Abstract
In this paper we report on our study of the changes in biomass, lipid composition, and fermentation end products, as well as in the ATP level and synthesis rate in cultivated potato (Solanum tuberosum) cells submitted to anoxia stress. During the first phase of about 12 h, cells coped with the reduced energy supply brought about by fermentation and their membrane lipids remained intact. The second phase (12–24 h), during which the energy supply dropped down to 1% to 2% of its maximal theoretical normoxic value, was characterized by an extensive hydrolysis of membrane lipids to free fatty acids. This autolytic process was ascribed to the activation of a lipolytic acyl hydrolase. Cells were also treated under normoxia with inhibitors known to interfere with energy metabolism. Carbonyl-cyanide-4-trifluoromethoxyphenylhydrazone did not induce lipid hydrolysis, which was also the case when sodium azide or salicylhydroxamic acid were fed separately. However, the simultaneous use of sodium azide plus salicylhydroxamic acid or 2-deoxy-d-glucose plus iodoacetate with normoxic cells promoted a lipid hydrolysis pattern similar to that seen in anoxic cells. Therefore, a threshold exists in the rate of ATP synthesis (approximately 10 μmol g−1 fresh weight h−1), below which the integrity of the membranes in anoxic potato cells cannot be preserved.
O2 deprivation becomes a frequent stress for plants submitted to unpredictable heavy rainfalls and flooding. The diffusion of O2 to their submerged underground organs is severely limited, so that plants must cope with hypoxic or even anoxic conditions. Whereas ATP is produced with a high efficiency by respiration in nongreen cells, its synthesis is much lower under anoxia, with fermentation as the sole energy provider. For most higher plants, the latter condition eventually becomes lethal. The multifarious effects of O2 deprivation stress on plants sensitive and resistant to anoxia are fairly well understood and have been extensively reviewed in this decade (Armstrong et al., 1994; Sachs, 1994; Ratcliffe, 1995; Crawford and Braendle, 1996; Drew, 1997; Vartapetian and Jackson, 1997).
Aside from the obvious interest given to the responses of energy metabolism, the role of macromolecules, and in particular, of gene expression and protein synthesis, has received considerable attention (Sachs, 1994; Drew, 1997). In contrast, the behavior of membrane lipids under anoxia has scarcely been investigated. This is surprising knowing how important it is for a living cell to maintain its membrane integrity. In potato (Solanum tuberosum) tubers, for instance, changes in membrane lipids have mostly been studied during aging, and have been related to overall lipid unsaturation, lipid degradation, and peroxidation processes (Knowles and Knowles, 1989; Spychalla and Desborough, 1990a, 1990b; Kumar and Knowles, 1993; Dipierro and De Leonardis, 1997). Although these effects are not directly relevant to anoxia (Kumar and Knowles, 1996), they are probably related to the effects likely to occur under anoxia and postanoxia. Nevertheless, our knowledge of the mechanisms responsible for membrane lipid alterations during anoxia is still limited. Lipid synthesis was shown to diminish and desaturation of acyl chains to be stopped because of their respective ATP and O2 requirements (Vartapetian et al., 1978; Brown and Beevers, 1987). Organelle damage, mainly to mitochondria, occurs under anoxia and upon re-aeration (Andreev et al., 1991).
Potato is an important crop with a high sensitivity to O2 deprivation, which is mostly studied in tubers. During the first 6 h of anoxia, the adenylate levels and the energy charge decrease continuously, and the ATP production rate becomes too low to sustain the basal metabolic requirement of the tuber in spite of its ample starch reserves (Sieber and Braendle, 1991). Membrane damage could be induced by ATP deprivation, which is suggested by the correlation between the leakage of electrolytes from the cells and the release of FFAs in anoxic tubers (Crawford and Braendle, 1996). A link should exist between the energy status of the tuber and its capability to maintain the integrity of its membrane lipids under anoxia.
However, because of their compactness, potato tubers are not well suited for some types of experiments. We have chosen to work with potato cell cultures as an alternative and practical model for anoxia studies. Cell suspensions allow an optimal diffusibility of gases and solutes and present an inherent homogeneity. In addition, the duration of anoxic treatments can be shortened to about 24 h.
Here we compare the changes in lipid composition and in energy metabolism between cells under anoxia and cells treated with inhibitors in the presence of O2. We conclude that a threshold in the ATP synthesis rate exists, below which potato cells become committed to hydrolysis of their membrane lipids.
MATERIALS AND METHODS
Chemicals
Most chemicals were high-purity products from Fluka or Sigma. Kits were from Boehringer Mannheim and Lumac (Landgraaf, The Netherlands).
Cells
Potato (Solanum tuberosum L. cv Bintje) cells (kindly given by Dr. J.L. Coquoz, University of Fribourg, Switzerland) were cultivated as 50-mL suspension cultures on Murashige and Skoog medium fortified with 10 mg L−1 thiamine-HCl, 0.1 mg L−1 kinetin, 3 mg L−1 2,4-D, and 90 mm Suc in 250-mL conical flasks. Cell cultures were incubated in a rotary shaker (120 rpm) at 24°C ± 1°C in the dark and renewed by a weekly inoculation of 3.5 mL of cell suspension in 50 mL of fresh medium. After transfer, cells were allowed to grow for 4 d under the above conditions. At this stage, they were in their mid-log phase and reached a concentration of about 15 mg fresh weight mL−1. Cell suspensions were aseptically pooled, distributed as 6- to 8-mL (or 25-mL) aliquots in 50-mL (or 100-mL) conical flasks, treated, and incubated as described below. The liquid surface area available for gas exchange was 1 to 2 cm2 mL−1.
Treatments of Cells
O2-free conditions were provided by incubating and filtrating cells in an anaerobic workbench (model 1029, Forma Scientific, Marietta, OH) filled with 85% N2, 5% CO2, and 10% H2. The residual O2 level (monitored with an O2 analyzer, model LF 700, Toray, Lippke, Neuwied, Germany) was <0.001%. Cells were frozen in liquid N2 and stored at −80°C.
Modulation of energy metabolism in the presence of O2 was achieved by aseptically feeding cells with 0.1 to 1 μm FCCP, 2 mm NaN3, 2 mm SHAM, 20 mm DeOGlc, 2 mm IAc, or 1 to 5 mm NaF, followed by incubation under normoxia. Ethanol, as a cosolvent for some of these compounds, had no effect on cell biomass changes or on lipid composition at least up to 100 mm (approximately 0.6%, v/v). In all of the cases, cells were separated from their incubation medium by filtration through filter paper (type LS 14, Schleicher & Schuell) under reduced pressure (50–100 mbars).
Lipid Analysis
Frozen cells (60–250 mg fresh weight) were placed in a 7-mL screw-cap glass tube, and 0.18 mL of 0.5 n HCl, 2.4 mL of methanol, and 1.2 mL of chloroform were added. After shaking, incubating for 10 min, and spinning (1000g) for 5 min, the supernatant was saved. The pellet was re-extracted once as above. We added 1.2 mL of chloroform and 1.8 mL of 0.5 m KCl to the supernatants. After shaking, the mixtures were spun down as described above. Pooled lower phases were filtered on glass wool and anhydrous Na2SO4 and dried under N2. The residue, taken up in 1 mL of chloroform:methanol (8:2, v/v), was the total lipid extract.
Total lipids were measured on a 200-μL aliquot of the total lipid extract, supplemented with 100 μg of nonadecanoic acid as the internal standard. FFAs were obtained as follows: The remaining total lipid extract (800 μL) received 100 μg of heptadecanoic acid as the recovery standard for FFAs. The mixture was spotted as a 3-cm streak (Linomat II, Camag AG, Muttenz, Switzerland) onto a TLC glass plate (10 × 20 cm) coated with a 0.25-mm-thick layer of silicagel 60 (E. Merck AG, Dietikon, Switzerland). After development with petroleum ether (boiling point = 50°C–70°C):diethylether:acetic acid (70:30:1, v/v), the plate was briefly air-dried, sprayed with primuline (0.01% in acetone:water, 4:6, v/v), and viewed under UV light. Bands corresponding to the FFAs were scraped off and transferred into 7-mL tubes together with 100 μg of nonadecanoic acid as an internal standard.
Total lipids and FFAs were methylated in 2 mL of 5% H2SO4:methanol under N2 for 60 min at 85°C. After cooling, we added 1.8 mL of pentane and 2 mL of water and shook and then centrifuged the mixture. The upper phase was washed once and transferred into glass inserts. Fatty acid methyl esters were isothermally (190°C) separated on a 25-m × 0.4-mm × 0.2-μm FFA phase-coated column (Macherey and Nagel, Oensingen, Switzerland) and quantified with an AutoSystem (Perkin-Elmer).
Determination of Respiratory Activity of Cells
We measured the O2 uptake of cells in a glass cuvette at 24°C using a Clark-type O2 electrode. The reaction medium contained 40 to 80 mg of cell fresh weight in 5 mL of Murashige and Skoog medium plus 90 mm Suc.
Measurement of ATP
ATP was extracted at 2°C from 250 mg of frozen cells by homogenization with 6 mL of 6% perchloric acid for 30 s in a Potter glass homogenizor. After centrifugation for 10 min at 24,000g at 2°C, the supernatant was neutralized with 5 m ice-cold K2CO3 in an ice bath. KClO4 was removed by spinning as described above. We determined ATP immediately with the luciferin-luciferase system, using a biocounter (model 2500, Lumac) as described by Sieber and Brändle (1991).
Determination of Fermentation End Products
We measured the amounts of ethanol, lactate, and Ala in cells and in their corresponding filtrates. The ethanol and lactate were extracted from frozen cells (0.5–2 g) in the presence of 3 to 6 mL of 6% perchloric acid as described above. We placed the filtrates in tightly closed vessels, inactivated them at 80°C for 15 min, and then froze and stored them until use. We performed the spectrophotometric determination of ethanol and lactate at 365 nm with enzymatic test kits (Boehringer Mannheim). We used the supernatants (centrifuged at 24,000g for 10 min) of cell homogenates and of the corresponding filtrates for Ala determination. After protein precipitation, we readied the amino acids for reaction with phenylisothiocyanate (Bidlingmeyer et al., 1984) and analysis by HPLC (Davey and Ersser, 1990) on a Novapak C18 column (150 × 3.9 mm; 4 μm, porosity 60 Å), using norleucine as an internal standard.
RESULTS
Time Course of Biomass Change
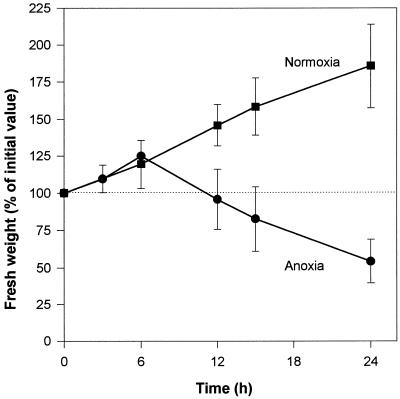
Under normoxic conditions cell biomass increased regularly, almost doubling over a 24-h period (Fig. 1). Anoxic cells behaved similarly for up to 6 h, but then their biomass started to decrease, reaching the initial 100% value after about 12 h. This decrease continued steadily for up to 24 h (Fig. 1). The filtration behavior of cells and their aspect changed dramatically after 12 h of anoxia. Before this time, anoxic cells were easy to filtrate and the packed cells had the typical yellowish and granulous aspect of normoxic cells. On the other hand, filtration of anoxic cells became increasingly tedious after 12 h, and packed cells appeared dark yellow to gray with a pasty consistency.
Figure 1.
Relative change of cell biomass during incubation time under normoxic (▪) and anoxic (•) conditions. The initial (100%) value ranged between 15 and 25 mg cell fresh weight mL−1. Each point is the average of up to five independent determinations.
Time Course of Lipid Hydrolysis under Anoxia
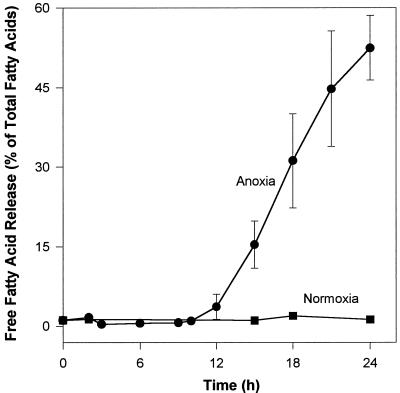
When potato cells were incubated under anoxia, FFAs were released in a time-dependent way. Lipid hydrolysis started after a time lag of about 10 to 12 h, and reached about 60% 12 h later (Fig. 2). In contrast, the lipid composition of normoxic cells did not change during this period, as indicated by the stable low level (approximately 1%) of endogenous FFAs (Fig. 2). To check whether lipids would be released from cells during the treatments, we performed control experiments to compare the lipid content of filtrates obtained from cells incubated for 24 h under normoxia with those under anoxia. We found a maximum of 1% and 6% of the total cell fatty acids in the filtrates of normoxic and anoxic cells, respectively. However, most of these lipids could be ascribed to small cell fragments (especially under anoxia) having leached through the filter and not to the net release of lipid molecules into the medium.
Figure 2.
Change in the hydrolysis extent of potato cell lipids during incubation time under normoxic (▪) and anoxic (•) conditions. The release of FFAs is expressed as a percentage of the total fatty acid content of cells. Each point is the average of up to 10 independent determinations. The total fatty acid content of potato cell lipids (100%) was 25.3 ± 1.2 μmol fatty acids g−1 fresh weight of which 76% ± 3% were phospholipids, 14% ± 2% were glycolipids, and 10% ± 1% were neutral, apolar lipids (mostly triacylglycerols and acylated sterols).
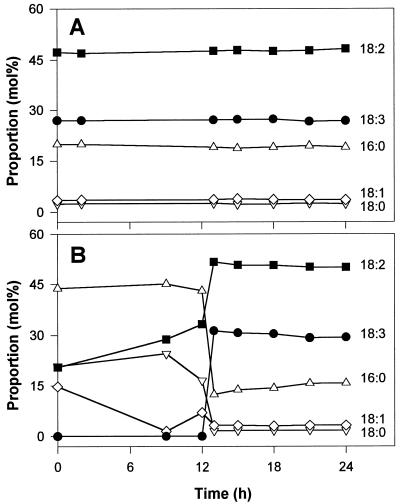
We also determined the acyl chain composition of total lipids and of the FFAs over a 24-h period (Fig. 3). The acyl composition of total lipids was very constant with time and essentially similar in normoxic and anoxic cells. In all cases, the three major fatty acyl chains were 18:2 (linoleic), 18:3 (linolenic), and 16:0 (palmitic) acids, with minor amounts of 18:1 (oleic) and 18:0 (stearic) acids (Fig. 3A). On the other hand, the acyl composition of the FFAs changed dramatically with the progress of lipid hydrolysis (see legend to Fig. 2). During the initial time lag of approximately 12 h, the low level of FFAs in the anoxic cells (Fig. 3B) was constant and essentially accounted for by saturated chains (16:0 and 18:0). This was also the case for the normoxic cells. The absence of lipid hydrolysis was thus reflected by the low DBI of the small endogenous FFA pool (see legend to Fig. 3) generated by neosynthesis and elongation (Harwood, 1988). When hydrolysis started in the anoxic cells, the acyl composition of the FFAs switched rapidly toward increased unsaturation and became similar to that of the total lipids (compare with the final fatty acid levels in Fig. 3). In this case, lipid hydrolysis was reflected by a much higher DBI value for the FFAs released, which was comparable to that of the total lipids (see legend to Fig. 3).
Figure 3.
Acyl chain composition (in mol %) of total cell lipids (A) and of the FFAs released (B) in potato cells incubated under anoxia. Each data point is the average of two experiments (agreeing within ±1%). The acyl composition of total lipids in normoxic cells is essentially similar to that of anoxic cells and is therefore not detailed here. By definition, DBI = (Σ[N × mol % fatty acid ])/100, where Σ N is the number of double bonds in each fatty acid molecule. Here, DBI is thus equal to (0 × mol % 16:0 + 0 × mol % 18:0 + 1 × mol % 18:1 + 2 × mol % 18:2 + 3 × mol % 18:3)/100. In A, the DBI values were 1.788 at 0 h, 1.804 at 12 h, and 1.986 at 24 h. In B, the DBI values were 0.575 at 0 h, 0.734 at 12 h, and 1.912 at 24 h. The constancy of the fatty acid composition of total lipids in anoxia-treated cells (A) is in agreement with the requirement of acyl chain desaturation for O2 (Harwood, 1988).
Nature of the Enzyme Responsible for Lipid Hydrolysis
We investigated the nature of the enzyme responsible for the lipid hydrolysis reported in Figure 2 and summarized our results in Tables I and II. On a molar basis, each phospholipid hydrolyzed gave rise to two FFAs, and lysophospholipids were not detected (Table I). When we assayed a cell extract for esterase activity in a Ca2+-free buffer using p-nitrophenylpalmitate as a substrate (Galliard, 1971), a substantial amount of palmitic acid was produced (Table I). Finally, after significant lipid hydrolysis, the acyl composition of released FFAs and that of total lipids matched closely (Table II; see also Fig. 3). Together, these data suggest that the FFA release described in Figures 2 and 3 is due to a LAH.
Table I.
Evidence for the occurrence of a lipolytic acyl hydrolase activity in potato cells
| Ratio of FFA to phospholipid degradationa | 1.88 mol:molb |
| Lysophospholipidsa | Not detectable on TLC plates |
| Hydrolytic activity of cell extractscwith p-nitrophenylpalmitate as the substrate | 440 ± 72dnmol Palmitate released g fresh wt−1 h−1 |
Phospholipids were separated from other lipids using silica gel cartridges. After loading the total lipid extract and washing with acetone, phospholipids were eluted with methanol, and either separated by TLC or transmethylated and analyzed by GC. Soluble cell extracts were prepared by homogenization of normoxic potato cells (0.2–0.5 g fresh wt) in 1 mL of 0.1 m potassium phosphate buffer (pH 7.5) using a Potter and spinning at 15,000g for 15 min. The supernatants were assayed for activity in a Ca2+-free phosphate buffer (Galliard, 1971).
Obtained after 24 h of anoxia.
Mean of two determinations.
From normoxic cells.
n = 6.
Table II.
Acyl composition of total lipids and of FFA released by potato cells under anoxia
| Acyl Chain | Total Lipidsa | FFAa |
|---|---|---|
| mol % | ||
| 16:0 | 19.7 ± 0.5 | 16.3 ± 0.4 |
| 18:0 | 2.4 ± 0.1 | 1.7 ± 0.1 |
| 18:1 | 3.1 ± 0.4 | 2.7 ± 0.3 |
| 18:2 | 47.4 ± 0.6 | 49.5 ± 0.4 |
| 18:3 | 27.0 ± 0.2 | 29.5 ± 0.2 |
Total lipids and FFA were determined as described in Methods. n = 4.
Values obtained after 24 h of anoxia, with hydrolysis extents ranging from 45% to 60%.
Energy Production Linked to the Formation of Fermentation Products under Anoxia
We measured the production of ethanol, lactate, and Ala together with the level of ATP in anoxic potato cells. There was a 45% decrease in the ATP level after 6 h of anoxia (Table III). Longer incubation times led to a further depletion of the ATP pool, which was reduced to 10% of the initial normoxic value after 12 h. Ethanol accumulated steadily during 24 h up to a level 4.5-fold higher than that of lactate or Ala. Lactate rose only during the first 6 h of anoxia and then remained stable. Ala increased up to 18 h, then stabilized at the same level as lactate. We calculated the ATP synthesis rates as described in the legend of Table III. Under normoxia the synthesis rates were high and insensitive to the method (via invertase or Suc synthase) of Suc metabolism (Plaxton, 1996). These rates were drastically reduced during the first 6 h of anoxia to 1.5% and 2.8% (via invertase or Suc synthase, respectively) of the maximum theoretical values attainable under normoxia; they diminished further in the next 6 h, and finally showed an essentially constant value between 12 and 24 h.
Table III.
Levels of ATP and fermentation products generated during incubation of potato cells under anoxia, and the corresponding calculated ATP synthesis rates
| Incubation Time under Anoxia | ATP | Ethanol | Lactate | Ala | Calculated
ATP Synthesis Rate
|
|
|---|---|---|---|---|---|---|
| a | b | |||||
| h | μmol g fresh wt−1 | μmol g fresh wt−1 h−1 | ||||
| 0 | 86 ± 13 | 1.4 ± 0.2 | 3.7 ± 0.3 | 4.6 ± 1.3 | ∼608 | ∼640 |
| 6 | 47 ± 22 | 37.8 ± 11.8 | 16.9 ± 2.4 | 9.7 ± 0.7 | 9.1 | 18.2 |
| 12 | 9 ± 6 | 45.2 ± 8.4 | 15.9 ± 4.1 | 10.5 ± 1.6 | 5.2 | 10.3 |
| 18 | 3 ± 1 | 66.9 ± 22.1 | 19.1 ± 2.1 | 16.5 ± 1.6 | 5.2 | 10.3 |
| 24 | 5 ± 3 | 92.7 ± 30.3 | 19.1 ± 3.4 | 17.4 ± 2.1 | 5.0 | 10.0 |
Levels were measured as described in Methods and are the average (±sd) of n = 5 determinations. Except for the zero-time samples, the rates of ATP synthesis were calculated by: (a) assuming an equimolar correspondence between each fermentation end product and ATP, (b) summing all end products after subtraction of their zero-time levels, and (c) expressing results on a 1-h basis. The ATP synthesis rate at time 0 was calculated from the O2 uptake of normoxic cells (96 ± 11 μmol O2 g fresh wt−1 h−1, n = 11) and assuming the production of 38 or 40 ATP molecules per 6 O2 molecules consumed. Cases a and b correspond to the feeding of the glycolytic pathway by the invertase or by the Suc-synthase, respectively (Plaxton, 1996).
Metabolic Inhibitors Can Induce FFA Release under Normoxia
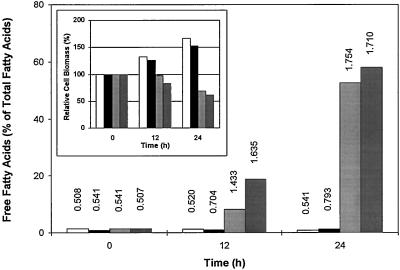
The results of Table III point to the obvious and important role played by energy metabolism. We attempted to mimic the effect of anoxia on membrane lipid degradation (Fig. 2) by treating potato cells under normoxia with different inhibitors of: (a) the glycolytic pathway (DeOGlc + IAc), (b) the mitochondrial electron transport in the Cyt chain (azide) and in the alternative pathway (SHAM), and (c) the membrane-linked ADP phosphorylation (FCCP). After incubation for 12 and 24 h, we determined the extent of lipid hydrolysis and the relative change in biomass (Fig. 4). FCCP had no effect on these parameters (as compared with normoxic controls), although it stimulated the O2 uptake rate by 50%. The DBI of the FFA fraction was <1. However, lipid hydrolysis occurred in a time-dependent manner when the mitochondrial inhibitor couple, azide plus SHAM, or the glycolytic inhibitor couple, DeOGlc plus IAc, was used. The acyl chain composition and the DBI of the FFAs released under normoxia (Fig. 4) resembled that obtained between 12 and 24 h of anoxia (Fig. 3B) and was accompanied by a decrease in the relative cell biomass. Glycolytic inhibitors were more efficient than mitochondrial inhibitors for both parameters (Fig. 4). Furthermore, both the change in the relative biomass and the extent of lipid hydrolysis obtained after 12 and 24 h of normoxia in the presence of inhibitors were consistent with those observed during the anoxic incubation of potato cells (compare Fig. 4 with Figs. 1 and 2). NaF (1–5 mm) could not be substituted to the glycolytic inhibitors. Finally, total lipids were constant, either on a fresh weight basis or as absolute amounts in a fixed cell-suspension volume. Lipid hydrolysis was thus achieved without further processing (e.g. β-oxidation) of the hydrolysis products. We must also point out that membrane degradation occurred independently in the absence (Figs. 2 and 3) or presence (Fig. 4) of O2.
Figure 4.
FFA release and relative biomass changes (inset) in potato cells incubated under normoxia for 0, 12, and 24 h (no addition, open bars), or in the presence of FCCP (0.1 μm, black bars), azide plus SHAM (2 mm each, light gray bars), and DeOGlc plus IAc (20 and 2 mm, dark gray bars). Figures above bars represent the DBI of the corresponding FFA pools. Data represent the mean value of two independent experiments.
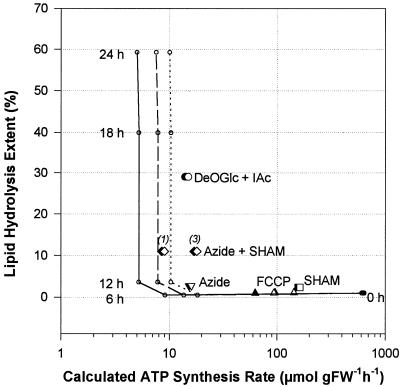
The Relation between Lipid Hydrolysis and ATP Synthesis Rates
One can see from the results in Figure 2 and Table III that lipid hydrolysis occurred under anoxia only after a threshold time of approximately 12 h, when the energy production was rather low. We have explored this relation over a wide range of ATP synthesis rates, and the results are presented in Figure 5. Under anoxia, lipid hydrolysis did not occur provided that the rates were higher than about 10 μmol g−1 fresh weight h−1, depending on how Suc could be metabolized. Below this threshold value anoxic cells became committed to lipid hydrolysis. When we treated potato cells with inhibitors under normoxia, we calculated the ATP-synthesis rates from the residual O2-uptake rates. As shown in Figure 5, these rates were indeed reduced by SHAM, FCCP, and azide, but each of the three compounds failed to induce lipid degradation when applied alone. In contrast, the combination of azide plus SHAM could elicit lipid hydrolysis in normoxic cells; the glycolytic inhibitors were even more efficient in this respect. We obtained similar results with 12 μm antimycin A instead of sodium azide (data not shown).
Figure 5.
Relation between calculated ATP synthesis rate (logarithmic scale) and the extent of lipid hydrolysis (linear scale) in potato cells. In the first series of experiments (○), cells were incubated for 0, 6, 12, 18, and 24 h under anoxia and the levels of ethanol, lactate, and Ala were determined together with lipid hydrolysis. From these data, ATP synthesis rates were calculated assuming that Suc is hydrolyzed via the invertase pathway, which gives a net yield of two ATP per hexose (continuous line), or via the Suc-synthase pathways, which can yield between three (dashed line) and four ATP (dotted line) per hexose, and plotted against the corresponding extent of lipid hydrolysis. In the second group of experiments (large symbols), cells were treated for 12 h under normoxia with 2 mm azide (▾ and ▿), 2 mm SHAM (▪ and □), or azide + SHAM (2 mm each, ♦ and ⋄), DeOGlc + IAc (20 and 2 mm, • and ○, respectively), and FCCP (0.1 and 1 μm, ▴ and ▵, respectively). ATP synthesis rates were then calculated from the O2 uptake rates (for SHAM and/or azide, for DeOGlc + IAc and for FCCP) measured after 12 h of incubation, and plotted against lipid hydrolysis extents. These calculations were based on an O2-to-hexose ratio of 6 and on the maximal theoretical yield of ATP:hexose derived from metabolic pathways. Filled and open symbols correspond to the two extreme Suc utilization scenarios (see above). In the case of the combined azide + SHAM treatment, italic numbers correspond to the number of mitochondrial “phosphorylation sites” used for the calculation. FW, Fresh weight.
DISCUSSION
We studied the changes in biomass, lipid composition, fermentation end products, ATP levels, and synthesis rates in cultivated potato cells submitted to anoxia stress. We then compared the changes with those obtained in cells treated in the presence of O2 with inhibitors that interfered with energy metabolism. The cells exhibited a two-phase behavior with respect to anoxia. The first phase (0–12 h) reflects the survival of cells that temporarily coped with the stress condition by switching to fermentation. There was no net loss in cell biomass, and cell viability was maintained (Fig. 1), although both the level and the rate of ATP synthesis were depressed to lower values because of fermentations (Table III). Cell membranes were still intact, as suggested by the absence of lipid degradation (Figs. 2 and 3B). The second phase was an autolytic process starting approximately 12 h after the onset of anoxia. It was characterized by a decrease in cell biomass (Fig. 1), a further reduction of ATP levels and production rates (Table III), and a massive accumulation of FFAs (Figs. 2 and 3B) at the expense of phospholipids (Table I).
The release of two fatty acids per phospholipid and the absence of lysophospholipids (Table I) suggest that an LAH was responsible for the extensive lipid degradation observed in Figure 2. This was confirmed by the close similarity of the acyl compositions of total lipids and FFAs (Table II; see also the 24-h point in Fig. 3B). This would not be the case if fatty acids had been released from a single position of the glycerol by a phospholipase A1 or A2 because 16- and 18-carbon fatty acids show a preferential localization on the sn2 and sn1 carbon atoms, respectively, of the glycerol backbone of plant phospholipids (Galliard, 1973). Furthermore, cell extracts hydrolyzed p-nitrophenylpalmitate; Ca2+ was not required for this activity, in contrast to phospholipases A. As shown in Table I, the extract from 1 g of cell fresh weight would form 5.3 μmol of palmitic acid from p-nitrophenylpalmitate in 12 h. In anoxic cells an equivalent FFA release would correspond to a hydrolysis extent of about 20%, which falls within the range of values shown in Figure 2. The autolytic phase of anoxic potato cells involves the activation of an LAH. This enzyme had already been implicated in the response of plant cells to mechanical or pathogenic wounding (Racusen, 1984; Slusarenko et al., 1991; Farmer and Ryan, 1992) and in the formation of autophagic vacuoles after Suc starvation (Aubert et al., 1996).
Because FFAs account for up to 60% of total lipids after 24 h of anoxia (Fig. 2), they must arise mainly from phospholipids and glycolipids, which make up 76% and 14%, respectively, of total cell lipids. This suggests that lipid degradation eventually affects most cellular membranes. The very sharp DBI change in the FFAs (Fig. 3B) is more illustrative of a general hydrolytic process than of selective degradation, such as that shown by cell membrane autophagy under Suc starvation (Aubert et al., 1996). FFAs remain in the membrane in which they were generated (Roelofsen, 1982); our control experiments agree with this view. Aside from the detergent/fusogen properties of FFAs, there is another consequence of LAH action: removal of the polar headgroups of membrane lipids, which dissolve in the aqueous medium. Clearly, membrane properties must be strongly altered by these structural changes. In potato tubers the anoxia-induced accumulation of FFAs was correlated with an increased electrolyte leakage, which reached almost 100% when the FFA level was 6% to 7% of total lipids (Crawford and Braendle, 1996). The change in the aspect of cell pellets after 12 h of anoxia may well reflect these alterations in membrane composition and structure.
Cell behavior during anoxia depends on how the energy requirements are fulfilled when the energy supply is exclusively supported by fermentation. The metabolic survival strategy of cultivated cells includes several aspects. ATP-consuming processes of lower priority can be suppressed, as illustrated by the arrest in biomass increase after 6 h of anoxia (Fig. 1). Entering into anaerobic retreat can slow down the ongoing ATP-consuming processes (Pradet and Raymond, 1983). The metabolism of Suc via Suc synthase rather than via invertase, for example, can improve residual ATP production (Stitt and Steup, 1985; Sachs, 1994), resulting in an increase in the ATP net yield of 1.5- to 2-fold, according to pyrophosphate availability (Mertens, 1991; Stitt, 1998). Finally, the efficiency of ATP processes can be enhanced, e.g. by raising the H+-to-ATP ratio of plasmalemma and tonoplast H+-ATPase pumps (Slayman, 1980). This strategy met with some success during the first 12 h of anoxia, because the cells did not undergo any membrane damage during this period, although both the ATP levels and the synthesis rates were reduced (Table III).
The ATP content of a cell cannot sustain itself for more than 1 to 2 min (Roberts et al., 1984). On a time scale of hours, the main determinant of energy balance must be the rate of ATP synthesis rather than the ATP level (Tadege et al., 1998). There is a threshold in fermentation and ATP synthesis rates below which survival is prejudiced (Xia et al., 1995). Our results are in agreement with this view and suggest that the integrity of membrane structure relies on a low threshold value of the ATP production rate of approximately 10 μmol g−1 fresh weight h−1. This value is about 1.5% of the maximal theoretical ATP synthesis rate under normoxia (Table III; Fig. 5). It is generally agreed, however, that only about half of the mitochondrial H+ electrochemical potential is used to drive ATP synthesis. Thus, the maximum reachable ATP yield per Glc is probably closer to 20 than to 38. The threshold of 10 μmol ATP g−1 fresh weight h−1 would then be 3% of the maximum ATP synthesis rate. The most important aspect of this threshold is not the sharpness of its absolute value, but rather its existence and its low value compared with control rates. However, this seemingly low value is higher than the cost of membrane maintenance in nongrowing plant cells, estimated to be approximately 1.7 mg Glc g−1 dry weight d−1 by Penning de Vries (1975), which is equivalent to approximately 0.16 μmol ATP g−1 fresh weight h−1. These modest rates merely emphasize the sobriety of membrane maintenance mechanisms.
Accordingly, treating normoxic cells with metabolic inhibitors should confirm the involvement of the energy production rate in the maintenance of membrane integrity. Cells can be fed with an uncoupler (e.g. FCCP). This will suppress membrane-linked phosphorylations, whereas substrate-level phosphorylations of the glycolytic pathway and of the tricarboxylic acid cycle will still occur and will even be stimulated by the increased O2 uptake rate. Clearly, uncoupling conditions allow sufficiently high rates of substrate-level phosphorylation (60–144 μmol ATP g−1 fresh weight h−1) to prevent any lipid hydrolysis during the whole incubation period (Figs. 4 and 5).
Azide (or antimycin A) and/or SHAM can inhibit mitochondrial electron transport pathways (Vanlerberghe and McIntosh, 1997). Membrane lipid degradation (Figs. 4 and 5) is then strictly dependent on the presence of both inhibitors, suggesting that degradation occurs only when all membrane-linked redox reactions in mitochondria are inhibited. DeOGlc plus IAc can block the glycolytic pathway (Jans et al., 1997). This treatment is more efficient than one that inhibits respiration (Figs. 4 and 5), probably because of the upstream localization of the inhibition sites. These inhibitors are usually used for short-term studies, and with isolated enzymes and organelles rather than with whole cells. Moreover, IAc is an oxidizing agent of sulphydryl groups. Secondary effects might contribute to overall inhibition during the 12-h incubation period and shift the lipolytic response toward higher values of the rate of ATP synthesis. The lack of effect of 1 to 5 mm NaF as an alternative glycolytic inhibitor is presumably due to restricted penetration within the cells. Nevertheless, we ascribe the release of FFAs in normoxic cells treated with glycolytic and respiratory inhibitors to a threshold in the rate of ATP synthesis, the value of which is comparable to that observed under anoxia (Fig. 5). In addition, the mere absence of O2 does not trigger lipid hydrolysis. Therefore, no O2 sensor is required in this process.
The similarity of the extent of lipid hydrolysis and of the acyl composition of FFAs obtained under anoxia (Figs. 2 and 3) and normoxia with inhibitors (Fig. 4) suggests that these hydrolytic processes stem from a common mechanism. The mechanism is triggered when the rate of ATP synthesis reaches a low threshold value (Fig. 5) and eventually activates an LAH. Clearly, membrane lipid integrity is a key factor in the survival of both anoxia-intolerant (this paper) and resistant plants (Henzi and Braendle, 1993). Accumulation of new mRNAs and a shift in protein synthesis (Sachs, 1994) would be useless without an intact membrane network. Finally, our results emphasize the prime importance of the events occurring under anoxia, as compared with those taking place after reaeration (Pfister-Sieber and Braendle, 1994). Indeed, with the hydrolysis values reported in Figure 2, a cell is dead. A further degradative process (e.g. peroxidation) would be purely (bio)chemical, without any physiological relevance for the actual cell. Overexpressing the key enzymes of the fermentation pathways can increase the energetic competence of cells. We are currently establishing potato cell lines with enhanced fermentation capacity from transformed plants. We hypothesize that such cells will not undergo lipid degradation altogether or will delay it significantly.
ACKNOWLEDGMENT
We are grateful to Urs Kaempfer (Chemistry Department, University of Bern, Switzerland) for his help with the HPLC analysis of amino acids.
Abbreviations:
- DBI
double-bond index
- DeOGlc
2-deoxy-d-Glc
- FCCP
carbonyl-cyanide-4-trifluoromethoxyphenylhydrazone
- FFA
free fatty acid
- IAc
sodium iodoacetate
- LAH
lipolytic acyl hydrolase
- SHAM
salicylhydroxamic acid
- X:Y
a fatty acyl group containing X carbon atoms and Y double bonds (cis unless otherwise specified)
Footnotes
This work was supported by the Swiss National Science Foundation.
LITERATURE CITED
- Andreev VY, Generozova IP, Vartapetian BB. Energy status and mitochondrial ultrastructure of excised pea root at anoxia and postanoxia. Plant Physiol Biochem. 1991;29:171–176. [Google Scholar]
- Armstrong W, Braendle R, Jackson B. Mechanisms of flood tolerance in plants. Acta Bot Neerl. 1994;43:307–358. [Google Scholar]
- Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R. Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrates. J Cell Biol. 1996;133:1251–1263. doi: 10.1083/jcb.133.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlingmeyer BA, Cohen SA, Tarvin TL. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984;336:93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Brown DJ, Beevers H. Fatty acid of rice coleoptiles in air and anoxia. Plant Physiol. 1987;84:555–559. doi: 10.1104/pp.84.2.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford RMM, Braendle R. Oxygen deprivation stress in a changing environment. J Exp Bot. 1996;47:145–159. [Google Scholar]
- Davey JF, Ersser RS. Amino acid analysis of physiological fluids by high-performance liquid chromatography with phenylisothiocyanate derivatization and comparison with ion-exchange chromatography. J Chromatogr. 1990;528:9–23. doi: 10.1016/s0378-4347(00)82358-0. [DOI] [PubMed] [Google Scholar]
- Dipierro S, De Leonardis S. The ascorbate system and lipid peroxidation in stored potato (Solanum tuberosum L.) tubers. J Exp Bot. 1997;48:779–783. [Google Scholar]
- Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliard T. The enzymic deacylation of phospholipids and galactolipids in plants: purification and properties of a lipolytic acyl hydrolase from potato tubers. Biochem J. 1971;121:379–390. doi: 10.1042/bj1210379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliard T. Phospholipid metabolism in photosynthetic plants. In: Ansell G, Hawthorne J, Dawson R, editors. Form and Function of Phospholipids, Ed 2. Amsterdam, The Netherlands: Elsevier; 1973. pp. 253–288. [Google Scholar]
- Harwood JL. Fatty acid metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1988;39:101–138. [Google Scholar]
- Henzi T, Braendle R. Long term survival of rhizomatous species under oxygen deprivation. In: Jackson M, Black C, editors. Interacting Stresses on Plants in a Changing Climate. NATO ASI Series, Vol 116. Berlin: Springer-Verlag; 1993. pp. 305–314. [Google Scholar]
- Jans SW, Willems J, van Bilsen M, Reutelingsperger CP, van der Vusse GJ. Phospholipid degradation in energy-deprived cardiac myocytes: does annexin V play a role? J Mol Cell Cardiol. 1997;29:1401–1410. doi: 10.1006/jmcc.1997.0378. [DOI] [PubMed] [Google Scholar]
- Knowles NR, Knowles LO. Correlations between electrolyte leakage and degree of saturation of polar lipids from aged potato (Solanum tuberosum L.) tuber tissue. Ann Bot. 1989;63:331–338. [Google Scholar]
- Kumar GNM, Knowles NR. Changes in lipid peroxidation and lipolytic and free radical-scavenging enzyme activities during aging and sprouting of potato (Solanum tuberosum) seed tubers. Plant Physiol. 1993;102:115–124. doi: 10.1104/pp.102.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GNM, Knowles NR. Nature of enhanced respiration during sprouting of aged potato seed tubers. Physiol Plant. 1996;97:228–236. [Google Scholar]
- Mertens E. Pyrophosphate-dependent phosphofructokinase, an anaerobic glycolytic enzyme? FEBS Lett. 1991;285:1–5. doi: 10.1016/0014-5793(91)80711-b. [DOI] [PubMed] [Google Scholar]
- Penning de Vries FWT. The cost of maintenance processes in plant cells. Ann Bot. 1975;39:77–92. [Google Scholar]
- Pfister-Sieber M, Braendle R. Aspects of plant behavior under anoxia and post-anoxia. Proc R Soc Edinb Sect B. 1994;102B:313–324. [Google Scholar]
- Plaxton WC. The organization and regulation of plant glycolysis. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:185–214. doi: 10.1146/annurev.arplant.47.1.185. [DOI] [PubMed] [Google Scholar]
- Pradet A, Raymond P. Adenine nucleotide ratios and adenylate energy charge in energy metabolism. Annu Rev Plant Physiol. 1983;34:199–224. [Google Scholar]
- Racusen D. Lipid acyl hydrolase of patatin. Can J Bot. 1984;62:1640–1644. [Google Scholar]
- Ratcliffe RG. Metabolic aspects of the anoxic response in plant tissue. In: Smirnoff N, editor. Environmental Plant Metabolism: Flexibility and Acclimation. Oxford, UK: Bios Scientific Publishers; 1995. pp. 111–127. [Google Scholar]
- Roberts JKM, Callis J, Jardetzky O, Walbot V, Freeling M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc Natl Acad Sci USA. 1984;81:6029–6033. doi: 10.1073/pnas.81.19.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelofsen B. Phospholipases as tools to study the localization of phospholipids in biological membranes: a critical review. J Toxicol Toxin Rev. 1982;1:87–197. [Google Scholar]
- Sachs M (1994) Gene expression during anoxia. In A Basra, ed, Stress-Induced Gene Expression in Plants. Harwood Academic Publishers, Chur, Switzerland, pp 87–102
- Sieber M, Braendle R. Energy metabolism in rhizomes of Acorus calamus (L.) and in tubers of Solanum tuberosum (L.) with regard to their anoxia tolerance. Bot Acta. 1991;104:279–282. [Google Scholar]
- Slayman CL (1980) Transport control phenomena in Neurospora. In R Spanswick, W Lucas, J Dainty, eds, Plant Membrane Transport: Current Conceptual Issues. Elsevier, Amsterdam, The Netherlands, pp 179–190
- Slusarenko AJ, Croft PK, Voisey CR (1991) Biochemical and molecular events in the hypersensitive response of bean to Pseudomonas syringae pv phaseolica. In C Smith, ed, Biochemistry and Molecular Biology of Plant-Pathogen Interactions. Clarendon Press, Oxford, UK, pp 126–143
- Spychalla JP, Desborough SL. Fatty acids, membrane permeability and sugars of stored potato tubers. Plant Physiol. 1990a;94:1207–1213. doi: 10.1104/pp.94.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spychalla JP, Desborough SL. Superoxide dismutase, catalase and α-tocopherol content of stored potato tubers. Plant Physiol. 1990b;94:1214–1218. doi: 10.1104/pp.94.3.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. Pyrophosphate as an energy donor in the cytosol of plant cells: an enigmatic alternative to ATP. Bot Acta. 1998;111:167–175. [Google Scholar]
- Stitt M, Steup M. Starch and sucrose degradation. In: Douce R, Day D, editors. Encyclopedia of Plant Physiology, New Series, Higher Plant Cells, Vol 18. Berlin: Springer-Verlag; 1985. pp. 347–390. [Google Scholar]
- Tadege M, Braendle R, Kuhlemeier C. Anoxia tolerance in tobacco roots: effect of overexpression of pyruvate decarboxylase. Plant J. 1998;14:327–335. [Google Scholar]
- Vanlerberghe GC, McIntosh L. Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- Vartapetian BB, Jackson MB. Plant adaptations to anaerobic stress. Ann Bot. 1997;79:3–20. [Google Scholar]
- Vartapetian BB, Mazliak P, Lance C. Lipid biosynthesis in rice coleoptiles grown in the presence or in the absence of oxygen. Plant Sci Lett. 1978;13:321–328. [Google Scholar]
- Xia JH, Saglio PH, Roberts JKM. Nucleotide levels do not critically determine survival of maize root tips acclimated to a low oxygen environment. Plant Physiol. 1995;108:589–595. doi: 10.1104/pp.108.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]