thus the efflux enhancement appears to be temporary.
The rapidity of the response to NH4+ on both the influx and efflux components of NO3− uptake provides a compelling argument that the NH4+ effect occurs directly at the plasma membrane. Lee and Drew (1989) demonstrated a logarithmic relationship between the inhibition of NO3− influx by NH4+ and external [NH4+], which led to the suggestion that membrane depolarization by NH4+ may inhibit the NO3−/2H+ cotransport system due to effects on the proton motive force (Ullrich et al., 1984; Ayling, 1993). However, the provision of K+, which also depolarizes the plasma membrane to an extent similar to that of NH4+, fails to inhibit NO3− uptake (Glass and Siddiqi, 1995; Wang et al., 1996), arguing for a more specific effect of NH4+. Given the rapidity of the response, the inhibition probably occurs allosterically, rather than by involving the products of NO3− reduction and N assimilation, or possibly the effects of transcription or translation.
Similar conclusions have been reached by others (Deane-Drummond and Glass, 1983; Ingemarsson et al., 1987; Lee and Drew, 1989; Warner and Huffaker, 1989; Aslam et al., 1994). In agreement with de la Haba et al. (1990) and Aslam et al. (1994), we found that pretreatment of barley and rice plants with the Gln synthetase inhibitor Met sulfoximine for 6 h at 1 mm did not alleviate the inhibitory effect exerted by externally added NH4+ (data not shown). Thus it seems unlikely that N assimilates downstream of NH4+ are involved in the inhibition of NO3− uptake. We must stress that these conclusions apply only to the rapid effects of NH4+ on NO3− uptake; some (Krapp et al., 1998; Zhuo et al., 1999) have suggested that there may be long-term effects of Gln and other amino acids at the transcription level.
Summary
Our analyses provide evidence that the inhibitory effect of NH4+ upon NO3− uptake is mediated primarily by inhibiting NO3− influx, with only a small contribution from the enhancement of NO3− efflux, which: (a) is both transient and reversible, (b) is associated with a large efflux only in uninduced plants and plants induced by NO2− (i.e. under conditions where influx is very low), (c) is dependent on [NO3−]o, (d) is strong for IHATS but small for CHATS and LATS, (e) occurs directly at the plasma membrane (i.e. it does not involve NO3− reduction or N-assimilation products in the short term, although it may in the long-term); and (f) cannot be modeled quantitatively by the use of NO2− as an analog of NO3−.
ACKNOWLEDGMENTS
We thank D.T. Britto, M. Okamoto, D. Zhuo, and the staff at the Tri-University Meson Facility particle accelerator for technical help and discussions. We also thank Prof. R.C. Huffaker for generously providing us with cv CM-72 seeds.
Abbreviations:
- CAE
compartmental analysis by efflux
- CHATS
constitutive high-affinity transport system
- IHATS
inducible high-affinity transport system
- LATS
low-affinity transport system
- [NO3−]o
external [NO3−] Φ, ionic (N) flux (component fluxes denoted by subscripts, as indicated in text)
Footnotes
This work was supported by a National Science and Engineering Research Council grant to A.D.M.G. and by a University of Western Ontario grant to H.J.K.
LITERATURE CITED
- Aslam M, Rosichan JL, Huffaker RC. Comparative induction of nitrate reductase by nitrate and nitrite in barley leaves. Plant Physiol. 1987;83:579–584. doi: 10.1104/pp.83.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Travis RL, Huffaker RC. Comparative kinetics and reciprocal inhibition of nitrate and nitrite uptake in roots of uninduced and induced barley (Hordeum vulgare L.) seedlings. Plant Physiol. 1992;99:1124–1133. doi: 10.1104/pp.99.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Travis RL, Huffaker RC. Comparative induction of nitrate and nitrite uptake and reduction systems by ambient nitrate and nitrite in intact roots of barley (Hordeum vulgare L.) seedlings. Plant Physiol. 1993;102:811–819. doi: 10.1104/pp.102.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Travis RL, Huffaker RC. Stimulation of nitrate and nitrite afflux by ammonium in barley (Hordeum vulgare L.) seedlings. Plant Physiol. 1994;106:1293–1301. doi: 10.1104/pp.106.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Travis RL, Rains DW, Huffaker RC. Differential effect of ammonium on the induction of nitrate and nitrite reductase activities in roots of barley (Hordeum vulgare L.) seedlings. Physiol Plant. 1997;101:612–619. [Google Scholar]
- Ayling SM. The effect of ammonium ion on membrane potential and anion flux in roots of barley and tomato. Plant Cell Environ. 1993;16:297–303. [Google Scholar]
- Bloom AJ, Finazzo J. The influence of ammonium and chloride on potassium and nitrate absorption by barley roots depends on time of exposure and cultivar. Plant Physiol. 1986;81:67–69. doi: 10.1104/pp.81.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breteler H, Siegerist M. Effect of ammonium on nitrate utilization by dwarf bean. Plant Physiol. 1984;75:1099–1103. doi: 10.1104/pp.75.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawse PA. The determination of nitrate in soil solutions by ultraviolet spectrophotometry. Analyst. 1967;92:311–315. [Google Scholar]
- Chaillou S, Rideout JW, Raper CD, Morot-Gaudry J-F. Responses of soybean to ammonium and nitrate supplied in combination to the whole root system or separately in a split-root system. Physiol Plant. 1994;90:259–268. [PubMed] [Google Scholar]
- de la Haba P, Aguera E, Maldonado JM. Differential effects of ammonium and tungsten on nitrate and nitrite uptake and reduction by sunflower plants. Plant Sci. 1990;70:21–26. doi: 10.1007/BF00239997. [DOI] [PubMed] [Google Scholar]
- Deane-Drummond CE. Regulation of nitrate uptake into Chara corallina cells via NH4+ stimulation of NO3− efflux. Plant Cell Environ. 1985;8:105–111. [Google Scholar]
- Deane-Drummond CE. Nitrate uptake into Pisum sativum L cv. Feltham First seedlings: commonality with nitrate uptake into Chara corallina and Hordeum vulgare through a substrate cycling model. Plant Cell Environ. 1986;9:41–48. [Google Scholar]
- Deane-Drummond CE, Glass ADM. Short-term studies of nitrate uptake into barley plants using ion-specific electrodes and 36ClO3−. II. Regulation of NO3− efflux by NH4+ Plant Physiol. 1983;73:105–110. doi: 10.1104/pp.73.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devienne F, Mary B, Lamaze T. Nitrate transport in intact wheat roots. I. Estimation of cellular fluxes and NO3− distribution using compartmental analysis from data of 15NO3− efflux. J Exp Bot. 1994;45:667–676. [Google Scholar]
- Doddema H, Telkamp GP. Uptake of nitrate by mutants of Arabidopsis thaliana disturbed in uptake or reduction of NO3−. II. Kinetics. Physiol Plant. 1979;45:332–338. [Google Scholar]
- Fried MF, Zsoldos F, Vose PB, Shatokin IL. Characterizing the NO3− and NH4+ uptake process of rice roots by use of 15N-labeled NH4NO3. Physiol Plant. 1965;18:313–320. [Google Scholar]
- Friemann A, Lange M, Hachtel W, Brinkmann K. Induction of nitrate assimilatory enzymes in the tree Betula pendula. Plant Physiol. 1992;99:837–842. doi: 10.1104/pp.99.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass ADM, Siddiqi MY (1995) Nitrogen absorption in higher plants. In HS Srivastava, RP Singh, eds, Nitrogen Nutrition in Higher Plants. Associated Publishing, New Delhi, India, pp 21–55
- Glass ADM, Thompson RG, Bordeleau L. Regulation of NO3− influx in barley. Studies using 13NO3−. Plant Physiol. 1985;77:379–381. doi: 10.1104/pp.77.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingemarsson B, Oscarson P, af Ugglas M, Larsson C-M. Nitrogen utilization in Lemna. III. Short-term effects of ammonium on nitrate uptake and nitrate reduction. Plant Physiol. 1987;85:865–867. doi: 10.1104/pp.85.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WA, Kwik KD, Volk RJ, Butz RG. Nitrate influx and efflux by intact wheat seedlings: effects of prior nitrate nutrition. Planta. 1976;132:149–156. doi: 10.1007/BF00388896. [DOI] [PubMed] [Google Scholar]
- King BJ, Siddiqi MY, Glass ADM. Studies of the uptake of nitrate in barley. V. Estimation of root cytoplasmic nitrate concentrations using nitrate reductase activity. Implications for nitrate influx. Plant Physiol. 1992;99:1582–1589. doi: 10.1104/pp.99.4.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BJ, Siddiqi MY, Ruth TJ, Warner RL, Glass ADM. Feedback regulation of nitrate influx in barley roots by nitrate, nitrite, and ammonium. Plant Physiol. 1993;102:1279–1286. doi: 10.1104/pp.102.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Fraisier V, Scheible W-R, Quesada A, Gojon A, Stitt M, Caboche M, Daniel-Vedele F. Expression studies of Nrt2:1Np, a putative high-affinity nitrate transporter: evidence for its role in nitrate uptake. Plant J. 1998;14:723–731. [Google Scholar]
- Kronzucker HJ, Glass ADM, Siddiqi MY. Nitrate induction in spruce: an approach using compartmental analysis. Planta. 1995a;196:683–690. [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Compartmentation and flux characteristics of nitrate in spruce. Planta. 1995b;196:674–682. [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Compartmentation and flux characteristics of ammonium in spruce. Planta. 1995c;196:691–698. [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Kinetics of NO3− influx in spruce. Plant Physiol. 1995d;109:319–326. doi: 10.1104/pp.109.1.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Analysis of 13NH4+-efflux in spruce roots. A test case for compartment identification in efflux analysis. Plant Physiol. 1995e;109:481–490. doi: 10.1104/pp.109.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Kinetics of NH4+ influx in spruce. Plant Physiol. 1996;110:773–779. doi: 10.1104/pp.110.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature. 1997;385:59–61. [Google Scholar]
- Lee RB, Clarkson DT. Nitrogen-13 studies of nitrate fluxes in barley roots. I. Compartmental analysis from measurements of 13N efflux. J Exp Bot. 1986;37:1753–1767. [Google Scholar]
- Lee RB, Drew MC. Rapid, reversible inhibition of nitrate influx in barley by ammonium. J Exp Bot. 1989;40:741–752. [Google Scholar]
- Lycklama JC. The absorption of ammonium and nitrate by perennial rye-grass. Acta Bot Neerl. 1963;12:361–423. [Google Scholar]
- MacKown CT, Jackson WA, Volk RJ. Restricted nitrate influx and reduction in corn seedlings exposed to ammonium. Plant Physiol. 1982a;69:353–359. doi: 10.1104/pp.69.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKown CT, Volk RJ, Jackson WA. Nitrate assimilation by decapitated corn root systems: effects of ammonium during induction. Plant Sci Lett. 1982b;24:295–302. [Google Scholar]
- Minotti PL, Williams DC, Jackson WA. Nitrate uptake by wheat as influenced by ammonium and other cations. Crop Sci. 1969;9:9–14. [Google Scholar]
- Oaks A, Stulen I, Boesel IL. Influence of amino acids and ammonium on nitrate reduction in corn seedlings. Can J Bot. 1979;57:1824–1829. [Google Scholar]
- Oscarson P, Ingemarsson B, af Ugglas M, Larsson C-M. Short-term studies of NO3− uptake in Pisum using 13NO3−. Planta. 1987;170:550–555. doi: 10.1007/BF00402990. [DOI] [PubMed] [Google Scholar]
- Pan WL, Jackson WA, Moll RH. Nitrate uptake and partitioning by corn root systems. Differential effects of ammonium among genotypes and stages of root development. Plant Physiol. 1985;77:560–566. doi: 10.1104/pp.77.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KP, Rains DW. Nitrate absorption by barley. I. Kinetics and energetics. Plant Physiol. 1976;57:55–58. doi: 10.1104/pp.57.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty TW, Raper CD, Jackson WA. Growth and nitrogen assimilation of soybeans in response to ammonium and nitrate nutrition. Bot Gaz. 1983;144:466–470. [Google Scholar]
- Schrader LE, Domska PE, Jung PE, Jr, Peterson LA. Uptake and assimilation of ammonium-N and nitrate-N and their influence on the growth of corn (Zea mays L.) Agron J. 1972;64:690. 695. [Google Scholar]
- Siddiqi MY, Glass ADM, Ruth TJ. Studies of the uptake of nitrate in barley. III. Compartmentation of NO3−. J Exp Bot. 1991;42:1455–1463. [Google Scholar]
- Siddiqi MY, Glass ADM, Ruth TJ, Fernando M. Studies on the regulation of nitrate influx by barley seedlings using 13NO3−. Plant Physiol. 1989;90:806–813. doi: 10.1104/pp.90.3.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi MY, King BJ, Glass ADM. Effects of nitrite, chlorate, and chlorite on nitrate uptake and nitrate reductase activity. Plant Physiol. 1992;100:644–650. doi: 10.1104/pp.100.2.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich WR, Larsson M, Larsson C-M, Lesch S, Novacky A. Ammonium uptake in Lemna gibba G1, related membrane potential changes, and inhibition of anion uptake. Physiol Plant. 1984;61:369–376. [Google Scholar]
- Volk RJ. Unidirectional fluxes of nitrate into and out of maize roots: measurements and regulation by prior nitrate nutrition. Plant Sci. 1997;123:1–7. [Google Scholar]
- Wang MY, Siddiqi MY, Glass ADM. Interactions between K+ and NH4+ effects on ion uptake by rice roots. Plant Cell Environ. 1996;19:1037–1046. [Google Scholar]
- Warner RL, Huffaker RC. Nitrate transport is independent of NADH and NAD(P)H nitrate reductases in barley seedlings. Plant Physiol. 1989;91:947–953. doi: 10.1104/pp.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman GS. Growth and nitrogen absorption of wheat seedlings as influenced by the ammonium:nitrate ratio and the hydrogen ion concentration. Am J Bot. 1950;37:725–738. [Google Scholar]
- Zhuo D, Okamoto M, Vidmar JJ, Glass ADM (1999) Regulation of a putative high-affinity nitrate transporter (Nrt2;1At) in roots of Arabidopsis thaliana. Plant J (in press) [DOI] [PubMed]