Abstract
Background
Higher serum ferritin levels may be influenced by iron use and inflammation, and are associated with higher mortality in hemodialysis (HD) patients. We hypothesized that a major rise in serum ferritin is associated with a higher risk of mortality, irrespective of baseline serum ferritin in incident HD patients.
Methods
In a cohort of 93,979 incident HD patients between 2007 and 2011, we examined the association of change in serum ferritin from the baseline patient quarter (first 91 days from dialysis start) to the subsequent quarter with mortality. Multivariable adjustments were done for case-mix and markers of the malnutrition, and inflammation complex and intravenous iron dose. Change in serum ferritin was stratified into 5 groups: <−400, −400 to <−100, −100 to <100, 100 to <400, and ≥400 ng/mL/quarter.
Results
The median change in serum ferritin was 89 ng/mL/quarter (interquartile range −55 to 266 ng/mL/quarter). Compared to stable serum ferritin (−100 to <100 ng/mL/quarter), a major rise (≥400 ng/mL/quarter) was associated with higher all-cause mortality (hazard ratio [95% CI] 1.07 [0.99–1.15], 1.17 [1.09–1.24], 1.26 [1.12–1.41], and 1.49 [1.27–1.76] according to baseline serum ferritin: <200, 200 to <500, 500 to <800, and ≥800 ng/mL in adjusted models, respectively. The mortality risk associated with a rise in serum ferritin was robust, irrespective of intravenous iron use.
Conclusions
During the first 6-months after HD initiation, a major rise in serum ferritin in those with a baseline ferritin ≥200 ng/mL and even a slight rise in serum ferritin in those with a baseline ferritin ≥800 ng/mL are associated with higher mortality.
Keywords: Ferritin, Transferrin saturation, Hemodialysis, Mortality
Introduction
Serum ferritin is a marker used to reflect body iron storage [1, 2] and to monitor iron therapy in chronic kidney disease patients [3, 4]. Ferritin levels are influenced by both endogenous and exogenous iron as well as inflammatory conditions such as chronic diseases, infection, and cancer [5–7]. An iron-mediated rise of ferritin stems from dietary iron absorption, macrophage recycling [5, 8], and oral or intravenous (IV) iron administration [9]. Under inflammatory conditions, a liver-derived acute phase protein hepcidin inhibits iron absorption from the bowel, and iron release from macrophages and hepatocytes, thus restricting iron utilization in the body thereby leading to an increase in ferritin [10].
Among US non-dialysis dependent chronic kidney disease stage 5 patients, mean ferritin levels are approximately 150–250 ng/mL [11]; however, the mean ferritin may be upwards of 400 ng/mL among maintenance dialysis patients, which is attributed mostly to the initiation of IV iron therapy [12, 13]. The mean ferritin levels have been on the rise in maintenance dialysis patients since 1990 [12, 13] to a mean ferritin in excess of 800 ng/mL, as of 2011 [14]. A study in incident hemodialysis (HD) patients showed that there was a sharp increase in the first year of HD, which was followed by a subsequent rise in ferritin over time on dialysis [15]. Although initial increases in ferritin may be attributed to the administration of IV iron, sustained higher levels in maintenance dialysis patients may be attributed to lowering of erythropoietin [14] or other unknown causes [15].
Higher ferritin levels ≥800 ng/mL may be detrimental to patient survival and has been found to be associated with higher mortality risk in dialysis patients after adjustment for iron supplementation, safety of iron administration, malnutrition, or inflammation [16–18]. However, no study has investigated the relationship of abrupt changes in ferritin with mortality in incident HD patients. Thus, we aimed to investigate the association between ferritin changes over time and mortality in an incident HD population. We hypothesized that a rapid rise in ferritin over the first 6 months upon transition to maintenance HD is associated with a higher risk of mortality, independent of IV iron administration. We also examined whether the change in ferritin across strata of higher versus lower dose of prescribed IV iron during the same period has a bearing on mortality.
Materials and Methods
Study Cohort
We conducted analyses using administrative data from all incident HD patients receiving dialysis treatment in one of the out-patient dialysis facilities of a large dialysis organization (LDO) from January 1, 2007 to December 31, 2011. The construction of this cohort has previously been described elsewhere [19]. Patients’ follow-up time was divided into consecutive 91-day intervals (patient quarters) from the time of patients’ first dialysis treatment over the entire study period. Over the follow-up period, 208,820 subjects started dialysis treatment. After excluding patients aged <18 or >99 years old at initiation, those who received less than 60 days of total treatment and who were treated with a dialysis modality other than thrice-weekly HD over the duration of follow-up, 133,156 incident HD patients remained. Patients were further excluded for missing ferritin measurements during the first 6 months of transition to maintenance HD. Therefore, the final study population consisted of 93,979 incident HD patients (online suppl. Appendix Fig. S1; for all online suppl. material, see www.karger.com/doi/10.1159/000478735).
The study was approved by the Institutional Review Committees of the University of California Irvine, Los Angeles Biomedical Research Institute at Harbor-UCLA, and the University of Washington. Given the large sample size, anonymity of studied patients, and nonintrusive nature of research, the requirement for written consent was exempted.
Demographic and Clinical Measures
Information on race/ethnicity, primary insurance, vascular access type, and the presence of comorbidities at baseline were obtained from the LDO database. The following 16 preexisting co-morbidities were considered: diabetes, hypertension, cystic kidney disease, autoimmune disease, dyslipidemia, chronic obstructive pulmonary disease, liver disease, atherosclerotic heart disease, other cardiac disease (pericarditis and cardiac arrhythmia), congestive heart disease, cerebrovascular disease, malignancy, thyroid disorders, human immunodeficiency virus, substance use, and alcohol abuse.
Laboratory Measures
Blood samples were drawn using standardized techniques in the LDO clinics and were transported to the LDO laboratory in Deland, Florida, typically within 24 h. Most laboratory values were measured monthly, including serum creatinine, albumin, hemoglobin, hematocrit, platelet, peripheral white blood cell, lymphocyte percentage, total iron binding capacity (TIBC), iron saturation (ISAT), calcium, phosphorus, bicarbonate, and alkaline phosphatase. Serum intact parathyroid hormone (iPTH) and ferritin levels were usually measured at least once during each calendar quarter. Most blood samples were collected before dialysis, except for post-dialysis blood urea nitrogen to calculate urea kinetics. The normalized protein catabolic rate (nPCR) was measured monthly as an indicator of daily protein intake. The dialysis dose was estimated by single pool Kt/V (spKt/V) using the urea kinetic model. To minimize measurement variability, all repeated measures for each patient during each patient quarter were averaged and summary estimates used in all models. The quarterly averaged values during the first 91-days of dialysis (Q1) were used as baseline values to attenuate the effect of short-term variation in laboratory measurement.
Change in ferritin was calculated by subtracting the mean ferritin concentration during the first quarter (Q1) from the mean ferritin concentration during the second quarter (Q2). Δ ferritin was divided into 5 groups: less than −400, −400 to <−100, −100 to <100, 100 to <400, and ≥400 ng/mL/quarter.
Statistical Analysis
Patients’ baseline demographics, clinical characteristics, and laboratory measurements across change in ferritin groups were summarized as proportions, mean (±SD), or median (interquartile range) and compared using chi-square tests, analysis of variance, Kruskal-Wallis tests, depending on data type. We used Cox proportional hazard models to evaluate the association of change in ferritin during the first 6 months of dialysis initiation (Δ ferritin) with all-cause mortality. We considered Δ ferritin of −100 to <100 ng/mL/quarter to be the reference group because it reflects a relatively stable change in ferritin over the first 6 months of dialysis initiation. To test for effect modification of baseline ferritin, we performed stratified analyses by baseline ferritin (<200, 200 to <500, 500 to <800, and ≥800 ng/mL).
For each analysis, three levels of multivariable adjustment were used: (1) a minimally adjusted model that included the main predictor (change in serum ferritin group) and the entry calendar quarter (time period of dialysis initiation); (2) a case-mix adjusted model that included covariates in the minimally adjusted model as well as age, sex, race/ethnicity (white, African-American, Hispanic, Asian, and others), primary insurance (Medicare, Medicaid, and others), access type (central venous catheter, arteriovenous fistula, arteriovenous graft, and others), spKt/V, and 16 aforementioned comorbidities; (3) a case-mix and malnutrition-inflammation cachexia syndrome (MICS) adjusted model that included covariates in the case-mix model as well as iron and erythropoiesis-stimulating agent (ESA) dose, and 12 surrogates of nutritional and/or inflammatory status: serum creatinine, hemoglobin, albumin, peripheral white blood cell, lymphocyte percentage, TIBC, calcium, phosphorus, bicarbonate, iPTH, body mass index, and nPCR. All laboratory covariates, spKt/V, and access type were adjusted for the Δ of each value between 1st and 2nd patient quarter as well as values from the 2nd patient quarter to account for the simultaneous change that may occur over time as well. The assumption of proportional hazards was assessed by log-log plots. Patients were followed after the 2nd patient quarter until death, transplantation, transfer to a non-affiliated clinic or end of the study period (December 31, 2011).
To account for the effect of IV iron use on the change in ferritin and mortality, the cohort was divided into IV iron use and no IV iron use groups over the first 6 months of dialysis. We used a singular referent group of stable levels of ferritin (Δ ferritin −100 to <100 ng/mL/quarter) and baseline ferritin 200 to <500 ng/mL as the referent group.
We used the Spearman correlation to examine the relationship between change in ferritin and change in ISAT, and stratified among the change in ferritin groups. The comparison of change in ISAT and other laboratory measurements across change in ferritin groups were compared using trend tests. To evaluate the effect of ISAT on the association between change in ferritin and mortality, patients were divided according to the level of change in ISAT.
In sensitivity analyses, 18 different combinations based on groups of change in ferritin and groups of baseline ferritin were used and compared to a singular referent group of stable ferritin (Δ ferritin −100 to <100 ng/mL/quarter) and baseline ferritin 200 to <500 ng/mL, based on Kidney Disease Improving Global Outcomes recommended guidelines. Due to extremely small sample sizes, combinations of the change in ferritin <−400 ng/mL/quarter group and baseline ferritin groups of <200 and 200–<500 ng/mL were not created.
To further examine the relationship between change in ferritin and mortality, we performed subgroup analyses and tested for interactions between Δ ferritin and covariates using Wald’s test.
Data on ferritin at the first and second patient quarters were available. Data on sex, race/ethnicity, and comorbidities were missing for <0.5% of the cohort and not imputed. Other covariates including spKt/V, serum creatinine, hemoglobin, albumin, peripheral white blood cell, lymphocyte percentage, TIBC, calcium, phosphorus, bicarbonate, iPTH, body mass index, and nPCR were missing <5% and were imputed by using multiple imputation methods. All analyses were carried out using STATA MP version 13.1 (Stata Corp., College Station, TX, USA).
Results
Baseline Demographics According to Δ Ferritin
The analytic cohort comprised of 93,979 incident HD patients in whom the median Δ ferritin was 89 ng/mL (interquartile range −55 to 266 ng/mL). Online supplementary Appendix Figure S2 shows the distribution of change in ferritin over the first 6 months of HD in our cohort. Baseline characteristics of the total cohort and stratified by Δ ferritin groups are presented in Table 1. Patients who experienced a higher increase in ferritin level (≥400 ng/mL/quarter) were more likely to be older, less likely to be African American, and had a higher prevalence of diabetes and other comorbidities (including hypertension, atherosclerotic heart disease, and dyslipidemia), had lower baseline ferritin, and higher baseline hemoglobin. Patients who experienced a greater decrease in ferritin level (<−400 ng/mL/quarter) had a lower prevalence of diabetes and were more likely to be African American. In addition, these patients tended to have higher baseline ferritin and ISAT, and lower TIBC and hemoglobin levels. As expected, the highest quarterly averaged baseline monthly iron dose was observed in patients with Δ ferritin level ≥400 ng/mL/quarter.
Table 1.
Baseline characteristics stratified by Δ serum ferritin in 93,979 incident HD patients
| Variables | Total (n = 93,979) | Δ Serum ferritin, ng/mL/quarter | p value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| <−400 (n = 4,503) | −400 to <−100 (n = 13,910) | −100 to <100 (n = 30,354) | 100 to <400 (n = 31,894) | ≥400 (n = 13,318) | |||
| Age, years | 63±15 | 63±15 | 62±15 | 61±15 | 63±15 | 65±14 | <0.001 |
| Female, % | 44 | 48 | 45 | 42 | 43 | 48 | <0.001 |
| Diabetes mellitus, % | 60 | 54 | 57 | 60 | 62 | 62 | <0.001 |
| Race/ethnicity, % | |||||||
| White | 46 | 41 | 43 | 46 | 47 | 45 | <0.001 |
| African-American | 32 | 38 | 35 | 32 | 31 | 31 | <0.001 |
| Hispanic | 15 | 12 | 15 | 15 | 16 | 16 | <0.001 |
| Asian | 3 | 4 | 4 | 3 | 3 | 4 | <0.001 |
| Others | 4 | 4 | 4 | 4 | 4 | 4 | 0.082 |
| Primary insurance, % | |||||||
| Medicare | 54 | 55 | 54 | 53 | 53 | 56 | <0.001 |
| Medicaid | 7 | 8 | 7 | 7 | 7 | 6 | <0.001 |
| Others | 39 | 37 | 38 | 40 | 40 | 38 | <0.001 |
| Initial access type, % | |||||||
| CVC | 74 | 81 | 79 | 74 | 71 | 72 | <0.001 |
| AVF | 15 | 7 | 10 | 15 | 18 | 17 | <0.001 |
| AVG | 4 | 3 | 3 | 4 | 5 | 5 | <0.001 |
| Others | <1 | <1 | <1 | <1 | <1 | <1 | 0.139 |
| Unknown | 6 | 9 | 7 | 6 | 6 | 6 | <0.001 |
| Baseline spKt/V | 1.47±0.32 | 1.51±0.34 | 1.47±0.32 | 1.45±0.32 | 1.47±0.31 | 1.51±0.31 | <0.001 |
| Comorbidities, % | |||||||
| Hypertension | 52 | 50 | 51 | 52 | 52 | 53 | 0.001 |
| Cystic kidney disease | 2 | 2 | 2 | 3 | 3 | 2 | 0.011 |
| Congestive heart failure | 39 | 37 | 39 | 40 | 39 | 36 | <0.001 |
| Atherosclerotic heart disease | 15 | 14 | 14 | 15 | 15 | 16 | <0.001 |
| Other cardiac disease | 15 | 15 | 15 | 15 | 15 | 16 | 0.006 |
| Cerebrovascular disease | 2 | 2 | 2 | 2 | 2 | 2 | 0.123 |
| Chronic obstructive pulmonary disease | 5 | 5 | 5 | 5 | 5 | 5 | 0.849 |
| Liver disease | 1 | 2 | 2 | 1 | 1 | 1 | <0.001 |
| Thyroid disease | 10 | 10 | 10 | 10 | 10 | 11 | <0.001 |
| Dyslipidemia | 26 | 25 | 25 | 26 | 27 | 27 | <0.001 |
| Autoimmune disease | 2 | 3 | 2 | 2 | 2 | 2 | <0.001 |
| Malignancy | 2 | 4 | 2 | 2 | 2 | 3 | <0.001 |
| HIV antibody positive status | <1 | 2 | <1 | <1 | <1 | <1 | <0.001 |
| Substance abuse | <1 | <1 | <1 | <1 | <1 | <1 | 0.417 |
| Alcohol abuse | <1 | <1 | <1 | <1 | <1 | <1 | 0.145 |
| Baseline laboratory measurements | |||||||
| BMI, kg/m2 | 28.2±7.3 | 26.3±6.7 | 27.7±7.2 | 28.8±7.6 | 28.4±7.3 | 27.3±6.9 | <0.001 |
| Albumin, g/dL | 3.5±0.5 | 3.3±0.5 | 3.4±0.5 | 3.5±0.5 | 3.6±0.4 | 3.5±0.4 | <0.001 |
| Creatinine, mg/dL | 5.9±2.4 | 5.9±2.4 | 6.0±2.5 | 6.0±2.4 | 5.9±2.3 | 5.7±2.2 | <0.001 |
| Bicarbonate, mEq/L | 24±3 | 24±3 | 24±3 | 24±3 | 23±3 | 23±3 | 0.001 |
| Blood HGB, g/dL | 11.2±1.2 | 10.5±1.3 | 10.8±1.2 | 11.1±1.2 | 11.4±1.1 | 11.4±1.1 | <0.001 |
| WBC, mm3 | 7.8±2.6 | 8.4±3.3 | 7.9±2.7 | 7.7±2.6 | 7.7±2.6 | 7.8±2.5 | <0.001 |
| Lymphocyte (% of WBC) | 20.9±7.4 | 19.9±8.3 | 20.6±7.6 | 21.0±7.3 | 21.0±7.3 | 20.7±7.5 | <0.001 |
| Platelet, ×109/L | 251±88 | 251±110 | 256±91 | 252±87 | 249±83 | 251±88 | <0.001 |
| iPTH, pg/mL | 317 (201–489) | 267 (155–425) | 306 (190–486) | 330 (210–507) | 323 (209–494) | 304 (194–460) | <0.001 |
| Alkaline phosphatase, μ/L | 87 (69–114) | 95 (74–133) | 89 (70–117) | 86 (68–113) | 86 (68–111) | 88 (69–116) | <0.001 |
| Calcium, mg/dL | 9.1±0.6 | 9.2±0.6 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | <0.001 |
| Phosphorus, mg/dL | 5.0±1.1 | 4.7±1.2 | 4.9±1.2 | 5.0±1.2 | 5.0±1.1 | 4.9±1.1 | <0.001 |
| Ferritin, ng/mL | 276 (160–473) | 1,037 (795–1,404) | 492 (351–696) | 238 (144–381) | 217 (131–349) | 256 (156–418) | <0.001 |
| TIBC, mg/dL | 226±48 | 188±46 | 207±46 | 229±47 | 234±46 | 230±47 | 0.001 |
| ISAT, % | 23±9 | 29±13 | 26±10 | 23±8 | 22±7 | 22±8 | <0.001 |
| nPCR, g/kg/day | 0.80±0.22 | 0.76±0.24 | 0.77±0.22 | 0.79±0.21 | 0.81±0.21 | 0.81±0.22 | <0.001 |
| Iron dose, mg/month | 333 (150–500) | 0 (0–133) | 150 (33–333) | 317 (150–433) | 400 (267–550) | 500 (333–667) | <0.001 |
| ESA dose, U/week | 4,700 (1,512–12,000) | 4,694 (1,540–12,100) | 4,669 (1,530–11,804) | 4,714 (1,500–12,099) | 4,653 (1,500–11,860) | 4,839 (1,584–12,278) | 0.004 |
Continuous values are expressed as mean ± SD if normally distributed or median (interquartile range) if skewed.
AVF, arteriovenous fistula; AVG, arteriovenous graft; BMI, body mass index; CVC, central venous catheter; ESA, erythropoiesis stimulating agent; HGB, hemoglobin; iPTH, intact parathyroid hormone; ISAT, iron saturation; nPCR, normalized protein catabolic rate; spKt/V, single pool Kt/V; TIBC, total iron binding capacity; WBC, white blood cell.
Association of Ferritin Variations with Mortality
A total of 24,177 (25.7%) all-cause deaths were reported over follow-up. The crude mortality rate was 162 per 1,000 patient-years (95% CI 160–164). Across all strata of baseline ferritin, a major rise in ferritin ≥400 ng/mL/quarter over the first 6 months was associated with higher death risk during the subsequent 5 years compared to stable ferritin (Δ ferritin −100 to <100 ng/mL/quarter; Fig. 1a–c). The highest mortality risk associated with a major rise in ferritin was observed among patients with baseline ferritin ≥800 ng/mL (hazard ratios [HR] 1.49 [95% CI 1.27–1.76]) after adjustment for case-mix and MICS covariates (Fig. 1c).
Fig. 1.
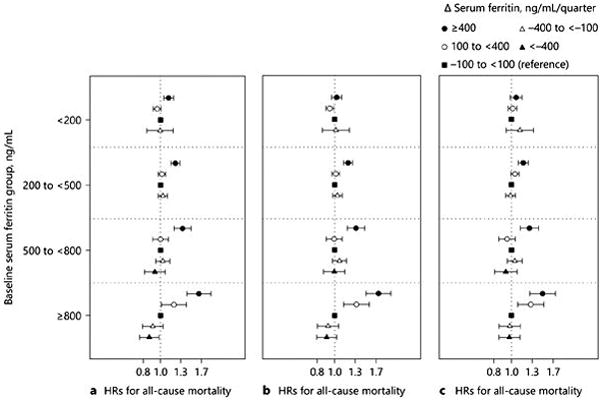
HRs (95% CIs) of all-cause mortality associated with change in serum ferritin over the first 6 months after maintenance HD initiation across 4 baseline serum ferritin groups in 93,979 incident HD patients. The models were adjusted (a) minimally with entry calendar quarter, additionally adjusted for (b) case-mix: age, sex, race/ethnicity, diabetes, insurance, dialysis access types, and dialysis dose Kt/V and comorbidities including hypertension, cystic kidney disease, autoimmune disease, dyslipidemia, chronic obstructive pulmonary disease, liver disease, atherosclerotic heart disease, other cardiac disease (pericarditis and cardiac arrhythmia), congestive heart disease, cerebrovascular disease, malignancy, thyroid disorders, human immunodeficiency virus, substance use and alcohol abuse); and additionally adjusted for (c) case-mix and MICS variables: case-mix variables plus serum creatinine, hemoglobin, albumin, peripheral white blood cell, lymphocyte percentage, total iron binding capacity, calcium, phosphorus, bicarbonate, iPTH, body mass index, normalized protein catabolic rate, iron dose, and ESA dose.
A drop in ferritin <−400 ng/mL/quarter in patients with baseline ferritin ≥800 ng/mL tended to be associated with lower mortality in the minimally adjusted model (HR 0.87 [95% CI 0.76–0.98]; Fig. 1a). However, this association was attenuated after additional adjustment for case-mix covariates (HR 0.91 [95% CI 0.80–1.04]) and case-mix and MICS covariates (HR 0.98 [95% CI 0.86, 1.10]; Fig. 1b, c).
Similar trends were observed in sensitivity analyses using 18 exposure groups consisting of combinations of baseline and change in ferritin with referent group of stable ferritin (Δ ferritin −100 to <100 ng/mL/quarter) and baseline ferritin 200 to <500 ng/mL) (online suppl. Appendix Fig. S3). This is analysis also showed that a major rise in ferritin ≥400 ng/mL/quarter was associated with higher mortality across increasing groups of baseline ferritin.
Effect Modifications by IV Iron Use, ISAT, Demographics, and Nutritional Parameters
The number of patients who never used IV iron during this period was relatively small (3,370 patients, or 3.6% of the cohort). Patients who never used IV iron had slightly lower levels of hemoglobin and iPTH, yet higher levels of serum ferritin and ISAT, compared to those using IV iron. We considered stable ferritin (Δ ferritin −100 to <100 ng/mL/quarter) and baseline ferritin 200 to <500 ng/mL as the referent groups in this analysis. In patients who never used IV iron during the first 6 months of HD, mortality HR for Δ ferritin ≥400 ng/mL/quarter appeared to be relatively similar: 2.55 (95% CI 1.23–5.29), 2.02 (95% CI 1.22–3.35), 1.67 (95% CI 0.74–3.74), and 2.04 (95% CI 1.41–2.97) according to baseline ferritin <200, 200 to <500, 500 to <800, and ≥800 ng/mL, respectively in case-mix + MICS adjusted models ( Fig. 2a). Conversely, in patients who were administered IV iron, the mortality risk associated with a rise in ferritin ≥400 ng/mL/quarter was incrementally higher across higher baseline ferritin strata (HR 1.05 [95% CI 0.98–1.12], HR 1.15 [95% CI 1.08–1.22], HR 1.18 [95% CI 1.07–1.30], and HR 1.42 [95% CI 1.23–1.63] in baseline ferritin <200, 200 to <500, 500 to 800, and ≥800 ng/mL, respectively; p for trend <0.001) in case-mix + MICS models (Fig. 2b). The mortality risk associated with the rise in ferritin ≥400 ng/mL/quarter in patients with higher baseline ferritin ≥ 800 ng/mL appeared to be robust irrespective of IV iron use. To account for the association between the change in IV iron dose and mortality, 18 different combination groups were used as shown in Figure 3. The mortality risk associated with rise in ferritin ≥ 400 ng/mL/quarter appeared independent of IV iron dose change (increase or decrease) over 6 months (Fig. 3a, b).
Fig. 2.
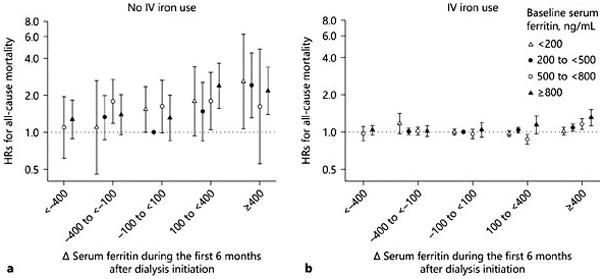
Case-mix and MICS-adjusted HRs (95% CIs) of all-cause mortality across 18 different combinations based on change in serum ferritin and baseline serum ferritin (a ) in the no IV iron use group (n = 3,354) and (b ) in the IV iron use group (n = 90,290). Reference group was stable serum ferritin (−100 to <100 ng/mL/quarter) and baseline serum ferritin 200 to <500 ng/mL.
Fig. 3.
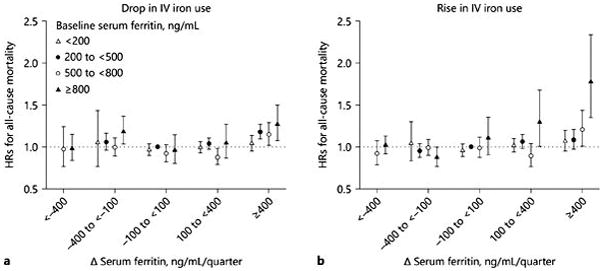
HRs (95% CIs) of all-cause mortality across the 18 different combinations based on change in serum ferritin and baseline serum ferritin with (a ) drop in IV iron use group (n = 50,808) and (b) rise in IV iron use group (n = 36,533) in case-mix and MICS adjusted models. Reference group was stable change in serum ferritin (−100 to <100 ng/mL/quarter) and baseline serum ferritin 200 to <500 ng/mL.
The change in ISAT (Δ ISAT) over the first 6 months after HD initiation tended to increase across increasing Δ ferritin groups (online suppl. Appendix Table S1), and was positively correlated with Δ ferritin in Spearman correlation analysis (rho = 0.41, p < 0.001). The rise in ferritin ≥100 ng/mL/quarter was still associated with higher mortality (reference: change in ferritin −100 to <100 ng/mL/quarter and rise in ISAT), irrespective of the change in ISAT (Table 2).
Table 2.
HRs (95% CIs) of all-cause mortality across different combinations based on change in serum ferritin and ISAT in case-mix + MICS adjusted models. Reference group was no change in serum ferritin (−100 to <100 ng/mL/quarter) and rise in ISAT
| Change in serum ferritin, ng/mL/quarter | Change in ISAT between PQ1 and PQ2 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| drop in ISAT | rise in ISAT | |||||
|
|
|
|||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
| <−400 | 0.99 | 0.91–1.08 | 0.86 | 1.03 | 0.94–1.13 | 0.57 |
| −400 to <−100 | 1.02 | 0.96–1.08 | 0.52 | 1.03 | 0.97–1.09 | 0.35 |
| −100 to <100 | 1.06 | 1.00–1.12 | 0.03 | Ref. | ||
| 100 to <400 | 1.08 | 1.01–1.15 | 0.02 | 1.06 | 1.02–1.10 | 0.01 |
| ≥400 | 1.11 | 1.02–1.22 | 0.02 | 1.22 | 1.17–1.28 | <0.01 |
HR, hazard ratio; ISAT, iron saturation; MICS, malnutrition-inflammation-cachexia syndrome; PQ, patient quarter; ref., reference.
Subgroup analysis showed that higher rise in ferritin during the first 6 months of HD (≥ 400 ng/mL/quarter) was associated with higher all-cause mortality compared to a stable ferritin (Δ ferritin −100 to <100 ng/mL/quarter) across a priori strata of demographics and nutritional parameters (Fig. 4).
Fig. 4.
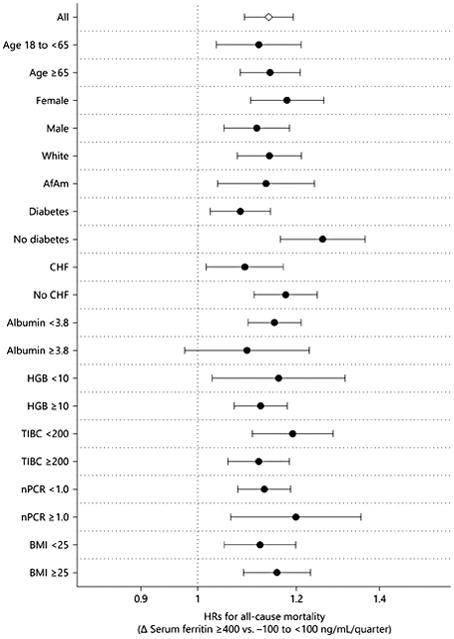
Case-mix and MICS-adjusted HRs (95% CIs) of all-cause mortality associated with increase of change in serum ferritin ≥400 ng/mL/quarter across subgroups of age (<65, ≥65 year-old), sex (female, male), race (white, African American), diabetes, CHF, serum albumin (<3.8, ≥3.8 g/dL), hemoglobin (<10, ≥10 g/dL), TIBC (<200, ≥200 mg/dL), nPCR (<1.0, ≥1.0 g/kg/day), and BMI (<25, ≥25 kg/m 2). AfAm, African American; BMI, body mass index; CHF, congestive heart failure; HGB, hemoglobin; nPCR, normalized protein catabolic rate; TIBC, total iron binding capacity.
Discussion
In a nationally representative contemporary cohort of 93,979 incident HD patients, we investigated the association between the change in ferritin over the first 6 months after HD initiation and mortality. We found that a major rise in ferritin ≥400 ng/mL/quarter was associated with higher mortality in patients with higher baseline ferritin compared to stable ferritin. These associations were still robust independent of the prescribed IV iron dose. Moreover, a rise in ferritin ≥100 ng/mL/quarter regardless of drop or rise in ISAT was associated with all-cause mortality.
In a prior study investigating the association of baseline ferritin with mortality in maintenance HD patients, ferritin <1200 ng/mL was associated with lower mortality risk after considering nutritional status and inflammation [18]. In another observational study, ferritin <800 ng/mL in incident dialysis patients was associated with lower mortality risk after iron supplementation [16]. Recently published guidelines recommended considering iron administration in dialysis patients with ferritin ≤500 ng/mL for safety [4, 20]. In the 6-week extension study of Dialysis Patients’ Response to IV Iron with Elevated Ferritin, a rise in ferritin <1,200 ng/mL by IV iron was beneficial in maintaining hemoglobin levels and keeping ESA use down, but survival outcomes were not evaluated due to the short study period [21]. In our analysis, we found that a major rise in ferritin ≥400 ng/mL/quarter during the first 6-months after HD initiation was significantly associated with higher mortality risk even in patients with baseline ferritin 200 to <500 ng/mL at HD initiation. These findings suggest that a more cautious approach in selecting patients needing IV iron treatment may be appropriate.
Ferritin is not a direct surrogate of administered iron dose [14], but can be used as a valuable marker to reflect the total body iron storage in clinical practice. Elevated ferritin can reflect exogenous iron overload by iron treatment as well as inflammation from conditions including acute or chronic inflammatory disorders, malignant disease, and liver disease [22, 23]. Serum ferritin is widely known as a nonspecific, acute phase reactant, which is affected by inflammatory pathways involving hepcidin upregulation and iron administration directly. Despite an increase of ferritin under inflammatory conditions, iron is sequestered in macrophage due to increased hepcidin [10] and is not available for erythropoiesis. Conversely, ESA therapy downregulates hepcidin synthesis and iron is rapidly mobilized out of the store, thus contributing to the decrease in serum ferritin [24]. Also, among incident dialysis patients, access type via a central venous catheter [25, 26] or malnutrition [6] may also lead to an inflammatory state in these patients. Previous studies have demonstrated higher serum ferritin levels among malnourished dialysis patients, and it is also a marker of morbidity, including infection, and mortality in dialysis patients [27]. In addition, HD treatment is associated with higher inflammatory biomarkers, leading to oxidative stress and inflammation [28–30]. It is difficult to differentiate what causes ferritin to rise in clinical practice because inflammatory status as well as iron treatment might occur simultaneously in dialysis patients. Therefore, higher baseline serum ferritin and a major rise of ferritin with iron or without iron therapy may be observed due to various factors related to the inflammatory and malnutrition processes, which are also associated with higher mortality. Our findings have shown that even in patients without IV iron treatment, a major rise in ferritin was associated with a higher mortality risk compared to stable ferritin even after adjusting for dialysis access type and nutrition and inflammatory covariates. This finding suggests that the association between a steep rise in ferritin and high mortality are robust and independent of markers of malnutrition and inflammation, and IV iron use. However, there may be additional unknown mechanisms that contribute to the rise in serum ferritin, as well as other inflammatory makers not captured by covariates available in our data. Thus, additional studies are needed to further examine mechanistically the causes of a rise in serum ferritin independent of IV iron use, and the relationship of this rise with mortality in dialysis patients.
ISAT, which is the ratio of serum iron and TIBC, is one of the markers used to represent functional iron deficiency or overloading. Previous studies showed the association between low ISAT (less than 20–24%) and higher mortality [31, 32], and between ISAT 35–50% and lower mortality with time-varying analyses in maintenance HD patients [18]. In our incident HD patients, we found that the rise in both ferritin and ISAT over the first 6 months was associated with high mortality as expected; however, we also found that a rise in ferritin with a drop in ISAT was also associated with higher mortality. Therefore, the rise or drop in ISAT over the first 6 months did not show independent associations with mortality across strata of ferritin change. These findings suggest that rise in ferritin may be a more potent predictor of mortality rather than the change in ISAT during the first 6 months of HD initiation. Even though it is unknown as to why the change in ferritin is associated more with mortality than the change in ISAT, it is suggested that ISAT is an indirect marker affected by serum iron and TIBC. Serum iron tends to be overestimated after IV iron administration [18, 33, 34], and TIBC, which is a negative acute phase reactant, is influenced by inflammatory conditions [32].
The strengths of this study included the size and contemporary nature of the cohort, the evaluation of the association between all-cause mortality and the change in ferritin as early as 6 months after maintenance HD initiation, and the adjustment for numerous covariates related to malnutrition and inflammation. However, our study also has several limitations. First, direct inflammatory markers such as C-reactive protein and interleukin-6 were not available in our data. Although we adjusted for several potential inflammatory markers including albumin and TIBC as negative acute phase reactants as well as WBC as a positive acute phase reactant, residual confounding with inflammatory status may exist. This residual confounding may also explain the lack of better survival observed in patients with decreased serum ferritin after adjustment for malnutrition and inflammation markers. Second, a relatively small proportion of patients never received IV iron over 6 months after HD initiation (3.6%). Therefore, we were only able to evaluate the association of change in ferritin with mortality, independent of IV iron administration in this small stratum. However, to protect dialysis patients from potential iron deficiency, clinicians prescribe iron to most incident HD patients. After changes in dialysis reimbursement bundling for IV drugs in 2011, IV iron treatment increased in dialysis patients [14, 35]. Considering the prescription rates and policies for IV iron, 3.6% patients who were included in the no IV iron treatment analysis may not be such a small number.
In conclusion, in a large HD cohort study we found that the rapid rise in ferritin ≥400 ng/mL over the first 6 months upon transition to HD is associated with higher mortality, in particular in patients with higher baseline ferritin. These associations persisted, independent of changes in prescribed dose of IV iron and use of IV iron over the same period. The rise in ferritin ≥400 ng/mL/quarter is also associated with higher mortality regardless of the change in ISAT. Further studies investigating the underlying pathophysiological mechanisms between the relationship of increasing ferritin, administration of IV iron, and mortality may be warranted.
Supplementary Material
Acknowledgments
We thank DaVita Clinical Research® for providing the clinical data for this study. The study was supported by Dr. Kamyar Kalantar-Zadeh’s research grants from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institute of Health (R01-DK95668, K24-DK091419, and R01-DK078106), and philanthropic grants from Mr. Harold Simmons, Mr. Louis Chang, Dr. Joseph Lee, and AVEO. C.P.K. is supported by the NIDDK grants R01-DK096920 and U01-DK102163. C.M.R. is supported by the NIDDK grant K23-DK102903. E.S. is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs (IK2-CX001266-01). Y.O. is supported by the Uehara Memorial Foundation Research Fellowship. T.K. is supported by a research grant from the Inje University.
Footnotes
Disclosure Statement
K.K.-Z. has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra-Zeneca, AVEO, Chugai, DaVita, Fresenius, Genetech, Hay-market Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor, ZS-Pharma, and was the medical director of DaVita Harbor-UCLA/MFI in Long Beach, CA during 2007–2012. C.P.K. has received honoraria from Abbott Nutrition, Sanofi-Aventis, Relypsa, and ZS Pharma. Other authors have not declared any conflicts of interest.
References
- 1.Lipschitz DA, Cook JD, Finch CA. A clinical evaluation of serum ferritin as an index of iron stores. N Engl J Med. 1974;290:1213–1216. doi: 10.1056/NEJM197405302902201. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs A, Miller F, Worwood M, Beamish MR, Wardrop CA. Ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. Br Med J. 1972;4:206–208. doi: 10.1136/bmj.4.5834.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kliger AS, Foley RN, Goldfarb DS, Goldstein SL, Johansen K, Singh A, Szczech L. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for anemia in CKD. Am J Kidney Dis. 2013;62:849–859. doi: 10.1053/j.ajkd.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 5.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Rodriguez RA, Humphreys MH. Association between serum ferritin and measures of inflammation, nutrition and iron in haemodialysis patients. Nephrol Dial Transplant. 2004;19:141–149. doi: 10.1093/ndt/gfg493. [DOI] [PubMed] [Google Scholar]
- 7.Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 8.Ganz T. Systemic iron homeostasis. Physiol Rev. 2013;93:1721–1741. doi: 10.1152/physrev.00008.2013. [DOI] [PubMed] [Google Scholar]
- 9.Coyne DW, Kapoian T, Suki W, Singh AK, Moran JE, Dahl NV, Rizkala AR. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the dialysis patients’ response to IV iron with elevated ferritin (DRIVE) study. J Am Soc Nephrol. 2007;18:975–984. doi: 10.1681/ASN.2006091034. [DOI] [PubMed] [Google Scholar]
- 10.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fishbane S, Pollack S, Feldman HI, Joffe MM. Iron indices in chronic kidney disease in the national health and nutritional examination survey 1988–2004. Clin J Am Soc Nephrol. 2009;4:57–61. doi: 10.2215/CJN.01670408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fishbane S, Mathew AT, Wanchoo R. Intravenous iron exposure and outcomes in patients on hemodialysis. Clin J Am Soc Nephrol. 2014;9:1837–1839. doi: 10.2215/CJN.09510914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charytan DM, Pai AB, Chan CT, Coyne DW, Hung AM, Kovesdy CP, Fishbane S. Considerations and challenges in defining optimal iron utilization in hemodialysis. J Am Soc Nephrol. 2015;26:1238–1247. doi: 10.1681/ASN.2014090922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karaboyas A, Zee J, Morgenstern H, Nolen JG, Hakim R, Kalantar-Zadeh K, Zager P, Pisoni RL, Port FK, Robinson BM. Understanding the recent increase in ferritin levels in United States dialysis patients: potential impact of changes in intravenous iron and erythropoiesis-stimulating agent dosing. Clin J Am Soc Nephrol. 2015;10:1814–1821. doi: 10.2215/CJN.02600315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim T, Rhee CM, Streja E, Obi Y, Brunelli SM, Kovesdy CP, Kalantar-Zadeh K. Longitudinal trends in serum ferritin levels and associated factors in a national incident hemodialysis cohort. Nephrol Dial Transplant. 2017;32:370–377. doi: 10.1093/ndt/gfw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zitt E, Sturm G, Kronenberg F, Neyer U, Knoll F, Lhotta K, Weiss G. Iron supplementation and mortality in incident dialysis patients: an observational study. PLoS One. 2014;9:e114144. doi: 10.1371/journal.pone.0114144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spinowitz BS, Kausz AT, Baptista J, Noble SD, Sothinathan R, Bernardo MV, Brenner L, Pereira BJ. Ferumoxytol for treating iron deficiency anemia in CKD. J Am Soc Nephrol. 2008;19:1599–1605. doi: 10.1681/ASN.2007101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalantar-Zadeh K, Regidor DL, McAllister CJ, Michael B, Warnock DG. Time-dependent associations between iron and mortality in hemodialysis patients. J Am Soc Nephrol. 2005;16:3070–3080. doi: 10.1681/ASN.2005040423. [DOI] [PubMed] [Google Scholar]
- 19.Kuttykrishnan S, Kalantar-Zadeh K, Arah OA, Cheung AK, Brunelli S, Heagerty PJ, Katz R, Molnar MZ, Nissenson A, Ravel V, Streja E, Himmelfarb J, Mehrotra R. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant. 2015;30:1208–1217. doi: 10.1093/ndt/gfv097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razeghi E, Parkhideh S, Ahmadi F, Khashayar P. Serum CRP levels in pre-dialysis patients. Ren Fail. 2008;30:193–198. doi: 10.1080/08860220701810539. [DOI] [PubMed] [Google Scholar]
- 21.Kapoian T. Challenge of effectively using erythropoiesis-stimulating agents and intravenous iron. Am J Kidney Dis. 2008;52(6 suppl):S21–S28. doi: 10.1053/j.ajkd.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Lopez A, Cacoub P, Macdougall IC, Peyrin-Biroulet L. Iron deficiency anaemia. Lancet. 2016;387:907–916. doi: 10.1016/S0140-6736(15)60865-0. [DOI] [PubMed] [Google Scholar]
- 23.Zumbrennen-Bullough K, Babitt JL. The iron cycle in chronic kidney disease (CKD): from genetics and experimental models to CKD patients. Nephrol Dial Transplant. 2014;29:263–273. doi: 10.1093/ndt/gft443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swinkels DW, Wetzels JF. Hepcidin: a new tool in the management of anaemia in patients with chronic kidney disease? Nephrol Dial Transplant. 2008;23:2450–2453. doi: 10.1093/ndt/gfn267. [DOI] [PubMed] [Google Scholar]
- 25.United States Renal Data System. 2015 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. [Google Scholar]
- 26.Banerjee T, Kim SJ, Astor B, Shafi T, Coresh J, Powe NR. Vascular access type, inflammatory markers, and mortality in incident hemodialysis patients: the choices for healthy outcomes in caring for end-stage renal disease (CHOICE) study. Am J Kidney Dis. 2014;64:954–961. doi: 10.1053/j.ajkd.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalantar-Zadeh K, Don BR, Rodriguez RA, Humphreys MH. Serum ferritin is a marker of morbidity and mortality in hemodialysis patients. Am J Kidney Dis. 2001;37:564–572. [PubMed] [Google Scholar]
- 28.Libetta C, Sepe V, Esposito P, Galli F, Dal Canton A. Oxidative stress and inflammation: implications in uremia and hemodialysis. Clin Biochem. 2011;44:1189–1198. doi: 10.1016/j.clinbiochem.2011.06.988. [DOI] [PubMed] [Google Scholar]
- 29.Borazan A, Aydemir S, Sert M, Yilmaz A. The effects of hemodialysis and peritoneal dialysis on serum homocysteine and C-reactive protein levels. Mediators Inflamm. 2004;13:361–364. doi: 10.1155/S0962935104000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danielski M, Ikizler TA, McMonagle E, Kane JC, Pupim L, Morrow J, Himmelfarb J. Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy. Am J Kidney Dis. 2003;42:286–294. doi: 10.1016/s0272-6386(03)00653-x. [DOI] [PubMed] [Google Scholar]
- 31.Koo HM, Kim CH, Doh FM, Lee MJ, Kim EJ, Han JH, Han JS, Oh HJ, Park JT, Han SH, Yoo TH, Kang SW. The relationship of initial transferrin saturation to cardiovascular parameters and outcomes in patients initiating dialysis. PLoS One. 2014;9:e87231. doi: 10.1371/journal.pone.0087231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Liu E, Kopple JD. A low serum iron level is a predictor of poor outcome in hemodialysis patients. Am J Kidney Dis. 2004;43:671–684. doi: 10.1053/j.ajkd.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 33.Kitsati N, Liakos D, Ermeidi E, Mantzaris MD, Vasakos S, Kyratzopoulou E, Eliadis P, Andrikos E, Kokkolou E, Sferopoulos G, Mamalaki A, Siamopoulos K, Galaris D. Rapid elevation of transferrin saturation and serum hepcidin concentration in hemodialysis patients after intravenous iron infusion. Haematologica. 2015;100:e80–e83. doi: 10.3324/haematol.2014.116806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wood JC. Guidelines for quantifying iron overload. Hematology Am Soc Hematol Educ Program. 2014;2014:210–215. doi: 10.1182/asheducation-2014.1.210. [DOI] [PubMed] [Google Scholar]
- 35.Charytan DM, Pai AB, Chan CT, Coyne DW, Hung AM, Kovesdy CP, Fishbane S. Dialysis Advisory Group of the American Society of Nephrology: Considerations and challenges in defining optimal iron utilization in hemodialysis. J Am Soc Nephrol. 2015;26:1238–1247. doi: 10.1681/ASN.2014090922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


