Abstract
Multidrug exporters are a class of membrane proteins that remove antibiotics from the cytoplasm of bacteria and in the process confer multidrug resistance to the organism. This chapter outlines the sample preparation and optimization of oriented solid-state NMR experiments applied to the study of structure and dynamics for the model transporter EmrE from the small multidrug resistance (SMR) family.
1. INTRODUCTION
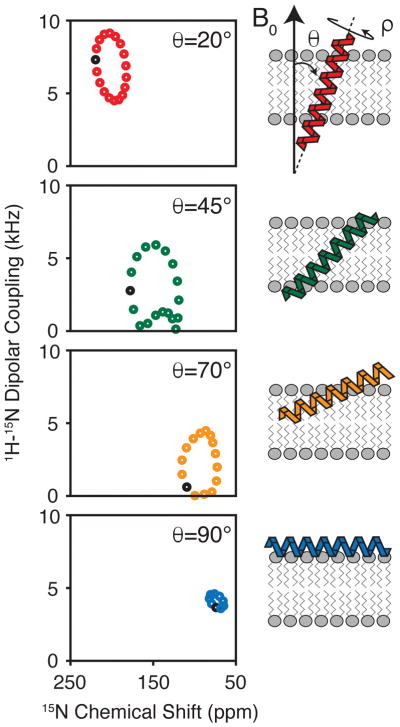
Drug efflux by membrane transport proteins is one of the primary mechanisms bacteria use to confer resistance to antiseptics and antibiotics (1–3). These multidrug transporters bind and efflux lethal compounds from the cytoplasm and in the process reduce the toxicity to the organism. Several transporter structures have been determined using X-ray crystallography which have provided detailed insight into the transport process (reviewed in (4)). A complementary method for resolving the mechanism of ion-coupled transport is through the use of nuclear magnetic resonance (NMR) spectroscopy. One major advantage of this approach is to study efflux pumps under conditions that better mimic the native membrane environment. This chapter will describe how oriented solid-state NMR (O-SSNMR) spectroscopy is used to directly interrogate the structure of the drug transporter EmrE from the small multidrug resistance (SMR) family. The anisotropic observables offered from these experiments (e.g., chemical shifts and dipolar couplings) are used as restraints in structure determination and are excellent reporters of the tilt and rotation angles for transmembrane proteins within the lipid bilayer (Figure 1) (5–11).
Figure 1.
Calculated separated local field spectra of 1H-15N dipolar coupling vs. 15N chemical shift for an ideal helix. The periodic spectral patterns are sensitive to the tilt and rotation angles of the secondary structures with respect to the magnetic field. These patterns have been termed PISA wheels (9,7).
The prerequisite for using O-SSNMR to characterize membrane protein structures is to align the samples within the magnetic field. This is most commonly accomplished using mechanical alignment of lipid bilayers absorbed onto glass plates (12,13) or magnetic alignment with lipid bicelles (14). The former approach has been successfully applied for multiple membrane proteins including M2 from the influenza A virus (15) and the muscle regulatory protein phospholamban (16). For EmrE, we utilize lipid bicelle technology due to the excellent control of sample hydration and improved alignment with respect to the magnetic field (17). Specifically, our experiments use a bicelle composition of DMPC (14:0) and DHPC (6:0) at a 3.2/1 molar ratio, which readily forms aligned liquid crystals at 37 °C (18–21). Replacing the DMPC component with a shorter (12:0) or longer (16:0) chain phosphatidylcholine lipid can be used to lower or raise the alignment temperature, respectively (22–24). In addition, the usage of POPC in combination with DMPC can also reduce the alignment temperature to 25 °C (17). One of the most important requirements of bicelle selection is to ensure protein stability in the chosen lipid composition (20). Finally, since the quality of protein alignment can be protein dependent, the lipid to protein ratio should be optimized in an empirical fashion to ensure bicelle alignment in the magnetic field. For EmrE, we find that a lipid to protein ratio of 150:1 (mol:mol) is effective to achieve good signal to noise without compromising the overall alignment with respect to the magnetic field.
Below we discuss the procedure of bicelle sample preparation containing EmrE and the set-up of the polarization spin exchange at the magic angle (PISEMA) experiment (25,26), which is a sensitive way to probe secondary structural information with respect to the membrane. Using this experiment and others, we have resolved the asymmetric monomer subunits within the EmrE homodimer and quantified conformational exchange dynamics of the transporter that are required for drug efflux (27,28).
2. MATERIALS
2.1 Sample Preparation
-
1
Isotopically 15N enriched protein: modify the expression conditions for your favorite protein with the addition of 15NH4Cl to M9 minimal media to obtain uniformly 15N enriched proteins or the addition of one or several 15N labeled amino acids for selectively enriched proteins.
-
2
n-dodecyl-β-D-maltoside (DDM)
-
3
n-octyl-β-D-glucoside (OG)
-
4
Bio-Beads™ SM-2 resin, Bio-Rad Laboratories
-
5
Ultracentrifuge: instrument should reach centrifugal forces of at least 150,000 x g.
-
6
1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC), Avanti Polar Lipids, Inc.
-
7
1,2-dihexanoyl-sn-glycero-3-phosphocholine (DHPC), Avanti Polar Lipids, Inc.
-
8
Ytterbium(III) chloride (YbCl3)
2.2 NMR Instrumentation and Data Analysis
-
9
Bicelle sample holder assembly (design by Peter Gor’kov at NHMFL, Tallahassee, FL):
5 mm open-ended glass tube, New Era Enterprises, part No. NE-RG5-P-17-OE-BOE.
B5 plugs with and without fill hole, Revolution NMR, part No. MP4763-001 and MP4764-001.
-
10
Solid-state NMR spectrometer: our experiments were carried out using an Agilent DD2 spectrometer operating at a 1H Larmor frequency of 600 MHz.
-
11
Solid-state NMR probes capable of 31P and 15N detection. Our experiments use the following:
BioStatic H-X probe with 5 mm bicelle coil tuned to 31P on the X-channel (Agilent).
Low-E 1H-15N probe with 5 mm bicelle coil for 15N detection (probe design by Peter Gor’kov) (29). This probe technology is commercially available from Revolution NMR, LLC (www.revolutionnmr.com).
-
12
NMR data processing software: NMRPipe (30) and Sparky v3.113 (T.D. Goddard and D. G. Kneller, SPARKY 3, University of California, San Francisco).
3. METHODS
3.1 Sample Preparation
EmrE is expressed from a pMAL™ vector (New England Biolabs) where maltose binding protein (MBP) is positioned on the N-terminal side of the EmrE gene. The expression is performed in E. coli BL21(DE3) cells in minimal media using selectively labeled 15N amino acids or 15NH4Cl to uniformly enrich the protein. EmrE is purified with an amylose affinity column, cleaved with TEV protease to remove MBP, and passed over a size exclusion column as previously described (27,28). Purified EmrE in DDM detergent micelles is then reconstituted into DMPC lipid bilayers. Below is the reconstitution procedure:
28 mg DMPC is hydrated with 1 mL of 20 mM sodium phosphate and 20 mM NaCl and subjected to multiple cycles of flash freezing.
Bath sonicate the sample for 15 min.
Add 5.6 mg OG to the mixture and equilibrate for 10 min.
Add 7 mL EmrE at a concentration of 0.5 mg/mL in DDM detergent to the sonicated lipids. Additional DDM may be added to ensure the lipids are solubilized, which will be evident when the mixture becomes clear. Typically, a total of ~30 mg DDM is added including the detergent with EmrE.
Incubate the sample for 1 hour at room temperature.
Add 2.25 grams of Bio-Beads to give a final ratio of 75 mg per mg of detergent.
Gently stir for 12 hours at 4 °C. Note that the removal of detergent by the Bio-Beads will instigate formation of proteoliposomes and lead to a slightly cloudy solution.
Remove Bio-Beads from the suspension.
Centrifuge proteoliposomes in a Beckman Optima MAX_XP TLA 110 rotor at 100,000 x g for 1.5 hrs.
Resuspend the liposome pellets in 100 mM HEPES and 20 mM NaCl (pH 7) buffer and freeze thaw 5–10 times to exchange the buffer.
Centrifuge the sample in the TLA 110 rotor for 2.5 hrs at 150,000 x g.
Add 25 μL of 425 mM DHPC in water to the proteoliposome pellet. This corresponds to a DMPC:DHPC molar ratio (“q value”) of ~3.2–3.5 (see Note 1).
Mix thoroughly by a combination of vortexing, flash freezing with liquid nitrogen, and heating to 37 °C. After the mixing is complete, the sample should be fluid on ice and a viscous gel at 37 °C (see Note 2).
Centrifuge the sample in a 1.5 mL microcentrifuge tube for 2 min at 1,500 x g using a benchtop centrifuge and place the supernatant in a new microcentrifuge tube.
For unflipped bicelle samples (bicelle normal perpendicular to magnetic field), proceed to step 15. For flipped samples (bicelle normal parallel to magnetic field), add 6 μL of 100 mM YbCl3 to give a final concentration of 4.5 mM (see Note 3).
Place the sample in the bicelle sample holder for O-SSNMR experiments.
3.2 Check Bicelle Alignment with 31P NMR
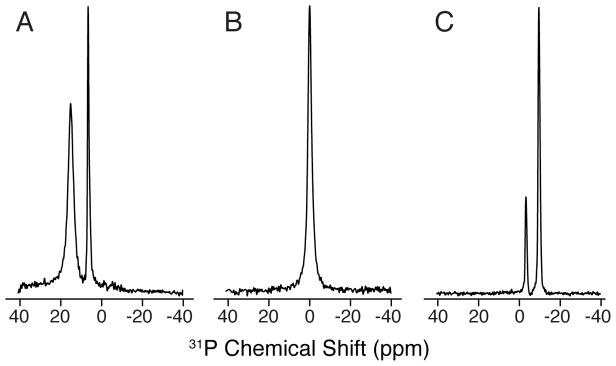
The alignment of the bicelle in the magnetic field is checked by recording a one-pulse 31P experiment optimized for a 90° tilt angle followed by acquisition of the free induction decay under 1H decoupling with SPINAL-64 (31) This experiment is a sensitive way to check the sample since 31P is 100% natural abundant, has a relatively high gyromagnetic ratio, and is present in both DMPC and DHPC headgroups. Representative 31P spectra of flipped, isotropic and unflipped bicelles are shown in Figure 2A, B, C, respectively (see Note 4).
Figure 2.
31P spectra of lipid bicelle samples. The panels show representative spectra for (A) flipped, (B) isotropic, and (C) unflipped bicelles. The flipped spectra were obtained by the addition of YbCl3.
3.3 Optimization of the polarization inversion spin exchange at the magic angle (PISEMA) experiment
PISEMA is a separated local field experiment to correlate dipolar couplings with anisotropic chemical shifts (25,26). The spectral patterns from PISEMA are directly sensitive to the tilt and rotation angles of the protein secondary structures with respect to the magnetic field (7,9). PISEMA begins with a cross-polarization (CP) sequence to transfer magnetization from 1H to 15N spins in order to increase the sensitivity of the experiment. Homonuclear dipolar decoupling in the indirect dimension is achieved by frequency-switched or phase-modulated Lee-Goldburg (FSLG or PMLG) (32–35). Both of these sequences enable evolution of a scaled heteronuclear dipolar coupling in the absence of 15N chemical shift evolution. After evolving the dipolar coupling in the indirect dimension, the PISEMA experiment detects the free precession of the X nucleus under high-power 1H decoupling. The most common application of PISEMA is to enrich the protein with 15N labeling and correlate the 1H-15N dipolar coupling with the 15N anisotropic chemical shift (see Note 5). Advancements in the original PISEMA experiment include developments to reduce the 1H offset dependence of the indirect dimension (36), a sensitivity-enhanced version to improve signal-to-noise (37,38) and a constant-time experiment to improve the resolution in the dipolar coupling dimension (39).
Prior to data acquisition on the protein sample, it is recommended to first optimize the pulse sequence parameters with two model compounds: (1) an aqueous buffer composed of 100 mM HEPES and 20 mM NaCl (pH 7.0) and (2) a single crystal of 15N-labeled N-acetyl-leucine (NAL).
3.3.1 Insert buffer sample to calibrate 1H 90° pulse parameters
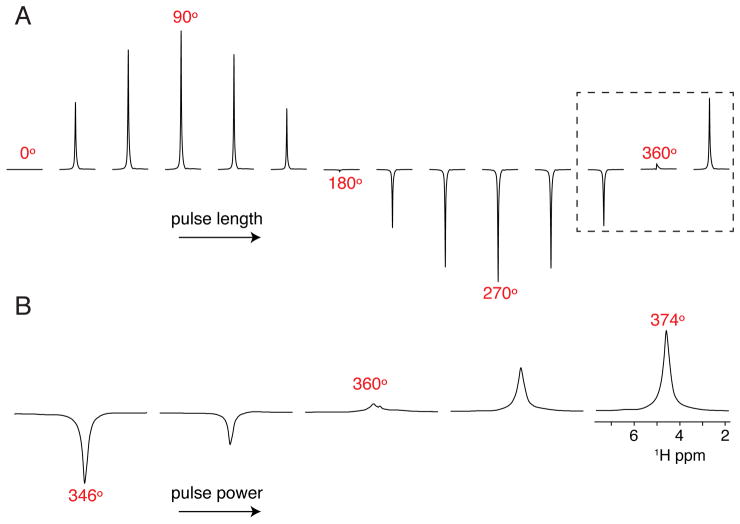
If the probe requires pulse length calibration for the first time, it is necessary to optimize 1H 90° pulses for a given pulse duration that is within the voltage handling of the probe circuit. This is typically specified by a minimum 90° pulse width that can be applied without damage to the probe. We typically optimize for 5 and 6 μsec pulse lengths on the water signal of a sample of 100 mM HEPES and 20 mM NaCl (pH 7.0) by carrying out a full nutation curve where the pulse length is varied at a constant power (Figure 3A). Next, the pulse power is more finely calibrated to the desired 360° pulse (e.g., 20 or 24 μsec, respectively) by adjusting the power level to identify the corresponding null in the spectrum (see Note 6). An example of the calibration is shown in Figure 3B.
Figure 3.
Optimization of the 1H 90° pulse on the water peak. (A) Nutation curve by varying the pulse length at a fixed B1 amplitude to determine an accurate 360° pulse. (B) Fine tune calibration of the 360° pulse by setting the pulse length to four times the desired 90° pulse and increasing the power level from left to right. The null value corresponds to the 360° pulse.
3.3.2 Insert the N-acetyl leucine single crystal
Optimize cross-polarization (CP) from 1H to 15N. There are a few variants of this sequence that provide efficient polarization transfers, including linear and adiabatic ramps as well as CP-MOIST (40,41) (see Note 7).
Optimize the 90° pulse for 15N. The experiment employs a 90° 15N pulse after the CP from 1H to 15N. The signal on 15N is detected under 1H decoupling. The flip back pulse is set to the desired length with the power adjusted in an iterative manner. The null in the spectrum gives the optimal power corresponding to the pulse length.
- Calculate the parameters for the indirect dimension of the PISEMA experiment. For the SEMA portion of PISEMA (i.e., t1 period), the effective frequency on 15N must match the effective frequency of 1H (i.e., ωeff,15N = ωeff,1H). The 15N spin-lock during t1 evolution is on resonance so the effective frequency is equal to applied frequency (ω1,15N = ωeff,15N) (see Note 8). Unlike the 15N effective frequency, the 1H effective frequency during FSLG or PMLG is not equal to the applied frequency. For this reason, the frequency offset (Δω) and applied frequency (ω1) need to be set so that the effective frequency of 1H will match that of 15N (ωeff,15N = ωeff,1H). A second requirement of PMLG or FSLG is that the effective frequency on 1H is applied at the magic angle in order to average out homonuclear dipolar couplings. Using the trigonometric relationships given in Eqns. 1 and 2 and the desired effective frequencies for 15N and 1H, Δω and ω1 can be calculated for 1H (see Note 9).
(1) (2) Collect the 2D PISEMA experiment. The PISEMA experiment is collected using the optimized pulse sequence parameters and Fourier transformed to give the resulting two-dimensional spectrum (see Note 10).
Adjust 1H offset to minimize the zero-frequency. Since the dipolar coupling frequency measured in the indirect dimension is sensitive to the 1H offset, one can adjust the transmitter frequency for 1H to minimize the zero-frequency signals for desired peaks in the single crystal sample. The optimal 1H offset may be different for the protein sample.
3.3.3 Acquire PISEMA spectrum on membrane protein sample
Optimize the 1H 90° pulse. Similar to the pulse calibration on the buffer sample, we optimize for 5 and 6 μsec 90° pulse lengths on the water signal in the protein/bicelle sample. As described above and shown in Figure 3B, the pulse length is set to 4 times the 90° pulse with the power levels adjusted to find the null.
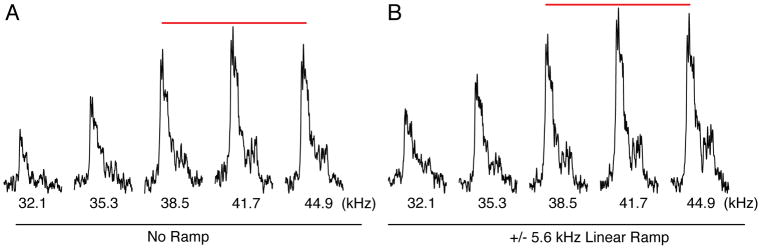
Optimize the CP condition for protein. In order to find optimal matching conditions for CP, it is recommended to have a uniformly 15N labeled sample that gives at least 10:1 signal-to-noise in ~256–512 scans. Similar to that described for the single crystal, the CP values can be optimized directly on the sample by adjusting the 1H power level (Figure 4) (see Note 11).
Optimize the length of the CP contact time. In addition to the power levels, the contact time of the CP should be iteratively adjusted to maximize signal-to-noise. EmrE in aligned bicelles shows an optimal value of ~0.75 msec.
1H offset and recycle delay. The 1H offset must be set correctly in order to reduce the zero-frequency peaks. For transmembrane helical proteins in flipped bicelles, a 1H offset in the range of 4.7 to 7 ppm will minimize the zero-frequency signals (37).
Find optimal recycle delay. The recycle delay should be set to 3–5 times the 1H T1, which can be measured using an inversion recovery experiment on the protein. For EmrE, we normally use a delay of 3 or 4 sec.
Acquisition of the PISEMA experiment. Using the calibrated 15N pulse powers from the single crystal and the 1H pulse power from the protein sample, one can calculate the applied frequency for 1H during FSLG or PMLG to match the desired effective frequency. Since the maximum 1H-15N dipolar couplings are ~10 kHz, we typically employ a t1 dwell time of 48 μsec for protein samples in flipped bicelles, which corresponds to an effective frequency ωeff/2π = 41.7 kHz. This spectral width is sufficient to cover the entire breadth of dipolar couplings in the indirect dimension. An example PISEMA spectrum for selectively labeled EmrE with 15N methionine is shown in Figure 5.
Assignment of PISEMA spectra. The acquired spectrum needs to be assigned in order to obtain site-resolved structural information. There are several viable approaches including the usage of site-directed mutagenesis (27), selectively labeled samples in combination with periodic wheel approximations (7,9,42,43), and spectroscopic methods that make use of magnetization transfers (44,45). Once the assignments are obtained, these can be used as restraints in structural refinement calculations.
Figure 4.
Optimization of 1H to 15N cross-polarization as demonstrated for EmrE in lipid bicelles. (A) The 1H power is arrayed around a constant 15N amplitude used for cross-polarization. As seen from the spectra, a linear ramp of 41.7 ± 5.6 kHz gave the highest signal/noise for all experiments. These data show that a linear ramp improves the signal/noise and emphasizes the importance of optimizing cross-polarization.
Figure 5.
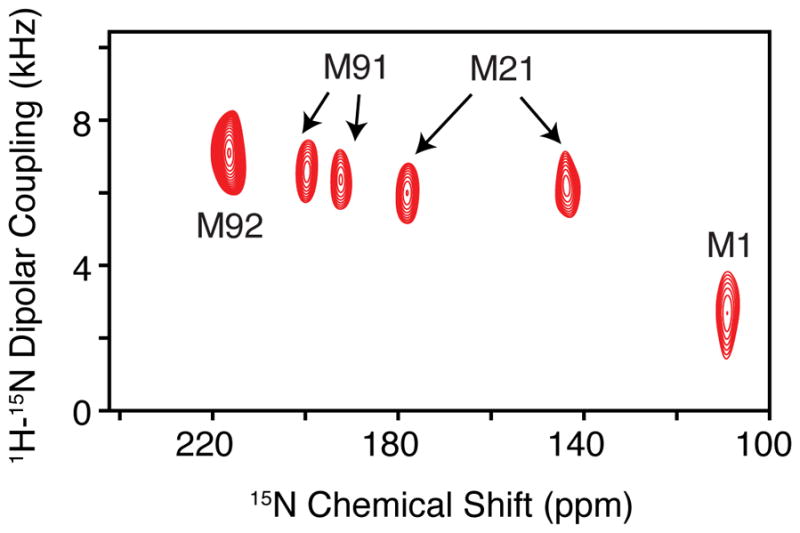
PISEMA of 15N methionine labeled EmrE in DMPC/DHPC aligned bicelles. Note that M21 and M91 show clear doubling in the spectrum indicative of an asymmetric dimer. (Reproduced with permission from Ref. (27). © WILEY-VCH Verlag GmbH & Co. KgaA, Weinheim, 2013).
We have successfully employed PISEMA to show that both monomers in the functional EmrE dimer give a separate set of peaks (see Figure 5). These results validated the asymmetric dimer structural models available from X-ray crystallography and cryoelectron microscopy (46,47,27). Furthermore, we recently applied the pure exchange (PUREX) method (48) to investigate the conformational dynamics of EmrE in aligned bicelles that can be applied to other membrane protein systems (28). Notably, we discovered that the change in protonation state of a conserved glutamate residue alters the conformational exchange rate of EmrE that plays a direct role in drug efflux (49).
Acknowledgments
This work was supported by NIH grant R01AI108889. M.L. acknowledges support from a Margaret-Strauss Kramer Fellowship.
Footnotes
The ability of a sample to align is very dependent on the molar ratio of DMPC:DHPC (“q-value”). If the q-value is significantly below 3.2 the bicelle will be isotropic.
The final volume of the sample should be 125 μL to ensure that the total lipid concentration is at least 25% (w/v). If the lipid concentration is too low the bicelles will not align.
The sample will readily align at 37 °C with the bilayer normal perpendicular with respect to the direction of the magnetic field (i.e., unflipped bicelles). Some proteins that undergo rapid uniaxial rotational diffusion can be characterized under these conditions (50). However, our experiments show that EmrE’s rotational diffusion is not sufficiently rapid to average out the residual anisotropy (51). For this reason, the bicelle normal is “flipped” by 90° using YbCl3, which orients the bilayer normal parallel to the magnetic field. These paramagnetic ions bind to the phosphate within the lipid headgroup and change the magnetic susceptibility of the bicelle (19,21).
It is important to note that these spectra do not guarantee that the protein will be properly aligned in the magnetic field. However, when the bicelle containing protein is unaligned or partially aligned, our experience is that the protein’s 15N spectrum will give a powder pattern or be of low quality.
The solid-state NMR probe for PISEMA spectroscopy needs to include a sensitive detection coil optimized for 15N detection. The probe designed by Gor’kov et al. (29) is the most sensitive available and uses a loop-gap resonator for the 1H channel that minimizes sample heating and uses a 15N solenoid for detection. The inner solenoid coil is positioned closest to the sample in order to maximize the filling factor and sensitivity for 15N detection.
It is important to acquire a full nutation curve when first optimizing power levels to ensure accurate measurement of the 360° pulse.
The optimal match condition for the single crystal is typically not the best for the protein sample; however, it should be optimized to ensure sufficient signal-to-noise for calibration of the 15N pulse and PISEMA experiment. The contact time can also be adjusted iteratively, but typically ~2 msec will give the highest signal-to-noise.
Note that the applied nutation frequency can be calculated by ω1/2π = 250 / t90, where t90 is equal to the length of the 90° pulse in μsec and ω1/2π is given in kHz. For example, a 6 μsec pulse corresponds to a frequency of 41.7 kHz.
We commonly employ an effective 1H frequency of ωeff/2π = 41.7 kHz, an offset Δω/2π = 24.1 kHz, and an applied frequency of ω1/2π = 34.0 kHz. Unlike most multidimensional NMR experiments, the dwell time during the t1 evolution period cannot be set independently from the FSLG or PMLG parameters. Specifically, the minimum t1 dwell time is the application of two 360° pulses. This works out to give a minimum dwell time equal to 8×250/(ωeff/2π). For example, with an effective frequency of ωeff/2π = 41.7 kHz, the dwell time is 48 μsec.
The indirect 1H-15N dipolar coupling is scaled due to the Lee-Goldburg sequence and needs to be adjusted by the scaling factor (0.82) to give the correct couplings (26). For example, a dwell time of 48 μsec during FSLG or PMLG gives a corrected spectral width of 25.4 kHz.
For selectively labeled samples, the signal-to-noise often prevents detailed optimization of pulse parameters. However, since the buffer composition is the same as that of the uniformly 15N labeled sample, the optimal values typically do not vary significantly.
References
- 1.Nikaido H. Multidrug resistance in bacteria. Annu Rev Biochem. 2009;78:119–146. doi: 10.1146/annurev.biochem.78.082907.145923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikaido H, Pages JM. Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev. 2012;36(2):340–363. doi: 10.1111/j.1574-6976.2011.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen HKD, Justin; Wan, Huimi Helena, Cloud-Hansen Karen A, Davies Julian, Handelsman Jo. Call of the wild: antibiotic resistance genes in natural environments. Nature Reviews Microbiology. 2010;(4):251–259. doi: 10.1038/nrmicro2312. [DOI] [PubMed] [Google Scholar]
- 4.Du D, van Veen HW, Murakami S, et al. Structure, mechanism and cooperation of bacterial multidrug transporters. Curr Opin Struct Biol. 2015;33:76–91. doi: 10.1016/j.sbi.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 5.Traaseth NJ, Shi L, Verardi R, et al. Structure and topology of monomeric phospholamban in lipid membranes determined by a hybrid solution and solid-state NMR approach. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(25):10165–10170. doi: 10.1073/pnas.0904290106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vostrikov Vitaly V, Grant Christopher V, Opella Stanley J, et al. On the Combined Analysis of (2)H and (15)N/(1)H Solid-State NMR Data for Determination of Transmembrane Peptide Orientation and Dynamics. Biophysical Journal. 2011;101(12):2939–2947. doi: 10.1016/j.bpj.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marassi FM, Opella SJ. A Solid-State NMR Index of Helical Membrane Protein Structure and Topology. Journal of Magnetic Resonance. 2000;144(1):150–155. doi: 10.1006/jmre.2000.2035. doi: http://dx.doi.org/10.1006/jmre.2000.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cross TA. A Solid State Nuclear Magnetic Resonance Approach for Determining the Structure of Gramicidin a without Model Fitting. Biophysical Journal. 1986;49(1):124–126. doi: 10.1016/S0006-3495(86)83620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Denny J, Tian C, et al. Imaging Membrane Protein Helical Wheels. Journal of Magnetic Resonance. 2000;144(1):162–167. doi: 10.1006/jmre.2000.2037. doi: http://dx.doi.org/10.1006/jmre.2000.2037. [DOI] [PubMed] [Google Scholar]
- 10.Buffy JJ, Traaseth NJ, Mascioni A, et al. Two-Dimensional Solid-State NMR Reveals Two Topologies of Sarcolipin in Oriented Lipid Bilayers†. Biochemistry. 2006;45(36):10939–10946. doi: 10.1021/bi060728d. [DOI] [PubMed] [Google Scholar]
- 11.Opella SJ, Marassi FM. Structure Determination of Membrane Proteins by NMR Spectroscopy. Chemical Reviews. 2004;104(8):3587–3606. doi: 10.1021/cr0304121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark NA, Rothschild KJ, Luippold DA, et al. Surface-induced lamellar orientation of multilayer membrane arrays. Theoretical analysis and a new method with application to purple membrane fragments. Biophysical Journal. 1980;31(1):65–96. doi: 10.1016/S0006-3495(80)85041-7. doi: http://dx.doi.org/10.1016/S0006-3495(80)85041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moll F, 3rd, Cross TA. Optimizing and characterizing alignment of oriented lipid bilayers containing gramicidin D. Biophysical Journal. 1990;57(2):351–362. doi: 10.1016/S0006-3495(90)82536-4. doi: http://dx.doi.org/10.1016/S0006-3495(90)82536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanders CR, II, Hare BJ, Howard KP, et al. Magnetically-oriented phospholipid micelles as a tool for the study of membrane-associated molecules. Progress in Nuclear Magnetic Resonance Spectroscopy. 1994;26(Part 5):421–444. doi: http://dx.doi.org/10.1016/0079-6565(94)80012-X. [Google Scholar]
- 15.Song Z, Kovacs FA, Wang J, et al. Transmembrane Domain of M2 Protein from Influenza A Virus Studied by Solid-State 15N Polarization Inversion Spin Exchange at Magic Angle NMR. Biophysical Journal. 2000;79(2):767–775. doi: 10.1016/S0006-3495(00)76334-X. doi: http://dx.doi.org/10.1016/S0006-3495(00)76334-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traaseth NJ, Verardi R, Torgersen KD, et al. Spectroscopic validation of the pentameric structure of phospholamban. Proceedings of the National Academy of Sciences. 2007;104(37):14676–14681. doi: 10.1073/pnas.0701016104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Angelis AA, Opella SJ. Bicelle samples for solid-state NMR of membrane proteins. Nat Protocols. 2007;2(10):2332–2338. doi: 10.1038/nprot.2007.329. [DOI] [PubMed] [Google Scholar]
- 18.Sanders CR, Schwonek JP. Characterization of magnetically orientable bilayers in mixtures of dihexanoylphosphatidylcholine and dimyristoylphosphatidylcholine by solid-state NMR. Biochemistry. 1992;31(37):8898–8905. doi: 10.1021/bi00152a029. [DOI] [PubMed] [Google Scholar]
- 19.Marcotte I, Auger M. Bicelles as model membranes for solid- and solution-state NMR studies of membrane peptides and proteins. Concepts in Magnetic Resonance Part A. 2005;24A(1):17–37. doi: 10.1002/cmr.a.20025. [DOI] [Google Scholar]
- 20.Warschawski DE, Arnold AA, Beaugrand M, et al. Choosing membrane mimetics for NMR structural studies of transmembrane proteins. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2011;1808(8):1957–1974. doi: 10.1016/j.bbamem.2011.03.016. doi: http://dx.doi.org/10.1016/j.bbamem.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Prosser RS, Hwang JS, Vold RR. Magnetically Aligned Phospholipid Bilayers with Positive Ordering: A New Model Membrane System. Biophysical Journal. 1998;74(5):2405–2418. doi: 10.1016/S0006-3495(98)77949-4. doi: http://dx.doi.org/10.1016/S0006-3495(98)77949-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto K, Pearcy P, Ramamoorthy A. Bicelles Exhibiting Magnetic Alignment for a Broader Range of Temperatures: A Solid-State NMR Study. Langmuir. 2014;30(6):1622–1629. doi: 10.1021/la404331t. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto K, Pearcy P, Lee DK, et al. Temperature-Resistant Bicelles for Structural Studies by Solid-State NMR Spectroscopy. Langmuir. 2015;31(4):1496–1504. doi: 10.1021/la5043876. [DOI] [PubMed] [Google Scholar]
- 24.Triba MN, Devaux PF, Warschawski DE. Effects of Lipid Chain Length and Unsaturation on Bicelles Stability. A Phosphorus NMR Study. Biophysical Journal. 91(4):1357–1367. doi: 10.1529/biophysj.106.085118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C, Ramamoorthy A, Opella S. High-resolution heteronuclear dipolar solid-state NMR spectroscopy. Journal of Magnetic Resonance. 1994;109A:270–272. [Google Scholar]
- 26.Ramamoorthy A, Wei Y, Lee D-K. Annual Reports on NMR Spectroscopy. Vol. 52. Academic Press; 2004. PISEMA Solid-State NMR Spectroscopy; pp. 1–52. doi: http://dx.doi.org/10.1016/S0066-4103(04)52001-X. [Google Scholar]
- 27.Gayen A, Banigan JR, Traaseth NJ. Ligand-Induced Conformational Changes of the Multidrug Resistance Transporter EmrE Probed by Oriented Solid-State NMR Spectroscopy. Angewandte Chemie International Edition. 2013;52(39):10321–10324. doi: 10.1002/anie.201303091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cho MK, Gayen A, Banigan JR, et al. Intrinsic Conformational Plasticity of Native EmrE Provides a Pathway for Multidrug Resistance. Journal of the American Chemical Society. 2014;136(22):8072–8080. doi: 10.1021/ja503145x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gor’kov PL, Chekmenev EY, Li C, et al. Using low-E resonators to reduce RF heating in biological samples for static solid-state NMR up to 900 MHz. J Magn Reson. 2007;185(1):77–93. doi: 10.1016/j.jmr.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Delaglio F, Grzesiek S, Vuister GW, et al. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. Journal of Biomolecular NMR. 6(3):277–293. doi: 10.1007/bf00197809. [DOI] [PubMed] [Google Scholar]
- 31.Fung BM, Khitrin AK, Ermolaev K. An Improved Broadband Decoupling Sequence for Liquid Crystals and Solids. Journal of Magnetic Resonance. 2000;142(1):97–101. doi: 10.1006/jmre.1999.1896. doi: http://dx.doi.org/10.1006/jmre.1999.1896. [DOI] [PubMed] [Google Scholar]
- 32.Bielecki A, K aC, De Groot HJM, Griffin RG, Levitt MH. Frequency-switched Lee-Goldburg sequence in solids. Advances in Magnetic Resonance. 1990;14:111–150. [Google Scholar]
- 33.Lee MWG. Nuclear magnetic resonance line narrowing by a rotation RF field. Phys Rev. 1965;140:1261–1271. [Google Scholar]
- 34.Vinogradov E, Madhu PK, Vega S. High-resolution proton solid-state NMR spectroscopy by phase-modulated Lee–Goldburg experiment. Chemical Physics Letters. 1999;314(5–6):443–450. doi: http://dx.doi.org/10.1016/S0009-2614(99)01174-4. [Google Scholar]
- 35.Fu R, Tian C, Cross TA. NMR Spin Locking of Proton Magnetization under a Frequency-Switched Lee–Goldburg Pulse Sequence. Journal of Magnetic Resonance. 2002;154(1):130–135. doi: 10.1006/jmre.2001.2468. doi: http://dx.doi.org/10.1006/jmre.2001.2468. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto K, Lee DK, Ramamoorthy A. Broadband-PISEMA solid-state NMR spectroscopy. Chemical Physics Letters. 2005;407(4–6):289–293. doi: 10.1016/j.cplett.2005.04.017. doi: http://dx.doi.org/10.1016/j.cplett.2005.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gopinath T, Traaseth NJ, Mote K, et al. Sensitivity Enhanced Heteronuclear Correlation Spectroscopy in Multidimensional Solid-State NMR of Oriented Systems via Chemical Shift Coherences. Journal of the American Chemical Society. 2010;132(15):5357–5363. doi: 10.1021/ja905991s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veglia TGaG. Sensitivity Enhancement in Static Solid-State NMR Experiments via Single- and Multiple-Quantum Dipolar Coherences. Journal of American Chemical Society. 2009;131(16):5754–5756. doi: 10.1021/ja900096d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gopinath T, Veglia G. Improved Resolution in Dipolar NMR Spectra Using Constant Time Evolution PISEMA Experiment. Chem Phys Lett. 2010;494(1–3):104–110. doi: 10.1016/j.cplett.2010.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koroloff SN, Nevzorov AA. Optimization of cross-polarization at low radiofrequency fields for sensitivity enhancement in solid-state NMR of membrane proteins reconstituted in magnetically aligned bicelles. Journal of Magnetic Resonance. 2015;256:14–22. doi: 10.1016/j.jmr.2015.03.016. doi: http://dx.doi.org/10.1016/j.jmr.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 41.Tang W, Nevzorov AA. Repetitive cross-polarization contacts via equilibration-re-equilibration of the proton bath: Sensitivity enhancement for NMR of membrane proteins reconstituted in magnetically aligned bicelles. Journal of Magnetic Resonance. 2011;212(1):245–248. doi: 10.1016/j.jmr.2011.06.028. doi: http://dx.doi.org/10.1016/j.jmr.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 42.Bertram R, Quine JR, Chapman MS, et al. Atomic Refinement Using Orientational Restraints from Solid-State NMR. Journal of Magnetic Resonance. 2000;147(1):9–16. doi: 10.1006/jmre.2000.2193. doi: http://dx.doi.org/10.1006/jmre.2000.2193. [DOI] [PubMed] [Google Scholar]
- 43.De Angelis AA, Howell SC, Nevzorov AA, et al. Structure Determination of a Membrane Protein with Two Trans-membrane Helices in Aligned Phospholipid Bicelles by Solid-State NMR Spectroscopy. Journal of the American Chemical Society. 2006;128(37):12256–12267. doi: 10.1021/ja063640w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Traaseth NJ, Gopinath T, Veglia G. On the performance of Spin Diffusion NMR Techniques in Oriented Solids: Prospects for Resonance Assignments and Distance Measurements from Separated Local Field Experiments. The journal of physical chemistry B. 2010;114(43):13872–13880. doi: 10.1021/jp105718r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nevzorov AA. Mismatched Hartmann–Hahn Conditions Cause Proton-Mediated Intermolecular Magnetization Transfer between Dilute Low-Spin Nuclei in NMR of Static Solids. Journal of the American Chemical Society. 2008;130(34):11282–11283. doi: 10.1021/ja804326b. [DOI] [PubMed] [Google Scholar]
- 46.Fleishman SJ, Harrington SE, Enosh A, et al. Quasi-symmetry in the cryo-EM structure of EmrE provides the key to modeling its transmembrane domain. J Mol Biol. 2006;364(1):54–67. doi: 10.1016/j.jmb.2006.08.072. [DOI] [PubMed] [Google Scholar]
- 47.Chen YJ, Pornillos O, Lieu S, et al. X-ray structure of EmrE supports dual topology model. Proceedings of the National Academy of Sciences. 2007;104(48):18999–19004. doi: 10.1073/pnas.0709387104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.deAzevedo ER, Bonagamba TJ, Schmidt-Rohr K. Pure-Exchange Solid-State NMR. Journal of Magnetic Resonance. 2000;142(1):86–96. doi: 10.1006/jmre.1999.1918. doi: http://dx.doi.org/10.1006/jmre.1999.1918. [DOI] [PubMed] [Google Scholar]
- 49.Gayen A, Leninger M, Traaseth NJ. Protonation of a glutamate residue modulates the dynamics of the drug transporter EmrE. Nat Chem Biol. 2016;12(3):141–145. doi: 10.1038/nchembio.1999. http://www.nature.com/nchembio/journal/v12/n3/abs/nchembio.1999.html - supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu GJ, Opella SJ. Resonance Assignments of a Membrane Protein in Phospholipid Bilayers by Combining Multiple Strategies of Oriented Sample Solid-State NMR. Journal of biomolecular NMR. 2014;58(1):69–81. doi: 10.1007/s10858-013-9806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Banigan JR, Gayen A, Traaseth NJ. Correlating lipid bilayer fluidity with sensitivity and resolution of polytopic membrane protein spectra by solid-state NMR spectroscopy. Biochimica et Biophysica Acta (BBA) - Biomembranes. 2015;1848(1 Part B):334–341. doi: 10.1016/j.bbamem.2014.05.003. doi: http://dx.doi.org/10.1016/j.bbamem.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]