Abstract
Background
Carbapenem-nonsusceptible Klebsiella pneumoniae (CnSKP) is rapidly emerging as a life-threatening nosocomial infection. The efficacy of tigecycline in the treatment of bloodstream infections (BSIs) remains controversial.
Methods
Data from a total of 428 patients with carbapenem-susceptible Klebsiella pneumoniae (CSKP) and CnSKP BSIs were collected at a single center between January 2013 and December 2015. A three-part analysis was conducted to identify the risk factors associated with CnSKP, explore prognosis, and evaluate treatments.
Results
Data from 428 patients with Klebsiella pneumoniae (KP) BSIs were included, 31.5% (n=135) of them with CnSKP. Multivariate analysis showed that prior hospitalization, urinary catheterization, the use of immunosuppressive agents, prior use of antibiotics, pulmonary disease, and high Acute Physiology and Chronic Health Evaluation (APACHE) II scores were independent risk factors for CnSKP-BSIs. The 30-day mortality was higher in patients with CnSKP than in those with CSKP (58.5% vs 15.4%; P<0.001). In patients with KP-BSIs, neutropenia, multiple organ dysfunction, respiratory failure, CnSKP infection, high APACHE II score, and tigecycline therapy were independently associated with higher mortality risk. Among patients whose APACHE II score was <15, higher mortality rates were observed in patients treated with tigecycline than in those treated with other antibiotics (45.3% vs 7.7%; P<0.001). Central venous catheterization, multiple organ dysfunction, and high APACHE II scores were independent risk factors for death from CnSKP.
Conclusion
A significant increase in the incidence of CnSKP-BSIs was observed during the study period, with a higher mortality rate found in these patients. Exposure to carbapenems and severe illness were independent risk factors for the development of CnSKP-BSIs, and tigecycline therapy resulted in a significant increase in mortality.
Keywords: Klebsiella pneumoniae, bloodstream infection, carbapenem nonsusceptible, risk factors, tigecycline
Introduction
Klebsiella pneumoniae (KP) is a pathogen that is mainly associated with community and nosocomial infections; after Escherichia coli, it is the second most common pathogen that leads to gram-negative bloodstream infections (BSIs).1,2 With more and more KP isolates producing extended-spectrum β-lactamase, and therefore exhibiting resistance to many penicillin and cephalosporin antibiotics, carbapenems are the most widely used first-line antibiotics for such infections.3 However, the widespread use of these antibiotics has caused the emergence of carbapenem-resistant strains, mostly because of the propagation of carbapenemhydrolyzing β-lactamases like the KP carbapenemase (KPC). KPC-producing KP was first reported in 1996,4 and in the People’s Republic of China, the first KPC-positive KP isolates were found in intensive care unit (ICU) from a 75-year-old patient in 2004;5 it has subsequently emerged as a global health care threat and is now endemic in many countries.6–8
As well as being a serious public health issue and infection control challenge, carbapenem-resistant Klebsiella pneumoniae (CRKP) is related to higher treatment failure rates, mortality, and cost.7,8 Prior studies show that BSIs caused by carbapenem-nonsusceptible Klebsiella pneumoniae (CnSKP) are associated with disappointing outcomes; the hospital death rates associating with these infections range from 40% to 72% compared with 20% to 30% in patients with carbapenem-susceptible Klebsiella pneumoniae (CSKP) infections.9–12 Furthermore, being older, hospital-acquired infections, ICU stay, illness severity, and inappropriate regimens have been identified as risk factors contributing to increased mortality rates in patients with CnSKP-BSI.13,14
In previous retrospective studies, tigecycline combined with colistin, carbapenems, or aminoglycosides was found to be the most common regimen used for the management of carbapenem-resistant Enterobacteriaceae infection,15,16 although the most beneficial of the regimens has not yet been identified.12,17,18 Therefore, studies recognizing risk factors for the development of CnSKP-BSI and exploring the most effective therapeutic approaches are required. In this study, a retrospective group of patients with KP-BSIs were analyzed to identify the risk factors accompanied by CnSKP, explore prognosis, and evaluate treatments.
Methods
Study design
This retrospective study was conducted at the First Affiliated Hospital, College of Medicine, Zhejiang University, a 2500-bed teaching hospital in Eastern China, after receiving approval from the Research Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University. This study did not directly interfere with the patients or show the patients’ name, medical record number, or other personal information. Moreover, there was no adverse effect on the rights of patients; therefore, consent to review their medical records was not required by the Institutional Review Board. The study population comprised patients treated for BSI caused by KP (KP-BSI) between January 1, 2013, and December 31, 2015. Patients whose age was <16 years were excluded. If there were more than two episodes of KP-BSI in one patient, only the first episode was included. For the mortality analysis, patients who did not accept >48 hours of antimicrobial treatment for any reason were excluded. Patient demographics; clinical and microbiological data; laboratory analyses; data on antimicrobial therapy, underlying diseases, and comorbidities; and other relevant information were retrieved from the hospital information system. Illness severity was assessed by using the Acute Physiology and Chronic Health Evaluation (APACHE) II scores calculated when BSIs attack.19 Charlson comorbidity index was used to determine comorbid conditions.20
Data analysis
In order to assess treatment outcomes, 30-day mortality was investigated. As illustrated in Figure S1, a three-part analysis was conducted: 1) to evaluate the risk factors associated with CnSKP-BSI, 428 patients were divided into CSKP and CnSKP patient groups; 2) to explore the prognosis of KP-BSI and antibiotic treatment programs, the patients were categorized as survivors if they were alive after 30 days of infection or nonsurvivors if they were not (patients whose treatment time was <48 hours were excluded); and 3) to assess the risk factors associated with the 30-day mortality and treatment among patients with CnSKP-BSI, a case-controlled study was conducted.
Microbiological assessment and definition of terms
KP-BSI onset was defined as the collection date of the first positive blood culture. The probable infectious source was determined by using Centers for Disease Control and Prevention/National Healthcare Safety Network surveillance definitions; primary BSI was recorded if no source was identified.21 When an absolute neutrophil count was <1500/μL on BSI onset, it was defined as neutropenia. Steroid therapy was defined as >20 mg/day prednisone or its equivalent administered for ≥7 days. Antimicrobial drug exposure referred to the use of antibiotics for >72 hours at any point 2 weeks prior to BSI diagnosis. Empirical therapy indicated all antimicrobial drugs administered to treat a suspected BSI. Definitive therapy referred to antimicrobial therapy administered after the susceptibility testing results were available and was classified as “appropriate” if an adequate dose of at least one drug was administered to which the pathogen was susceptible (as indicated by in vitro susceptibility testing) or “inappropriate” if these criteria were not met.22 Overall mortality included all causes of death during hospitalization. During the study period, tigecycline was used to treat CnSKP-BSI, and its dosing was classified into conventional (100 mg loading dose, followed by 50 mg every 12 hours) or high dose (100 mg every 12 hours).23
The identification and antimicrobial susceptibility of KP were determined by using the Vitek2 system (bioMérieux, Marcy-l’Etoile, France). The minimum inhibitory concentration (MIC) of tigecycline was determined by using standard broth microdilution tests with fresh (<12 hours) Mueller–Hinton II Broth (cation-adjusted; Solarbio Science and Technology Ltd., Beijing, People’s Republic of China). According to the guidelines of the Clinical and Laboratory Standards Institute standards (2015), carbapenem-non-susceptibility is defined as an MIC of ≥1 mg/L for ertapenem or ≥2 mg/L for imipenem or meropenem.24 The US Food and Drug Administration (FDA) break points were used to judge tigecycline susceptibilities.25
Statistical analysis
In order to evaluate continuous variables, the Student’s t-test (for normally distributed variables) or Mann–Whitney U test (for variables that are not normally distributed) was used. Categorical variables were analyzed by using the χ2 test or two-tailed Fisher’s exact test appropriately. For continuous variables, results are expressed as median (interquartile range) or mean ± standard deviation, and categorical variables are expressed using the percentages of the group. The strength of all associations that emerged was determined using odds ratios (ORs) and 95% confidence intervals (CIs). Two-tailed tests were used to determine statistical significance. For multivariate analysis to identify independent predictors, variables with a P-value ≤0.05 in the univariate analysis were used in binary logistic regression. Kaplan–Meier product limit method was used to estimate the survival distribution function; nonparametric (log rank and Wilcoxon) tests were used to compare survival functions in different groups. In all analyses, P-values ≤0.05 were considered significant. All statistical analyses were carried out by using the SPSS Version 23.0 (IBM Corporation, Armonk, NY, USA).
Results
During the 3-year study period, 436 patients with at least one positive blood culture for Klebsiella were evaluated; 8 patients aged <16 years were excluded. Of the 428 patients included, 31.5% (n=135) had CnSKP. The overall incidence of KP-BSI was 0.154/1000 patient-days during the 3-year period (Figure S2). The overall incidence of CnSKP-BSI increased from 0.037/1000 patient-days in 2013 to 0.062/1000 patient-days in 2015, with the highest incidence occurring in the ICU (1.030/1000 patient-days). The results of antimicrobial susceptibility testing showed that the resistance rate of KP isolates to most antimicrobial agents was 35.0%–60%.
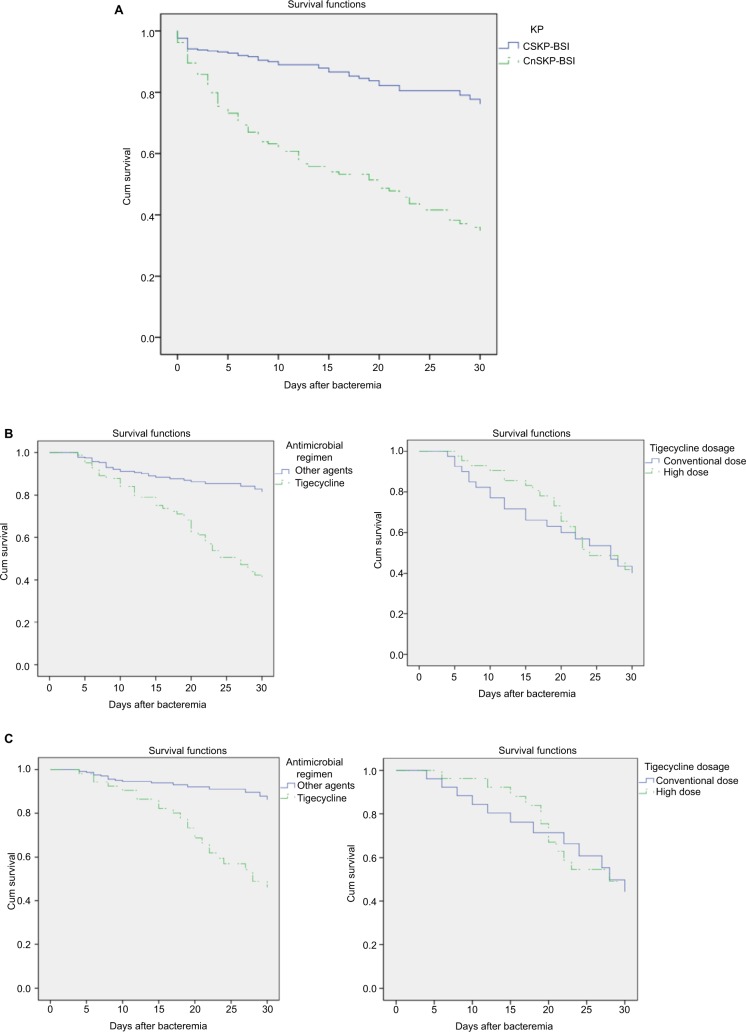
Table 1 shows the patient demographics and clinical characteristics. Regarding the probable infectious source of KP-BSI, intra-abdominal infection was most common (38.3%), followed by respiratory tract infection (31.8%) and primary bacteremia (17.5%). The overall all-cause 30-day mortality rate of KP-BSI patients was 29% (124 of 428); this was found to be significantly higher in patients with CnSKP-BSI (58.5%) than in those with CSKP (15.4%). Survival curve analysis confirmed the higher risks of mortality related to CnSKP-BSI (χ2=63.180, P<0.001; Figure 1A).
Table 1.
Clinical and demographic characteristics of patients with BSI caused by Klebsiella pneumoniae
| Univariate analysis
|
Multivariable analysis
|
||||||
|---|---|---|---|---|---|---|---|
| CSKP (n=293) | CnSKP (n=135) | P-values | Sig. | Exp(B) | 95% CI for Exp(B)
|
||
| Lower | Upper | ||||||
| Demographic | |||||||
| Gender, male, n (%) | 198 (67.6) | 101 (74.8) | 0.129 | ||||
| Age, years, mean ± SD | 58.7±16.4 | 59.1±15.4 | 0.799 | ||||
| Duration before bacteremia, days (IQR) | 4 (1–16) | 16 (6–37) | <0.001 | ||||
| Preexisting medical conditions | |||||||
| Pulmonary disease | 33 (11.3) | 51 (37.8) | <0.001 | 0.008 | 2.599 | 1.280 | 5.280 |
| Hepatic disease | 90 (30.8) | 30 (22.2) | 0.066 | ||||
| Hepatapostema | 45 (15.4) | 6 (4.4) | 0.001 | ||||
| Solid tumor | 60 (10.5) | 13 (9.6) | 0.006 | ||||
| CCI score (≥3), n (%) | 105 (35.8) | 60 (44.8) | 0.078 | ||||
| Likely source of bacteremia | |||||||
| Catheter-related | 7 (2.4) | 9 (6.7) | 0.030 | ||||
| Pneumonia | 69 (23.5) | 67 (49.7) | <0.001 | ||||
| Intra-abdominal | 123 (42) | 41 (30.4) | 0.022 | ||||
| Urinary tract | 6 (2.0) | 1 (0.7) | 0.441 | ||||
| Intracranial infection | 3 (1.0) | 4 (3.0) | 0.214 | ||||
| Mixed infection | 13 (4.4) | 14 (10.4) | 0.019 | ||||
| Primary bloodstream infection | 69 (23.5) | 6 (4.4) | <0.001 | ||||
| Hospital-acquired infection | 253 (86.3) | 135 (100) | <0.001 | ||||
| Prior hospitalizationa | 127 (43.3) | 92 (68.1) | <0.001 | 0.004 | 2.395 | 1.326 | 4.328 |
| Prior ICU stayb | 52 (17.7) | 87 (64.4) | <0.001 | ||||
| Prior surgeryb | 95 (32.4) | 65 (48.1) | 0.002 | ||||
| Previous transplantationsb | 5 (1.7) | 21 (15.6) | <0.001 | ||||
| Invasive procedure or devicesb | 96 (32.8) | 74 (54.8) | <0.001 | ||||
| Mechanical ventilation | 58 (19.8) | 100 (74.1) | <0.001 | ||||
| Central venous catheterization | 64 (21.8) | 101 (74.8) | <0.001 | ||||
| Urinary catheterization | 77 (26.3) | 110 (81.5) | <0.001 | <0.001 | 5.277 | 2.748 | 10.134 |
| Percutaneous tube | 61 (20.8) | 58 (43) | <0.001 | ||||
| Prior hemodialysisb | 19 (6.5) | 36 (26.7) | <0.001 | ||||
| Prior chemotherapy or radiotherapyb | 38 (13) | 6 (4.4) | 0.007 | ||||
| Prior corticosteroid useb | 39 (13.3) | 42 (31.1) | <0.001 | ||||
| Prior immunosuppressant useb | 21 (7.2) | 28 (20.7) | <0.001 | 0.001 | 4.093 | 1.734 | 9.661 |
| Use of antibiotics within 14 days prior to BSIc | 128 (43.7) | 120 (88.9) | <0.001 | 0.007 | 2.739 | 1.311 | 5.721 |
| Number of antibiotics | 0 (0–1) | 2 (1–3) | <0.001 | ||||
| Cephalosporin | 12 (4.1) | 17 (12.6) | 0.001 | ||||
| β-lactam and/or β-lactamase inhibitor | 84 (28.7) | 66 (48.9) | <0.001 | ||||
| Tigecycline | 10 (3.4) | 26 (19.4) | <0.001 | ||||
| Carbapenem | 36 (12.3) | 73 (54.1) | <0.001 | <0.001 | 4.591 | 2.331 | 9.044 |
| Fluoroquinolone | 22 (7.5) | 25 (18.5) | 0.001 | ||||
| Laboratory examination | |||||||
| Serum total protein, g/L | 61.8 (54.7–66.8) | 57.9 (53.0–65.4) | 0.023 | ||||
| Serum albumin <30 g/L | 93 (31.7) | 52 (38.5) | 0.169 | ||||
| Mean APACHE II score ± SD | 8.9±4.4 | 12.6±5.6 | <0.001 | 0.001 | 1.100 | 1.042 | 1.162 |
Notes: Data are expressed as numbers (%) unless otherwise stated;
During the 3 months preceding the BSI onset;
during the 30 days preceding BSI onset.
during the 14 days preceding BSI onset.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BSI, bloodstream infection; CCI, Charlson comorbidity index; CI, confidence interval; CnSKP, carbapenem-nonsusceptible Klebsiella pneumoniae; CSKP, carbapenem-susceptible Klebsiella pneumoniae; ICU, intensive care unit; IQR, interquartile range; SD, standard deviation.
Figure 1.
Kaplan–Meier survival estimates: (A) patients with BSI caused by CSKP and CnSKP (P<0.001); (B) KP-BSI patients treated with tigecycline (or other agents) and its dose effect; (C) KP-BSI patients (APACHE II score <15) treated with tigecycline (or other agents) and its dose effect.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BSI, bloodstream infection; CnSKP, carbapenem-nonsusceptible KP; CSKP, carbapenem-susceptible KP; KP, Klebsiella pneumoniae.
Risk factors associated with the development of CnSKP-BSIs
The univariate analysis showed that, compared with patients with CSKP-BSIs, those with CnSKP-BSIs were more likely to have nosocomial infection, respiratory tract origination, prior hospitalization, prior ICU hospitalization, or previous transplantations or to have undergone a nonsurgical invasive procedure, hemodialysis, chemotherapy, or radiotherapy. They also had lower total protein and high APACHE II scores and were more likely to have received corticosteroid therapy, immunosuppression, or prior exposure to drugs in the previous 14 days. In the multivariate analysis, logistic regression analysis (Table 1) showed the following factors to be independent risk factors for CnSKP-BSIs: hospitalization within 90 days before infection (OR =2.395, P=0.004), prior Foley catheterization (OR =5.277, P<0.001), immunosuppressive exposure (OR =4.093, P=0.001), prior use of antibiotics within 14 days prior to BSI (OR =2.739, P<0.001), previous carbapenem exposure (OR =4.591, P<0.001), pulmonary disease comorbidity (OR =2.599, P=0.008), and high APACHE II score (OR =1.100, P=0.001).
Risk factors for 30-day mortality in patients with KP-BSI
Of the 428 patients, 292 were classified as survivors and 78 as nonsurvivors; 58 patients were excluded as their treatment time was <48 hours. In the multivariate analysis (Table 2), factors independently associated with a higher risk of mortality were as follows: neutropenia, multiple organ dysfunction, respiratory failure, CnSKP infection, high APACHE II score, and tigecycline therapy after BSI. As shown in Table 2, carbapenem (n=254, 68.6%) was the most commonly used agent, followed by β-lactam and/or β-lactamase inhibitor (n=180, 48.6%) and tigecycline (n=84, 22.7%). Among KP-BSI patients treated with tigecycline, 48.8% received conventional dosing and 51.2% were treated with the high-dose regimen; no significant differences were seen in terms of 30-day mortality between the groups (Figure 1B). For patients with APACHE II scores <15 at the onset of bacteremia, the 30-day mortality rate of patients receiving tigecycline was higher than that of patients receiving other antibiotics (45.3% vs 7.7%; Figure 1C).
Table 2.
Analysis of risk factors for 30-day mortality in 370 patients with KP-BSI
| Univariate analysis
|
Multivariable analysis
|
||||||
|---|---|---|---|---|---|---|---|
| Survivors (292) | Nonsurvivors (78) | P-values | Sig. | Exp(B) | 95% CI for Exp(B)
|
||
| Lower | Upper | ||||||
| Demographic | |||||||
| Gender, male, n (%) | 202 (69.2) | 57 (73.1) | 0.504 | ||||
| Age, years, mean ± SD | 58.5±16.6 | 58.6±15.8 | 0.951 | ||||
| Hospital stay before bacteremia, days (IQR) | 5 (1–19) | 13.5 (3–30.25) | 0.001 | ||||
| Preexisting medical conditions | |||||||
| Pulmonary disease | 43 (14.7) | 25 (32.1) | <0.001 | ||||
| Hepatic disease | 87 (29.8) | 16 (20.8) | 0.117 | ||||
| Hepatapostema | 46 (15.8) | 2 (2.6) | 0.002 | ||||
| Comorbid conditions | |||||||
| CCI score (≥3) n (%) | 96 (32.9) | 38 (48.7) | 0.010 | ||||
| Respiratory failure | 6 (2.1) | 13 (16.7) | <0.001 | 0.014 | 5.266 | 1.396 | 19.866 |
| Multiple organ failure | 12 (4.1) | 30 (38.5) | <0.001 | 0.008 | 4.104 | 1.438 | 11.709 |
| Hospital-acquired infection | 254 (87) | 78 (100) | 0.001 | ||||
| Prior hospitalizationa | 135 (46.2) | 49 (62.8) | 0.009 | ||||
| Prior ICU stayb | 75 (25.7) | 44 (56.4) | <0.001 | ||||
| Prior surgeryb | 106 (36.3) | 36 (46.2) | 0.112 | ||||
| Previous transplantationb | 10 (3.4) | 13 (16.7) | <0.001 | ||||
| Invasive procedure or devicesb | 105 (36.0) | 43 (55.1) | 0.002 | ||||
| Mechanical ventilationb | 82 (28.1) | 54 (69.2) | <0.001 | ||||
| Central venous catheterizationb | 86 (29.5) | 52 (66.7) | <0.001 | ||||
| Urinary catheterizationb | 100 (34.2) | 56 (71.8) | <0.001 | ||||
| Percutaneous tubeb | 75 (25.7) | 34 (43.6) | 0.002 | ||||
| Invasive procedure or devices after BSIc | 79 (27.1) | 16 (20.5) | 0.240 | ||||
| Mechanical ventilationc | 54 (18.5) | 53 (67.9) | <0.001 | ||||
| Central venous catheterizationc | 73 (25.0) | 57 (73.1) | <0.001 | ||||
| Urinary catheterizationc | 103 (35.3) | 62 (79.5) | <0.001 | ||||
| Prior hemodialysisb | 25 (8.6) | 18 (23.1) | <0.001 | ||||
| Prior corticosteroid useb | 39 (13.4) | 25 (32.1) | <0.001 | ||||
| Prior immunosuppressant useb | 24 (8.2) | 16 (20.5) | 0.002 | ||||
| Hemodialysis after BSI | 19 (6.5) | 18 (23.1) | <0.001 | ||||
| Corticosteroid use after BSI | 46 (15.8) | 27 (34.6) | <0.001 | ||||
| Immunosuppressant use after BSI | 18 (6.2) | 11 (14.1) | 0.020 | ||||
| Prior receipt of antibiotics within 14 days prior to BSIc | |||||||
| Number of antibiotics | 0 (0–2) | 2 (1–3) | <0.001 | ||||
| Cephalosporin | 20 (6.8) | 8 (10.3) | 0.312 | ||||
| β-lactam and/or β-lactamase inhibitor | 88 (30.1) | 40 (51.3) | <0.001 | ||||
| Tigecycline | 17 (5.8) | 14 (17.9) | 0.001 | ||||
| Carbapenem | 52 (17.8) | 36 (46.2) | <0.001 | ||||
| Fluoroquinolone | 29 (9.9) | 7 (9.0) | 0.800 | ||||
| Carbapenem nonsusceptible | 55 (18.8) | 52 (66.7) | <0.001 | 0.009 | 2.847 | 1.302 | 6.227 |
| Laboratory examination | |||||||
| Neutropenia | 21 (7.2) | 12 (15.4) | 0.024 | 0.008 | 4.104 | 1.438 | 11.709 |
| Serum fibrinogen, d | 3.9 (2.7–5.1) | 3.5 (1.7–4.7) | 0.015 | ||||
| Serum albumin <30 g/L | 91 (31.2) | 34 (43.6) | 0.039 | ||||
| Severity of illness at time of BSI | |||||||
| Mean APACHE II score ± SD | 9.2±4.4 | 14.2±5.8 | <0.001 | 0.018 | 1.990 | 0.988 | 4.007 |
| Total antimicrobial regimen after BSI | |||||||
| β-lactam and/or β-lactamase inhibitor | 144 (49.3) | 36 (46.2) | 0.620 | ||||
| Tigecycline | 41 (14.0) | 43 (55.1) | <0.001 | 0.034 | 2.300 | 1.065 | 4.969 |
| <0.2 g/day | 20 (6.8) | 21 (26.9) | |||||
| ≥0.2 g/day | 21 (7.2) | 22 (28.2) | |||||
| a. Monotherapy | 7 (2.4) | 13 (3.9) | |||||
| b. Combination therapy | 34 (11.6) | 40 (51.9) | |||||
| Carbapenem | 198 (67.8) | 56 (71.8) | 0.500 | ||||
| Fluoroquinolone | 45 (15.5) | 14 (17.9) | 0.595 | ||||
| Appropriate empirical treatment | 237 (81.2) | 33 (42.3) | <0.001 | ||||
| 1) Monotherapy | 232 (79.5) | 33 (42.3) | <0.001 | ||||
| 2) Combination therapy | 60 (20.5) | 45 (57.7) | |||||
| Appropriate definitive treatment | 246 (84.2) | 50 (64.1) | <0.001 | ||||
Notes: Data are expressed as numbers (%) unless otherwise stated;
During the 3 months preceding BSI onset;
during the 30 days preceding BSI onset;
during the 14 days preceding BSI onset.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BSI, bloodstream infection; CCI, Charlson comorbidity index; CI, confidence interval; CnSKP, carbapenem-nonsusceptible KP; CSKP, carbapenem-susceptible KP; ICU, intensive care unit; KP, Klebsiella pneumoniae; IQR, interquartile range; SD, standard deviation.
Risk factors for 30-day mortality in patients with CnSKP-BSI
A total of 107 patients (excluding 28 patients with CnSKP-BSI who died within 48 hours of diagnosis) were included in this analysis; 65.4% of patients stayed in the ICU after infection, and the 30-day mortality rate was 48.6%. Table S1 shows the main characteristics of the CnSKP-BSI survivor and nonsurvivor subgroups. The logistic regression analysis indicated that prior indwelling central venous catheter (OR =3.704, 95% CI =1.325–10.356, P=0.013), multiple organ dysfunction (OR =5.498, 95% CI =1.727–17.504, P=0.004), and a high APACHE II score (OR =1.154, 95% CI =1.054–1.263, P=0.002) were independent risk factors for 30-day death from CnSKP infection.
The assessment of empirical treatment in the CnSKP-BSI group showed that 69 (64.5%) patients received at least two drugs within 48 hours of the onset of bacteremia, while 38 (35.5%) patients received monotherapy; no significant differences in the 30-day mortality were found among these two subgroups (36.8% vs 55.1%, P=0.071) or between those who received appropriate empirical treatment versus inappropriate empirical treatment (P=0.896). For definitive treatment, 61 (55.2%) patients received therapy with no active drug due to multiresistance, 37 patients (34.6%) received one active drug, and 9 patients (8.4%) received at least two active drugs; no significant differences were found in mortality between appropriate definitive treatment and inappropriate definitive treatment (54.3% vs 44.3%, P=0.220).
Discussion
KP is one of the most important pathogens of nosocomial infection, and while carbapenem antibiotics are an effective treatment approach,3 surveillance data showed that the rate of CnSKP has increased year on year in the People’s Republic of China, an has increased worldwide over the past 10 years.6–8 In the present study, we also observed an increase in CnSKP-BSI during the study period, rising from 26.9% in 2013 to 33.3% in 2015. With the emergence of antibiotic-resistant strains, effective clinical treatment and control of infection are likely to present an increasing challenge.
This study represents the largest 3-year evaluation of KP-BSIs in Mainland China up to present. Data from 428 KP-BSI patients were evaluated, demonstrating prior hospitalization, urinary catheterization, and high APACHE II scores to be independent risk factors for the development of CnSKP-BSI, which reflects risk factors reported in previous studies.13,14 The highest incidence of CnSKP infections was observed in the ICU, with 65.4% of CnSKP-BSI patients admitted to the ICU before infection. It is well known that KP often colonizes in the respiratory tract or intestinal tract and can invade the body when immunity is compromised. In the present study, recent solid organ or stem cell transplantation was associated with invasive CnSKP infection independently, and prior studies showed KP infection to be a greater cause of BSIs in liver transplant recipients.11 In our hospital, we found the second highest incidence of CnSKP-BSI in the department of liver transplantation, which may be due to having frequent hospitalization of the patients and long-term exposure to immunosuppressive agents.
Antimicrobial use prior to BSI is known as an important factor in drug-resistant infections,3,11 although some studies showed no association between CnSKP infection and prior antibiotic therapy.14 Our results also demonstrated that the use of cephalosporin, β-lactam and/or β-lactamase inhibitors, fluoroquinolones, tigecycline, or carbapenem in the 14 days prior to BSI differed between the CSKP and CnSKP groups, with multivariate analysis showing that antibiotic exposure, particularly carbapenem use, in this period was an independent risk factor for CnSKP.
In order to explore the high mortality rate associated with KP-BSI further, we evaluated patient characteristics and treatments. In this study, the 30-day death rate associated with CnSKP-BSI was 58.5%, significantly higher than that associated with CSKP (15.4%); these data are similar to figures found in previous reports.9,10 In addition, resistance to carbapenem was associated with an increased risk of mortality, which is in contrast to some previous studies.13 As previously reported,9,22 multiple organ dysfunction, respiratory failure, or high APACHE II scores were found as independent predictors of death; our analysis also found that KP-BSI patients with neutropenia were likely to have a poor outcome.
Tigecycline has a broad spectrum of action and excellent in vitro antimicrobial activity. It has been commonly used in infections caused by mixed pathogens or multidrug-resistant pathogens and is approved primarily for use in respiratory tract infections, complicated skin and skin structure infections, and complicated intra-abdominal infections caused by Enterobacteriaceae. Because of the lack of appropriate antibiotics for the treatment of multidrug-resistant bacteria such as CnSKP, tigecycline has become more widely used, although its efficacy in the treatment of KP-BSI remains controversial.15,16,26–28 The present study demonstrated significantly higher mortality rates in the tigecycline group than in controls (51.2% and 12.2%, P<0.001); for patients with APACHE II scores <15, the 30-day mortality rate of patients receiving tigecycline was 45.3%, versus 7.7% in patients receiving other antibiotics. These data are consistent with the FDA warning and a previous meta-analysis,26,28 which showed that the proportion of patients with septic shock was significantly higher in those treated by tigecycline than in the controls (relative risk =7.01). For patients whose infection is not resolved by conventional doses of tigecycline, an increased dose is often used, which is an approach that has also been recommended in a recent consensus statement.15 However, a study of mortality among patients receiving conventional versus higher-dose regimens suggested that differences were dependent on the underlying infection severity, and there is limited clinical evidence to support high-dose tigecycline regimens.23 Indeed, there were similarities in mortality between KP-BSI patients with APACHE II scores <15 treated with conventional dosing of tigecycline and higher dosing regimens in our analysis. Based on these observations, tigecycline does not appear to be superior to standard antimicrobial agents to treat KP-BSI, and physicians should exercise caution when selecting tigecycline for the therapy of multidrug-resistant infections.
In this study, deaths among patients with CnSKP-BSI were directly and independently related to the severity of infection, multiple organ dysfunction, and high APACHE II score. At present, a best practice treatment program for patients with CnSKP-BSI has not been established. In this study, most patients received carbapenem, with mono-therapy accounting for 23.9% of regimens and combination treatments accounting for 76.1%; no significant difference in CnSKP 30-day mortality was found among the two treatment regimens. The previous examination of the outcome of KPC-KP bacteremia in 125 patients treated at three large Italian teaching hospitals showed the overall 30-day mortality rate to be 42%, while mortality in patients receiving colistin, tigecycline, and meropenem combination regimens was significantly lower (34%, vs 54% with monotherapy; P=0.02).22 By contrast, a review of 141 patients with CRKP-BSIs found that there were similarities in the 30-day mortality of patients who were treated with monotherapy and those with combination regimens (38% vs 26%, P=0.1).12 Several clinical studies suggest that CRKP-BSI patients who were treated by carbapenem-containing combination regimens have significantly lower mortality rates than those treated by non-carbapenem-containing regimens, especially in cases where the MIC of KP was <4 mg/L.29,30 In patients treated with carbapenem combination therapy, we found successful treatment in 75% of patients with meropenem MIC ≤4 μg/mL, compared with 47.9% with meropenem MIC ≥8 μg/mL, while 54.7% of patients who received non-carbapenem-containing regimens were successfully treated, although the difference was not statistically significant. In future studies, it would be valuable to expand the sample size to explore the efficacy of carbapenem in a larger group of patients with an MIC ≤4 μg/mL. Because of the retrospective nature and selection bias of our study and lack of appropriate antibiotics, we cannot comment on the effectiveness of appropriate empirical and definitive therapy among patients with CnSKP infection.
We acknowledge a number of limitations to this study. First, our analysis was a retrospective study, and it is possible that there may have been some degree of misclassification of the source of infection. Second, it was a single center study with a high incidence of CnSKP. Clone spread of KPC-2 and KPC-3 may make the hospital dissemination of CnSKP and influence therapy or prognosis; therefore, certain observations may not be applicable to other settings.
Conclusion
CnSKP is emerging as a serious health care issue associated with high mortality rates and limited treatment options. This study demonstrated that prior hospitalization, urinary catheterization, receipt of immunosuppression agents, pulmonary disease, high APACHE II score, and exposure to carbapenems represent significant risk factors for the development of CnSKP-BSI. Neutropenia, low serum albumin, multiple organ dysfunction, respiratory failure, carbapenem-non-susceptibility, tigecycline therapy, and high APACHE II score are independent risk factors for mortality in patients with KP-BSI. With a higher observed mortality rate, we suggest that tigecycline may not be as effective as other antibiotics and that tigecycline should be used with caution for the treatment of multidrug-resistant KP.
Supplementary materials
Flowchart of the case selection process.
Abbreviations: BSI, bloodstream infection; CLSI, Clinical and Laboratory Standards Institute, CnSKP, carbapenem-nonsusceptible KP; CSKP, carbapenem-susceptible KP; KP, Klebsiella pneumoniae.
Annual incidence of Klebsiella pneumoniae bloodstream infections (KP and CnSKP) in hospital departments.
Abbreviations: CnSKP, carbapenem-nonsusceptible KP; CSKP, carbapenem-susceptible KP; GS, Department of General Surgery; HT, Department of Hematology; ICU, intensive care unit; ID, Department of Infectious Diseases; KP, Klebsiella pneumoniae; LT, Department of Liver Transplantation; NE, Department of Nephrology.
Table S1.
Analysis of risk factors for mortality in patients with BSI caused by CnSKP
| Univariate analysis
|
Multivariable analysis
|
||||||
|---|---|---|---|---|---|---|---|
| Survivors (55) | Nonsurvivors (52) | P-values | Sig. | Exp(B) | 95% CI for Exp(B)
|
||
| Lower | Upper | ||||||
| Demographic | |||||||
| Gender, male, n (%)q | 42 (76.4) | 39 (75) | 0.869 | ||||
| Age, years, mean ± SD | 57.8±16.5 | 59.1±15.2 | 0.679 | ||||
| Duration before bacteremia, days (IQR) | 19 (6–32) | 18 (7–49.5) | 0.781 | ||||
| Comorbid conditions | |||||||
| CCI score (≥3), n (%) | 25 (45.5) | 24 (46.2) | 0.942 | ||||
| Respiratory failure | 1 (1.8) | 8 (15.4) | 0.014 | ||||
| Heart failure | 1 (1.8) | 5 (9.6) | 0.106 | ||||
| Kidney failure | 3 (5.5) | 4 (7.7) | 0.711 | ||||
| Multiple organ failure | 5 (9.1) | 20 (38.5) | <0.001 | 0.004 | 5.498 | 1.727 | 17.504 |
| Prior ICU staya | 35 (63.6) | 37 (71.2) | 0.407 | ||||
| Invasive procedure or devicesa | 30 (54.5) | 33 (63.5) | 0.349 | ||||
| Mechanical ventilation | 38 (69.1) | 45 (86.5) | 0.031 | ||||
| Central venous catheterization | 36 (65.5) | 45 (86.5) | 0.011 | 0.013 | 3.704 | 1.325 | 10.356 |
| Urinary catheterization | 42 (76.4) | 47 (90.4) | 0.053 | ||||
| Invasive procedure or devices after BSI | 18 (32.7) | 9 (17.3) | 0.066 | ||||
| Mechanical ventilation | 25 (45.5) | 42 (80.8) | <0.001 | ||||
| Central venous catheterization | 29 (52.7) | 44 (84.6) | <0.001 | ||||
| Urinary catheterization | 36 (65.5) | 47 (90.4) | 0.002 | ||||
| Prior receipt of antibiotics within 14 days before BSI | b | ||||||
| Number of antibiotics | 2 (1–3) | 2 (2–3) | 0.157 | ||||
| Severity of illness at the time of BSI | |||||||
| Mean APACHE II score ± SD | 11.55±5.266 | 15.62±5.15 | <0.001 | 0.002 | 1.154 | 1.054 | 1.263 |
| Total antimicrobial regimen after BSI | |||||||
| Tigecycline | 30 (54.5) | 34 (65.4) | 0.253 | ||||
| <0.2 g/day | 14 (25.5) | 17 (32.7) | 0.790 | ||||
| ≥0.2 g/day | 16 (29.1) | 17 (32.7) | |||||
| Carbapenem | 37 (67.3) | 34 (65.4) | 0.836 | ||||
| MIC <4 μg/mL | 6 (16.2) | 1 (2.9) | 0.109 | ||||
| MIC ≥8 μg/mL | 31 (83.8) | 31 (91.2) | 0.482 | ||||
| Aminoglycoside | 11 (20) | 13 (25) | 0.535 | ||||
| Fluoroquinolone | 9 (16.4) | 10 (19.2) | 0.698 | ||||
| Appropriate empirical treatment | 9 (16.4) | 9 (17.3) | 0.896 | ||||
| 1) Monotherapy | 24 (43.6) | 14 (26.9) | 0.071 | ||||
| 2) Combination therapy | 31 (56.4) | 38 (73.1) | |||||
| Appropriate definitive treatment | 20 (36.4) | 25 (48.1) | 0.220 | ||||
| 1) No active drug | 34 (61.8) | 27 (51.9) | 0.236 | ||||
| 2) At least two active drugs | 3 (5.5) | 6 (11.5) | |||||
| 3) One active drug | 18 (32.7) | 19 (36.5) | |||||
| Antimicrobial regimen | |||||||
| 1) Tigecycline monotherapy | 6 (10.9) | 2 (3.8) | 0.272 | ||||
| 2) Tigecycline combination therapy | 24 (43.6) | 32 (61.5) | 0.064 | ||||
| APACHE II <15 | 16 (66.7) | 16 (50) | 0.212 | ||||
| APACHE II ≥15 | 8 (33.3) | 16 (50) | |||||
| 3) Carbapenem monotherapy | 11 (20) | 6 (11.5) | 0.231 | ||||
| 4) Carbapenem-containing regimen | 26 (47.3) | 28 (53.8) | 0.497 | ||||
| MIC <4 μg/mL | 3 (11.5) | 1 (3.6) | 0.342 | ||||
| MIC ≥8 μg/mL | 23 (88.5) | 25 (89.3) | 1.000 | ||||
Notes: Data are expressed as number (%) unless otherwise stated;
During the 30 days preceding BSI onset
during the 14 days preceding BSI onset.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BSI, bloodstream infection; CCI, Charlson comorbidity index; CI, confidence interval; CnSKP, carbapenem-nonsusceptible KP; CSKP, carbapenem-susceptible KP; ICU, intensive care unit; KP, Klebsiella pneumoniae; IQR, interquartile range; MIC, minimum inhibitory concentration; SD, standard deviation.
Acknowledgments
This work was partially supported by grants from the Key Research and Development Program of Zhejiang Province (No. 2015C03032). The abstract of this paper has been presented at the 11th International Symposium on Antimicrobial Agents and Resistance and the 3rd International Interscience Conference on Infection and Chemotherapy, September 14–16, 2017, and been published in the International Journal of Antimicrobial Agents, Volume 50, Supplement.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med. 2007;167:834–839. doi: 10.1001/archinte.167.8.834. [DOI] [PubMed] [Google Scholar]
- 2.Meatherall BL, Gregson D, Ross T, Pitout JD, Laupland KB. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med. 2009;122:866–873. doi: 10.1016/j.amjmed.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Orsi GB, Garcia-Fernandez A, Giordano A, et al. Risk factors and clinical significance of ertapenem-resistant Klebsiella pneumoniae in hospitalised patients. J Hosp Infect. 2011;78:54–58. doi: 10.1016/j.jhin.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie FM, Forbes KJ, Doraijohn T, Amyes SG, Gould IM. Emergence of a carbapenem-resistant Klebsiella pneumoniae. Lancet. 1997;350:783. doi: 10.1016/s0140-6736(05)62567-6. [DOI] [PubMed] [Google Scholar]
- 5.Wei ZQ, Du XX, Yu YS, Shen P, Chen YG, Li LJ. Plasmid-mediated KPC-2 in a Klebsiella pneumoniae isolate from China. Antimicrob Agents Chemother. 2007;51:763–765. doi: 10.1128/AAC.01053-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumonia carbapenemases. Lancet Infect Dis. 2013;13:785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infect. 2016;22:S9–S14. doi: 10.1016/j.cmi.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Ben-David D, Kordevani R, Keller N, et al. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect. 2012;18:54–60. doi: 10.1111/j.1469-0691.2011.03478.x. [DOI] [PubMed] [Google Scholar]
- 10.Tian LJ, Tan RM, Chen Y, et al. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control. 2016;5:48. doi: 10.1186/s13756-016-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol. 2008;29:1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-simmonds A, Nelson B, Eiras DP, et al. Combination regimens for the treatment of carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother. 2016;60:3601–3607. doi: 10.1128/AAC.03007-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shilo S, Assous MV, Lachish T, et al. Risk factors for bacteriuria with carbapenem-resistant Klebsiella pneumoniae and its impact on mortality: a case-control study. Infection. 2013;41:503–509. doi: 10.1007/s15010-012-0380-0. [DOI] [PubMed] [Google Scholar]
- 14.Debby BD, Ganor O, Yasmin M, et al. Epidemiology of carbapenem resistant Klebsiella pneumoniae colonization in an intensive care unit. Eur J Clin Microbiol Infect Dis. 2012;31:1811–1817. doi: 10.1007/s10096-011-1506-5. [DOI] [PubMed] [Google Scholar]
- 15.Chinese XDR Consensus Working Group. Guan X, He L, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug-resistant Gram-negative bacilli: a Chinese consensus statement. Clin Microbiol Infect. 2016;22:S15–S25. doi: 10.1016/j.cmi.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Kelesidis T, Karageorgopoulos DE, Kelesidis I, Falagas ME. Tigecycline for the treatment of multidrug-resistant Enterobacteriaceae: a systematic review of the evidence from microbiological and clinical studies. J Antimicrob Chemother. 2008;62:895–904. doi: 10.1093/jac/dkn311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect. 2011;17:1135–1141. doi: 10.1111/j.1469-0691.2011.03553.x. [DOI] [PubMed] [Google Scholar]
- 18.van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;75:115–120. doi: 10.1016/j.diagmicrobio.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention CDC/NHSN surveillance definitions for specific types of infections. 2015. [Accessed January 15, 2014]. Available from: http://www.cdc.gov/nhsn/PDFs/pscManual/17pscNosInfDef_current.pdf.
- 22.Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumonia carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 23.Falagas ME, Vardakas KZ, Tsiveriotis KP, Triarides NA, Tansarli GS. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents. 2014;44:1–7. doi: 10.1016/j.ijantimicag.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing, 25th informational supplement. Wayne: CLSI; 2015. CLSI document M100-S25. [Google Scholar]
- 25.Tygacil® (tigecycline) [package insert] Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2005. [Google Scholar]
- 26.Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother. 2011;66:1963–1971. doi: 10.1093/jac/dkr242. [DOI] [PubMed] [Google Scholar]
- 27.Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin Infect Dis. 2005;41:S333–S340. doi: 10.1086/431674. [DOI] [PubMed] [Google Scholar]
- 28.Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with Tigecycline after approval based on noninferiority trials. Clin Infect Dis. 2012;54:1699–1709. doi: 10.1093/cid/cis270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25:682–707. doi: 10.1128/CMR.05035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58:2322–2328. doi: 10.1128/AAC.02166-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart of the case selection process.
Abbreviations: BSI, bloodstream infection; CLSI, Clinical and Laboratory Standards Institute, CnSKP, carbapenem-nonsusceptible KP; CSKP, carbapenem-susceptible KP; KP, Klebsiella pneumoniae.
Annual incidence of Klebsiella pneumoniae bloodstream infections (KP and CnSKP) in hospital departments.
Abbreviations: CnSKP, carbapenem-nonsusceptible KP; CSKP, carbapenem-susceptible KP; GS, Department of General Surgery; HT, Department of Hematology; ICU, intensive care unit; ID, Department of Infectious Diseases; KP, Klebsiella pneumoniae; LT, Department of Liver Transplantation; NE, Department of Nephrology.
Table S1.
Analysis of risk factors for mortality in patients with BSI caused by CnSKP
| Univariate analysis
|
Multivariable analysis
|
||||||
|---|---|---|---|---|---|---|---|
| Survivors (55) | Nonsurvivors (52) | P-values | Sig. | Exp(B) | 95% CI for Exp(B)
|
||
| Lower | Upper | ||||||
| Demographic | |||||||
| Gender, male, n (%)q | 42 (76.4) | 39 (75) | 0.869 | ||||
| Age, years, mean ± SD | 57.8±16.5 | 59.1±15.2 | 0.679 | ||||
| Duration before bacteremia, days (IQR) | 19 (6–32) | 18 (7–49.5) | 0.781 | ||||
| Comorbid conditions | |||||||
| CCI score (≥3), n (%) | 25 (45.5) | 24 (46.2) | 0.942 | ||||
| Respiratory failure | 1 (1.8) | 8 (15.4) | 0.014 | ||||
| Heart failure | 1 (1.8) | 5 (9.6) | 0.106 | ||||
| Kidney failure | 3 (5.5) | 4 (7.7) | 0.711 | ||||
| Multiple organ failure | 5 (9.1) | 20 (38.5) | <0.001 | 0.004 | 5.498 | 1.727 | 17.504 |
| Prior ICU staya | 35 (63.6) | 37 (71.2) | 0.407 | ||||
| Invasive procedure or devicesa | 30 (54.5) | 33 (63.5) | 0.349 | ||||
| Mechanical ventilation | 38 (69.1) | 45 (86.5) | 0.031 | ||||
| Central venous catheterization | 36 (65.5) | 45 (86.5) | 0.011 | 0.013 | 3.704 | 1.325 | 10.356 |
| Urinary catheterization | 42 (76.4) | 47 (90.4) | 0.053 | ||||
| Invasive procedure or devices after BSI | 18 (32.7) | 9 (17.3) | 0.066 | ||||
| Mechanical ventilation | 25 (45.5) | 42 (80.8) | <0.001 | ||||
| Central venous catheterization | 29 (52.7) | 44 (84.6) | <0.001 | ||||
| Urinary catheterization | 36 (65.5) | 47 (90.4) | 0.002 | ||||
| Prior receipt of antibiotics within 14 days before BSI | b | ||||||
| Number of antibiotics | 2 (1–3) | 2 (2–3) | 0.157 | ||||
| Severity of illness at the time of BSI | |||||||
| Mean APACHE II score ± SD | 11.55±5.266 | 15.62±5.15 | <0.001 | 0.002 | 1.154 | 1.054 | 1.263 |
| Total antimicrobial regimen after BSI | |||||||
| Tigecycline | 30 (54.5) | 34 (65.4) | 0.253 | ||||
| <0.2 g/day | 14 (25.5) | 17 (32.7) | 0.790 | ||||
| ≥0.2 g/day | 16 (29.1) | 17 (32.7) | |||||
| Carbapenem | 37 (67.3) | 34 (65.4) | 0.836 | ||||
| MIC <4 μg/mL | 6 (16.2) | 1 (2.9) | 0.109 | ||||
| MIC ≥8 μg/mL | 31 (83.8) | 31 (91.2) | 0.482 | ||||
| Aminoglycoside | 11 (20) | 13 (25) | 0.535 | ||||
| Fluoroquinolone | 9 (16.4) | 10 (19.2) | 0.698 | ||||
| Appropriate empirical treatment | 9 (16.4) | 9 (17.3) | 0.896 | ||||
| 1) Monotherapy | 24 (43.6) | 14 (26.9) | 0.071 | ||||
| 2) Combination therapy | 31 (56.4) | 38 (73.1) | |||||
| Appropriate definitive treatment | 20 (36.4) | 25 (48.1) | 0.220 | ||||
| 1) No active drug | 34 (61.8) | 27 (51.9) | 0.236 | ||||
| 2) At least two active drugs | 3 (5.5) | 6 (11.5) | |||||
| 3) One active drug | 18 (32.7) | 19 (36.5) | |||||
| Antimicrobial regimen | |||||||
| 1) Tigecycline monotherapy | 6 (10.9) | 2 (3.8) | 0.272 | ||||
| 2) Tigecycline combination therapy | 24 (43.6) | 32 (61.5) | 0.064 | ||||
| APACHE II <15 | 16 (66.7) | 16 (50) | 0.212 | ||||
| APACHE II ≥15 | 8 (33.3) | 16 (50) | |||||
| 3) Carbapenem monotherapy | 11 (20) | 6 (11.5) | 0.231 | ||||
| 4) Carbapenem-containing regimen | 26 (47.3) | 28 (53.8) | 0.497 | ||||
| MIC <4 μg/mL | 3 (11.5) | 1 (3.6) | 0.342 | ||||
| MIC ≥8 μg/mL | 23 (88.5) | 25 (89.3) | 1.000 | ||||
Notes: Data are expressed as number (%) unless otherwise stated;
During the 30 days preceding BSI onset
during the 14 days preceding BSI onset.
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; BSI, bloodstream infection; CCI, Charlson comorbidity index; CI, confidence interval; CnSKP, carbapenem-nonsusceptible KP; CSKP, carbapenem-susceptible KP; ICU, intensive care unit; KP, Klebsiella pneumoniae; IQR, interquartile range; MIC, minimum inhibitory concentration; SD, standard deviation.