Abstract
Research suggests that altered emotion processing may be one important pathway linking social risk factors and depressive symptoms. We examined the extent to which neural response to negatively valenced social information might help to account for the relationship between social risk and depressive symptoms in youth. Forty-nine youth were scanned while identifying the emotional valence of words that connoted social status. They also completed questionnaires assessing self-reported social risk factors and depressive symptoms. Mediation analysis revealed that reduced dorsolateral prefrontal cortex activity in response to negative social status words explained the positive association between social risk and depressive symptoms. These findings suggest that social risk factors present during adolescence may contribute to depressive symptoms by influencing the neural substrates of emotion processing.
The transition into and across adolescence is characterized by significant changes in social structure and relationships as youth spend more time with peers, increasingly value peer affiliation, become more concerned with social status and position, and encounter greater peer stressors (Dijkstra, Cillessen, & Borch, 2013; Hankin, Mermelstein, & Roesch, 2007; Lam, McHale, & Crouter, 2012; Rubin, Bukowski, & Parker, 2006). These social changes coincide with increasing rates of depression. Depression rates begin to increase in early adolescence, and continue to rise throughout adolescence (Costello, Mustillo, Erkanli, Keeler, & Angold, 2003; Hankin et al., 2015). Interpersonal theories of depression emphasize the role of social risk factors in the development and maintenance of depression (Joiner & Coyne, 1999; Rudolph, Flynn, & Abaied, 2008). Specifically, these theories posit that behaviors and characteristics of depressed and depression-prone individuals increase the likelihood of experiencing social risk factors (Rudolph et al., 2008). Social risk, in turn, is thought to contribute to increases in depressive symptoms (Rudolph et al., 2008).
Social risk factors may include exposure to negative social experiences or social-cognitive vulnerabilities. This study focuses on two social risk factors that are developmentally salient in adolescence: peer victimization, a type of social experience strongly associated with increases in depressive symptoms (Hawker & Boulton, 2000; Sweeting, Young, West, & Der, 2006), and fear of negative evaluation, a social-cognitive vulnerability linked to internalizing problems, including anxiety and depression (Lipton, Weeks, & De Los Reyes, 2016; Nonterah et al., 2015; O’Connor, Berry, Weiss, & Gilbert, 2002; Weeks et al., 2005). The construct of fear of negative evaluation plays a key role in motivating individuals to expect and perceive criticism from others, and is similar to other depressogenic social-cognitive styles characterized by sensitivity to social feedback and negatively biased interpretations of social cues (e.g., rejection sensitivity, critical self-referential biases; Liu, Kraines, Massing-Schaffer, & Alloy, 2014; Prinstein, Cheah, & Guyer, 2005). Thus, both peer victimization and sensitivity to rejection/negative evaluation are well established key factors associated with risk for depression. Furthermore, both peer problems and sensitivity to rejection increase in adolescence, consistent with the other social changes that occur during this developmental period (Hankin et al., 2007; Hazel, Oppenheimer, Technow, Young, & Hankin, 2014; Marston, Hare, & Allen, 2010; Silk et al., 2014).
Interpersonal theorists have further speculated about potential processes through which social risk factors may contribute to the etiology and maintenance of depression among youth (Hammen, Rudolph, & Abaied, 2014). One potential pathway linking social risk factors, such as peer victimization and fear of negative evaluation, to increased depressive symptoms is altered emotion processing (i.e., heightened emotional reactivity to negative information and/or alterations in the ability to modulate negative emotion; Repetti, Taylor, & Seeman, 2002). This study is the first to examine whether altered neural activation in affective salience and prefrontal regions involved in emotional information processing accounts for the association between these social risk factors and depressive symptoms among clinically depressed and nondepressed youth during the transition into and across adolescence.
Emotion Processing as a Mechanism Linking Social Risk and Depressive Symptoms
Theory and research on social risk in adolescence suggest that negative social experiences and social-cognitive vulnerabilities, such as peer victimization and fear of negative evaluation, may sensitize youth to negative social interactions, leading to more intense emotional responses to social information which then tax youth’s ability to effectively regulate emotions (McLaughlin, Hatzenbuehler, & Hilt, 2009; Silvers et al., 2012; Troop-Gordon, Rudolph, Sugimura, & Little, 2015). Previous findings suggest that both peer victimization and fear of negative evaluation are associated with aberrant emotion processing, heightened emotional distress (e.g., negative emotions and high arousal) and/or emotion regulation difficulties (e.g., Herts, McLaughlin, & Hatzenbuehler, 2012; McLaughlin et al., 2009; Rossignol, Campanella, Bissot, & Philippot, 2013; Schwarz, Wieser, Gerdes, Muhlberger, & Pauli, 2013; Winton, Clark, & Edelmann, 1995). There is also growing evidence that altered emotion processing, including increased emotional reactivity and emotion regulation difficulties, is associated with internalizing and depressive symptoms during the transition into and across adolescence (Charbonneau, Mezulis, & Hyde, 2009; Garber, Braafladt, & Weiss, 1995; Hatzenbuehler, McLaughlin, & Nolen-Hoeksema, 2008; Silk, Steinberg, & Morris, 2003; Yap, Schwartz, Byrne, Simmons, & Allen, 2010). Finally, studies have shown that social risk (e.g., peer victimization) is associated with increased depressive symptoms through self-reported emotional processing problems among youth (Kochenderfer-Ladd, 2004; Koval & Kuppens, 2012; McLaughlin et al., 2009). Taken together, research supports that altered emotion processing is a potential mechanism linking social risk factors, such as peer victimization and fear of negative evaluation, and depressive symptoms during the adolescent developmental period.
Neurobiological Factors Linking Social Risk With Depressive Symptoms
Given the accumulating behavioral evidence that social risk may be associated with depressive symptoms through altered emotion processing, it is important to investigate whether neurobiological factors associated with altered emotion processing may account for the link between social risk factors and depressive symptoms. Brain structural and functional changes continue throughout adolescence and into adulthood, suggesting that adolescence encompasses a period of neural plasticity and mutability in neural systems (Crone & Dahl, 2012; Guyer, Silk, & Nelson, 2016; Ladouceur, 2012; Luna, Marek, Larsen, Tervo-Clemmens, & Chahal, 2015). Consistent with this, research shows age-related changes in emotion processing, such as increased affective responses to social information, and suggests that affective functioning is more flexible and strongly influenced by contextual factors in adolescence (Compas, Connor-Smith, Saltzman, Thomsen, & Wadsworth, 2001; Guyer, Choate, Pine, & Nelson, 2012; Silk et al., 2014; Troop-Gordon et al., 2015). Thus, social risk, such as peer victimization and fear of negative evaluation, may play a more formative role in social-affective development by exerting influence on neural substrates of emotional processing during adolescence, especially given increases in the significance of social relationships and exposure to social stressors during this developmental period. Altered neural functioning associated with maladaptive emotional processing may in turn contribute to the emergence and chronicity of depressive symptoms among youth.
Additionally, the use of neurobiological measures of emotion processing addresses limitations of previous research. First, prior studies examining associations among social risk factors, emotion processing, and depressive symptoms typically rely on the exclusive use of self-report measures, which can inflate associations among variables. Second, self-report measures of emotion processing often confound multiple emotion processes, including initial emotion reactivity and cognitive aspects of emotion processing (e.g., appraisal/evaluation and regulation), because they fail to capture the temporal dynamics of emotion processing, which unfolds over time (Gross, Sheppes, & Urry, 2011). Neurobiological measures allow us to explore the temporal dynamics of neural activation in brain regions differentially associated with emotional reactivity and cognitive aspects of emotion processing. Thus, research incorporating multiple methods at multiple levels of analyses may enhance our understanding of how altered emotion processing may explain the association between social risk factors and depressive symptoms.
Neuroimaging research has delineated the neural substrates underpinning emotion processing, including emotional reactivity and cognitive processing of emotion. Specifically, emotion processing (e.g., emotional reactivity) of negative information recruits affective salience regions including the amygdala, insula, and anterior cingulate cortex (ACC) (e.g., Lindquist, Satpute, Wager, Weber, & Barrett, 2016; Touroutoglou, Lindquist, Dickerson, & Barrett, 2015). Adolescents display heightened amygdala, insula, and subgenual ACC (sgACC) activity in response to negative (relative to neutral or positive) information such as fearful faces, social exclusion, and maternal criticism (e.g., Hare et al., 2008; Lee, Siegle, Dahl, Hooley, & Silk, 2015; Masten et al., 2009). Emotion processing is also often associated with activation of prefrontal regions including dorsolateral prefrontal cortex (DLPFC), ventrolateral prefrontal cortex (VLPFC), medial prefrontal cortex, and caudal ACC (Buhle et al., 2014; Kober et al., 2008; Lee & Siegle, 2012; Phillips, Ladouceur, & Drevets, 2008). These brain regions are known to be involved in various forms of cognitive processing of emotion such as emotional evaluation or appraisal and emotion regulation. Studies have demonstrated that adolescents show elevated activation in prefrontal regions (e.g., DLPFC and VLPFC) during labeling of emotions using static adults’ faces (Telzer et al., 2014) and dynamic peer faces (Flannery, Giuliani, Flournoy, & Pfeifer, 2017), and during cognitive reappraisal (Belden, Luby, Pagliaccio, & Barch, 2014; McRae et al., 2012).
The Current Study
The current study investigates whether neural function subserving emotion processing could help explain the link between social risk (i.e., peer victimization and fear of negative evaluation) and depressive symptoms during the transition into and across adolescence. We used social status words as our emotional stimuli because social information is particularly salient during the adolescent developmental period. Given evidence that negative information generates greater saliency and higher cognitive/regulatory demands than positive information (Barrett, Gross, Christensen, & Benvenuto, 2001; Lee & Siegle, 2014), we were particularly interested in examining whether altered emotion processing of negative information (i.e., brain response to negative social status words compared with positive social status words) was associated with social risk factors and depressive symptoms. We employed a word valence identification (WVID) task that instructed participants to label emotions associated with words. Previous studies have shown that emotion labeling tasks engage both affective salience (e.g., amygdala) and prefrontal regions (e.g., VLPFC and DLPFC) in adults (Burklund, Creswell, Irwin, & Lieberman, 2014; Lange et al., 2003; Lieberman et al., 2007) and in adolescents (Flannery et al., 2017; Telzer et al., 2014).
Emotion labeling tasks have also been used to explore the temporal course of physiological responses (i.e., early pupillary reactivity during labeling of negative emotion) in youth (Silk et al., 2007) and neural activation (i.e., sustained amygdala activation during labeling of negative emotion) in depressed adults (Siegle, Steinhauer, Thase, Stenger, & Carter, 2002). However, whether affective salience and prefrontal regions are activated at the same time or different times during emotion labeling in youth still remains unclear. Theories and empirical studies support the idea that emotion processing related to novelty and saliency temporally precedes processes that require more effortful and controlled processing, such as goal-related appraisal (Grandjean & Scherer, 2008; Scherer, 2001). Other research investigating explicit emotion regulation has also shown differential neural involvement in early emotion reactivity and subsequent cognitive processing of emotion (e.g., Eippert et al., 2007). For example, the amygdala was activated by the initial emotion induction phase (i.e., presentation of threat stimuli), whereas prefrontal regions were activated during the subsequent emotion regulation phase. Thus, we explored the time course of neural responses in affective salience and prefrontal regions during emotion labeling.
We tested the following three hypotheses.
Hypothesis 1: Affective salience regions (e.g., amygdala, insula, sgACC) and prefrontal regions (e.g., DLPFC and VLPFC) would show greater neural activity in response to negative social status words compared to positive social status words, but their peak activation would occur at different times. Specifically, we expected that neural response to negative (vs. positive) social status words would occur earlier in affective salience regions and later in prefrontal regions.
Hypothesis 2: Given that social risk and depressive symptoms are associated with increased emotion reactivity and decreased cognitive processing of emotion (e.g., more emotion regulation difficulties; Charbonneau et al., 2009; McLaughlin et al., 2009; Winton et al., 1995), we predicted that early activity in response to negative social status words in affective salience regions would be positively associated with self-reported social risk and self-reported depressive symptoms whereas late prefrontal activity would be negatively associated with social risk and depressive symptoms.
Hypothesis 3: Altered activation in affective salience regions and in prefrontal cortical regions would account for the relationship between these social risk factors and depressive symptoms.
METHOD
Participants
Fifty-eight youth spanning the transition into and across adolescence (42 female, aged 9–17 years [M = 14.47, SD = 1.88]) were recruited from community advertisements, pediatric offices, and existing research projects. Two participants were excluded due to poor task performance (e.g., no responding and lower accuracy rate < 60%) and seven participants were excluded due to excessive head movement (over 30% of scans with greater than ±5 mm and ±5° movement from a reference image and ±1 mm and ±1° incremental (scan-to-scan) movement). Forty-nine participants (35 female, ages 9–17, M [SD] = 14.61 [1.85]) were thus included for our final analysis. To increase variability in social risk factors, our study included both healthy youth and youth diagnosed with major depressive disorder (MDD). Twenty-nine participants were low-risk with no psychiatric history and 20 participants had a current diagnosis of MDD based on DSM-IV (American Psychiatric Association, 1994) criteria. MDD youth were included if they were on a stable dose of selective serotonin reuptake inhibitor (SSRI) medication but still met criteria for MDD (N = 2). Participants were excluded if they were taking psychoactive medications other than SSRIs or had metal objects in their body. MDD youth were excluded if they had a current diagnosis of obsessive–compulsive disorder, post-traumatic stress disorder, conduct disorder, substance abuse or dependence and ADHD combined type or predominantly hyperactive–impulsive type, or a lifetime diagnosis of bipolar disorder, psychotic depression, schizophrenia, schizoaffective disorder, or a pervasive developmental disorder. Eleven youth with MDD had a current diagnosis of one or more comorbid anxiety disorders, including panic disorder (N = 2), specific phobia (N = 4), generalized anxiety disorder (N = 6), social phobia (N = 2), separation anxiety disorder (N = 1), and agoraphobia (N = 1). One MDD youth had a comorbid diagnosis of oppositional defiant disorder.
Procedure and Experimental Paradigm
Procedure
The parents provided informed consent and participants provided assent using forms approved by the University of Pittsburgh Institutional Review Board. They then completed two laboratory visits. During the first visit, each participant and his or her parent(s) completed a structured diagnostic interview using the Schedule for Affective Disorders and Schizophrenia in School-Age Children—Present and Lifetime version (K-SADSPL; Kaufman et al., 1997). Parents and youth were interviewed separately, with clinicians integrating data from both informants to arrive at a final diagnosis. All interviews were carried out by trained BA- and MA-level clinicians. Fifteen percent of interviews were double-coded and there were no diagnostic disagreements (κα= 1.0). Participants and their parents completed a questionnaire to assess depressive symptoms. Participants also completed questionnaires to assess self-reported social risk (i.e., peer victimization and fear of negative evaluation) and emotion regulation. The fMRI assessment was completed during their second visit (approximately 2 weeks later). They were asked to lie as still as possible during the structural imaging acquisition and then to perform a WVID task during the functional imaging acquisition. After the fMRI assessment, participants were asked to complete a post-scan valence rating of each word, (“How emotional is this word for you?”) on a 7-point scale ranging from 1 (very negative) to 7 (very positive). To confirm that the social status words were considered relevant words to describe adolescent social status, participants were also asked to answer two questions: (1) Is this a word that kids your age would use to describe another kid they admire or look up to (high social status rating)? and (2) Is this a word that kids your age would use to describe another kid they do NOT admire or do NOT look up to (low social status rating)? The response was “yes” or “no”. Participants were carefully debriefed following completion of the scan.
Experimental paradigm
Participants were asked to complete a WVID task during fMRI assessment. Participants were instructed to identify the emotional valence of social status words (13 positive social status [e.g., accepted, popular, liked, invited] and 13 negative social status [e.g., ignored, loser, disliked, unwanted]) and 13 neutral nonsocial words (e.g., table, pencil, paper, book) by pressing a corresponding button for each valence (positive, negative, and neutral) using a Psychology Software Tools glove. Reaction time (RT) was measured from the onset of the button press to the word stimulus. Stimuli were displayed in black on a gray background via a back-projection screen (.88° visual angle) and presented using E-prime 1.0 software (Psychology Software Tools, Pittsburgh, PA). The social status words were generated by a focus group of six adolescents who were asked to generate a list of words that could be used to describe a person who the adolescent admired or looked up to (positive social status words) or a person they did not admire or look up to (negative social status words). We selected social status words with multiple nominations across adolescents that could be balanced with neutral words for word length and frequency of use in the English language.
A slow event-related paradigm was used to allow examination of the time course of event-related neural responses. Each trial began with a cue (a row of Xs) for 1,000 ms, followed by presentation of the word for 5,000 ms, and followed by a mask (another row of Xs) for 5,690 ms. A 5,690 ms inter-trial interval was used to provide sufficient time for blood oxygen level-dependent (BOLD) response to return the baseline between trials. The total trial duration is <12 s to clearly show resolution of the hemodynamic response function. There were 39 trials randomly presented with 26 social status words and 13 neutral words. Trials with neutral words were included as fillers.
Self-Report Measures
Depressive symptoms
Child and parent report of depressive symptomatology were obtained using the full version of the Mood and Feelings Questionnaire (MFQ; Angold, Costello, Messer, & Pickles, 1995), which is a widely used self-report measure of children’s and adolescents’ depressive symptoms, with excellent psychometric properties (Cronbach’s α= .90). The MFQ includes 33 items (child version) and 34 items (parent version). Participants rate how true each item is of them (child version) or their child (parent version) over the past 2 weeks (0 = not true, 2 = true). Internal consistency of this scale in this study sample was excellent (child version: α= .98, parent version: α= .95).
Social risk
Self-reports of peer victimization and fear of negative evaluation were assessed using the Peer Relations Questionnaire (PRQ; Rigby & Slee, 1993) and the Brief Fear of Negative Evaluation Scale (Brief-FNE; Leary, 1983), respectively. The PRQ is a 20-item scale that includes three subscales to assess peer relationships: bullying, victimization, and prosocial behavior. We used the victimization subscale, consisting of five items (e.g., “I get called names by others”). Participants rated on a scale ranging from 1 (never) to 5 (very often) how true each item is of them. This scale demonstrated adequate reliability, Cronbach’s α= .77–.83 (Rigby, 1998). Internal consistency of this subscale in this study sample was excellent (α= .95).
The Brief-FNE includes 12 items consisting of statements regarding concerns and apprehension about being negatively perceived and evaluated by others. Participants rated on a scale ranging from 1 (not at all) to 5 (extremely) how much they like each statement (e.g., “I am afraid that others will not like me”). This measure demonstrated strong reliability, Cronbach’s α= .90–.97 (Collins, Westra, Dozois, & Stewart, 2005; Leary, 1983). This scale had good internal consistency in this sample (α= .78).
Emotion regulation
Self-reports of emotion regulation were assessed using the Children’s Emotion Management Scale (CEMS; Zeman, Shipman, & Penza-Clyve, 2001), specifically sadness (12 items) and anger (11 items) emotion regulation strategies. The CEMS is comprised of three subscales including inhibition, dysregulated expression, and emotion regulation coping. We used the emotion regulation coping subscale, which is assumed to measure adaptive emotion regulation, consisting of four items (anger: e.g., “when I am feeling mad, I control my temper”) and five items (sadness: e.g., “When I am feeling sad, I can control my crying and carry on”). Participants responded to these items using a 3-point Likert scale (1 = hardly ever, 2 = sometimes, 3 = often). This scale demonstrated adequate psychometric properties, α= .60–.81 (Becker, Luebbe, & Joyce, 2015; Zeman et al., 2001). Internal consistency of this subscale in this study sample was good (sadness: α= .77, anger: α= .71).
Imaging Acquisition and Preprocessing
Imaging acquisition
Images were acquired on a 3T Trio scanner (Siemens, Erlangen, Germany). Thirty-two 3.2-mm slices were acquired parallel using a posterior-to-anterior echo planar (EPI) pulse sequence (T2*-weighted imaged depicting BOLD signal; TR = 1,670 ms, TE = 29 ms, FOV = 205 mm, flip angle = 75). Each image was acquired in 1.67 s, allowing 7 scans per 11.69 s trial (duration was determined by multiples of our TR, 1.67 s). High-resolution T1-weighted MPRAGE images (1 mm, axial) were also collected for use in cross-registration.
fMRI data preprocessing
fMRI analyses were conducted using locally developed NeuroImaging Software (NIS) (Fissell et al., 2003) and Analysis of Functional Neuroimaging (AFNI) software (Cox, 1996). Functional imaging data were corrected for motion using 3dVolReg implemented in AFNI using the first image as a reference. Linear and quadratic trends within runs were regressed out of fMRI time series to eliminate effects of scanner drift, unrelated to brain activity using nis-correct from NIS. Data were temporally smoothed using a 4-point Gaussian filter and converted to %-change based on the median of all imaging data. Data were co-registered to the Colin-27 Montreal Neurological Institute template using the Automated Image Registration package’s (Woods, Mazziotta, & Cherry, 1993) 32-parameter nonlinear automated warping algorithm and spatially smoothed using a 6 mm full width at half maximum (FWHM) filter.
Preliminary Statistical Analyses
fMRI data analysis: Whole-brain analysis to identify regions involved in negative emotion processing
Model-free analysis was employed to account for empirical variation in the shape of the hemodynamic response (e.g., early or sustained activation) rather than relying on hemodynamic responses to have a canonical shape (e.g., as we have done for similar designs in Siegle, Thompson, Carter, Steinhauer, and Thase (2007)). The long duration of each trial implemented in our slow event-related design enabled us to use scan-within-trial (defined as a time factor) as a repeated measure, eliminating the need for potential misleading event deconvolution. To identify brain regions (functional regions of interest [ROIs]) specifically involved in emotion processing of negative information compared to positive information, we conducted a random-effects whole-brain voxel-wise analysis of variance (ANOVA) with participant as a random factor, and valence (negative vs. positive) and time (seven scans spanning 11.69 s, the duration of each trial) as fixed factors.
We did not compare the negative and neutral conditions at the neural level for several reasons. First, literature suggests that neutral information can trigger brain activation associated with uncertainty or ambiguity (Pfeifer et al., 2011), making it difficult to interpret this contrast. Second, there is evidence that depressed individuals often show biased responses to neutral (nonemotional) information (Douglas & Porter, 2010; Leppanen, Milders, Bell, Terriere, & Hietanen, 2004), further making this contrast difficult to interpret. Evidence, in fact, suggests that youth compared to adults are particularly likely to show affective responses to neutral information (Silk et al., 2009; Thomas et al., 2001).
The functional map resulting from the voxel-wise whole-brain ANOVA was thresholded at voxel-wise p < .005 and corrected for multiple comparisons using an empirically determined minimum cluster size to achieve a brain-wise corrected p < .05, via AFNI’s 3dClustSim with smoothing estimated via AFNI’s 3dFWHMx, version 16.1.04 “acf” procedure. The recent version of 3dClustSim responds to a recent methods critique (Eklund, Nichols, & Knutsson, 2016) by accounting for non-Gaussian autocorrelation in estimating smoothness of the data and fixing a historical bug. Our cluster size was determined using 5,000 Monte Carlo simulations, third-nearest neighbor (NN3) clustering, and one-sided thresholding. Both the uncorrected voxel-wise p-value and contiguity threshold necessary to achieve a brain-wise corrected p < .05 are reported below.
Testing temporal dynamics in functional ROIs identified from the whole-brain analysis
To further examine temporal dynamics of brain activity, we extracted each participant’s time courses from functional ROIs remaining significant after-correcting for multiple comparisons. We explored specific temporal windows (e.g., sustained activity after the onset of stimuli) showing significant valence differences in time series by comparing time courses between valence at each time point (scan). To control Type I error, we used Guthrie and Buchwald (1991)’s method to account for autocorrelation: Temporal windows of time series were considered statistically significant, when two consecutive scans were significant at p < .05. Thus, temporal windows with significant differences between two conditions represented continuous series of time points that reliably differed in time courses. We averaged activity (signal % change from baseline [the first scan of each trial] in the negative condition to find negative trial-related responses) across these significant temporal windows for each functional ROI and each participant. Then we used the averaged activity for subsequent correlation and mediation analyses.
Correlation analysis: Correlations among variables
Before we tested our mediation models, correlation analyses were conducted to explore the relationships between variables (self-reported measures of social risk and depressive symptoms and brain activity) included in the mediation model.
Mediation Analysis
Neural response to negative information accounted for the relationship between social risk and depressive symptoms. A mediation analysis using the PROCESS macro for SPSS (Hayes, 2012) was conducted to test whether the association between social risk (i.e., self-reported peer victimization and fear of negative evaluation) and depressive symptoms (child-reported MFQ scores) was accounted for by activity (signal % change from baseline) in functional ROIs identified from the whole-brain analysis. Using an indirect approach due to our relatively small sample size, we performed a mediation analysis using bootstrapping (i.e., 95% bias-corrected bootstrap confidence intervals [CI] for the indirect effects based on 10,000 bootstrap resamples) across the entire sample. Indirect effects were considered statistically significant if the 95% bias-corrected CI did not include zero (Preacher & Hayes, 2008).
RESULTS
Manipulation Check
A repeated measures ANOVA on post-scan valence ratings revealed that there was a significant main effect of valence, F(1.43, 63) = 547.57, p < .001, χp2 = .93 with Greenhouse-Geisser correction used for sphericity. Positive social status words were rated as more positive (M = 5.65, SD = 0.55) than neutral words (M = 4.04, SD = 0.44) and negative social status words were rated as more negative (M = 2.11, SD = 0.51; lower ratings signify greater negativity) than neutral words (M = 4.04, SD = 0.44). Chi-square tests showed that social status words (both positive and negative) were more likely to be endorsed by participants as words they would use to describe social status compared to neutral words (high social status rating: χ2(1) = 664.68, p < .0001 and low social status rating: χ2(1) = 512.44, p < .0001).
Preliminary Results
fMRI results: Whole-brain analysis to identify regions involved in negative emotion processing
As presented in Table 1, only two clusters including lateral prefrontal (i.e., DLPFC) and parietal areas showed greater activation in response to negative social status information compared to positive social status information. Consistent with our hypothesis, the right DLPFC showed significantly elevated and sustained responses (6.68–11.69 s) to negative social status words compared to positive social status words (Figure 1a). Contrary to our hypothesis, none of the hypothesized affective salience regions showed significant early activity in response to negative compared to positive social status words. This result did not allow us to test further hypotheses regarding the affective salience regions.
TABLE 1.
Brain Regions Showing Significant Valence × Time Interactions (p < .005, 43 Voxels Contiguity)
| Brain region | BA | Size (mm3) | Tal coordinates of centroid
|
F-value of centroid | Temporal regions: Significant valence difference (s) | ANOVA: Significant valence difference | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| NEG > POS | ||||||||
| Middle/Superior frontal gyrus (DLPFC)a | 9 | 2,165 | 39 | 35 | 27 | 3.64 | 6.68 –11.69 | F(1,48) = 6.99, p = .01 |
| Superior frontal gyrus | 6 | 1,968 | 25 | 16 | 58 | 3.95 | 8.35 –11.69 | F(1,48) = 10.42, p = .00 |
| POS > NEG | ||||||||
| Inferior frontal gyrus | 47 | 1,607 | −35 | 26 | −1 | 7.56 | 6.68 –11.69 | F(1,48) = 7.07, p = .01 |
| Superior temporal gyrus | 21 | 1,764 | −58 | −10 | −1 | 6.34 | 8.35 –11.69 | F(1,48) = 8.39, p = .01 |
| Middle temporal gyrus | 21 | 2,165 | 57 | 2 | −14 | 3.72 | 6.68 –11.69 | F(1,48) = 12.37, p = .00 |
Notes. BA, Brodmann area; xyz, Talairach coordinates of centroid; F-value, F-value of centroid; NEG, negative social status words; POS, positive social status words.
Time series in dorsolateral prefrontal cortex (DLPFC) regions is presented in Figure 1a.
FIGURE 1.
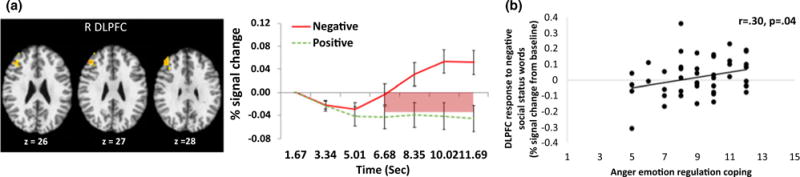
(a) Valence × Time interaction effects: DLPFC regions showing greater activity in response to negative social status words than positive social status words. Time series across scans associated with the interaction of valence and time in empirically derived DLPFC regions. Significant differences in time series between negative and positive social status words are highlighted below the x axis, pink = p < .05. R DLPFC: right dorsolateral prefrontal cortex, (b) A correlation between self-report anger emotion regulation coping (measured by a subscale of Children’s Emotion Management Scale) and DLPFC activity in response to negative social status words (average % signal change across 6.68 s–11.69 s from baseline [the first scan]).
The averaged late DLPFC activity (6.68–11.69 s) in response to negative social information was therefore used in our mediation model to test whether prefrontal activity accounted for the relationship between social risk factors and depressive symptoms. Although we used functional ROIs’ activity for our mediation analysis, functional ROIs were identified by an inherently independent analysis regardless of associations with self-reported measures, thus allowing us to minimize the potential risk of “double dipping” (Kriegeskorte, Simmons, Bellgowan, & Baker, 2009).
Secondary analysis: Brain activity associated with behavioral measures
To more clearly understand the potential role of prefrontal regions in negative relative to positive emotion processing, we conducted correlation analyses between late prefrontal activity and RT, and between late prefrontal activity and emotion regulation coping scores. We hypothesized that a significant positive correlation between late prefrontal activity and slower RT to negative social status words would represent effortful cognitive/appraisal processing and a significant positive correlation between late prefrontal activity and regulation coping scores would represent greater emotion regulatory function in prefrontal regions. We found that late DLPFC activity in response to negative social status words was not significantly correlated with RT (r = .05, p = .76). Late DLPFC activity was significantly correlated with anger emotion regulation coping, r = .30, p < .05 (see Figure 1b), but not with sadness emotion regulation coping, r = .09, p = 54. Adolescents who showed increased DLPFC activity in response to negative social information reported higher scores in anger emotion regulation coping.
Correlations among variables
There were significant correlations between variables included in our mediation model (Table 2). Self-reported peer victimization and fear of negative evaluation were positively correlated with depressive symptoms, respectively. Consistent with our hypothesis, late activity in the DLPFC was significantly negatively correlated with peer victimization, fear of negative evaluation, and depressive symptoms. Chronological age and gender were not correlated with any variables (all ps > .10), so they were not included in our mediation analysis.
TABLE 2.
Descriptive Statistics and Correlations Among Variables
| Variable | M | SD | Self-reported social risk
|
Self-reported depressive
symptoms 3 |
Brain
activation 4 |
|
|---|---|---|---|---|---|---|
| 1 | 2 | |||||
| 1. Fear of negative evaluation (BFNE) | 29.23 | 7.96 | – | |||
| 2. Peer victimization (PRQ) | 6.83 | 2.79 | .64*** | – | ||
| 3. Depressive symptom (child) (MFQ) | 16.83 | 18.15 | .62*** | .48** | – | |
| 4. DLPFC activitya | 0.03 | 0.12 | .36* | .38** | .39** | – |
Notes. BFNE, Brief Fear of Negative Evaluation Scale; PRQ, Peer Relations Questionnaire; MFQ, Mood and Feelings Questionnaire; DLPFC, dorsolateral prefrontal cortex; CEMS, Children’s Emotion Management Scale.
Average % signal change across 6.68 s–11.69 s from baseline (the first scan).
p < .05;
p < .01;
p < .001.
Mediation Analysis
Neural response to negative information accounted for the relationship between social risk and depressive symptoms. The mediation analysis revealed significant indirect effects of peer victimization on depressive symptoms through late activity in the DLPFC, 0.48, SE = 0.41, 95% Bootstrap CI (0.03, 1.78) (Figure 2) and of fear of negative evaluation on depressive symptoms through late activity in the DLPFC, 0.22, SE = 0.13, 95% Bootstrap CI (0.03, 0.58) (Figure 2). Reduced late DLPFC activity to negative social status words accounted for significant amounts of variance in the relationship between peer victimization and depressive symptoms, and between fear of negative evaluation and depressive symptoms.
FIGURE 2.

The mediation model of the relation between peer victimization, depressive symptoms, and DLPFC function (left) and the relation between fear of negative evaluation, depressive symptoms, and DLPFC function (right).
Sensitivity Analyses
Controlling for age
We tested whether our main findings remain significant after controlling for age, although there were no significant associations between age and other variables (self-report measures and brain activity). Our results remained significant after controlling for age (indirect effects of peer victimization on depressive symptoms through late DLPFC activity: 0.43, SE = 0.39, 95% Bootstrap CI [0.003, 1.76] and of fear of negative evaluation on depressive symptoms through the late DLPFC activity: 0.20, SE = 0.12, 95% Bootstrap CI [0.03, 0.56]).
Indirect effect of social risk on depressive symptoms through functional connectivity
We also investigated whether functional connectivity between the affective salience regions and prefrontal regions accounted for the association between social risk and depressive symptoms. We first conducted functional connectivity analyses using the DLPFC as a seed region. Specifically, we tested whether late and sustained DLPFC activity (6.68–11.69 s) was associated with early activity (3.34–6.68 s) in affective salience regions. This analysis was based on the idea that early reactivity in affective salience regions may be related to subsequent late activity in prefrontal regions. None of these regions showed functional connectivity with late DLPFC activity after controlling for multiple comparisons using AFNI’s 3dClustSim (voxel-wise p < .005, 47 voxels contiguity to achieve a brain-wise corrected p < .05). Due to the lack of functional connectivity, we were not able to test the indirect effect of social risk on depressive symptoms through functional connectivity between affective salience and prefrontal regions.
Indirect effect of social risk on anxiety symptoms through DLPFC activity
Given the higher levels of comorbidity with anxiety disorder in our depressed sample, we performed additional mediation analyses to examine whether findings were specific to depression or were more general to both depression and anxiety. We tested our proposed mediation models using anxiety symptoms, assessed by the Screen for Childhood Anxiety Disorder (Birmaher et al., 1997) as the dependent variable. Results showed that DLPFC activity accounted for the relationship between fear of negative evaluation and anxiety symptoms (indirect effect: 0.17, SE = 0.09, 95% Bootstrap CI [0.03, 0.38]), but not for the relationship between peer victimization and anxiety symptoms (indirect effect: 0.47, SE = 0.36, 95% Bootstrap CI [−0.08, 1.40]).
DISCUSSION
Findings from this study showed that youth exhibit greater but later occurring activation in the right DLPFC in response to negative social status words compared to positive social status words on the WVID task. This suggests that the DLPFC is involved in emotion processing that occurs after initial reactivity to negative social words. Late DLPFC activity was associated with increased presence of two types of social risk factors, peer victimization and fear of negative evaluation, and depressive symptoms. Importantly, DLPFC activity in response to negative social status words also accounted for the relationship between both these types of social risk and depressive symptoms in youth.
The finding showing elevated and sustained DLFPC activity in response to negative social status words compared to positive social status words is consistent with previous studies showing DLPFC involvement in cognitive processing of emotion during labeling emotions (Flannery et al., 2017) and cognitive emotion regulation (Belden et al., 2014; McRae et al., 2012). Relatively late elevated DLPFC activity may represent more cognitively effortful processing or cognitive emotion regulation associated with labeling negative social information, compared to positive social information. Additional analyses revealed that DLPFC response to negative social status words was positively correlated with management of negative emotion assessed by a self-report measure of anger emotion regulation coping. Thus, findings suggest that DLFPC activity underlying emotion regulation of anger may account for the association between social risk and depressive symptoms among youth. This is consistent with research showing that anger and irritability are especially relevant for depression in youth populations (Emslie, Mayes, & Ruberu, 2005; Sheeber et al., 2009; Silk et al., 2011). Nevertheless, because the task was not designed to examine explicit emotion regulation (e.g., task instructions to regulate emotion), and because we did not collect data related to specific emotion ratings during the task (e.g., how angry they felt and how sad they felt), we cannot be certain that the observed DLPFC activity reflects emotion regulation as opposed to other cognitive processes such as emotion appraisal and labeling, or reflects regulation of anger versus sadness.
Significant associations among both social risk factors (peer victimization and fear of negative evaluation), DLPFC activity in response to negative social status words, and depressive symptoms are consistent with prior research suggesting that social risk factors are associated with altered emotion processing (McLaughlin et al., 2009; Rossignol et al., 2013; Schwarz et al., 2013; Winton et al., 1995) and with depressive symptoms and disorders (Hatzenbuehler et al., 2008; Silk et al., 2003). Findings further suggest that altered DLPFC activity in response to negative social information may be a neural marker associated with both social risk and depression during the transition into and across adolescence.
Finally, our mediation analyses suggest that social risk may contribute to depressive symptoms by interfering with cognitive processes that support effective emotion processing and regulation. This is in line with previous behavioral studies demonstrating that emotion regulation difficulties account for the association between social risk factors (e.g., peer victimization) and depressive symptoms in adolescence (Doyle & Sullivan, 2017; McLaughlin et al., 2009), but reveals that altered functioning of prefrontal cortical regions implicated in processing negative social information may underlie this relationship, particularly blunted DLPFC function. It is important to note that DLPFC response to negative social information explained the associations between two important aspects of social risk, negative social experience (i.e., peer victimization), and social-cognitive vulnerability (i.e., fear of negative evaluation), which suggests that aberrant DLPFC function may be a general neurobiological factor for linking multiple types of social risk factors to the development and maintenance of depression.
We did not find significant functional connectivity among affective salience and prefrontal regions, particularly functional connectivity between early activity in affective salience regions and later activity in prefrontal regions. This finding limits our ability to examine whether social risk may contribute to aberrant connectivity between affective salience and prefrontal regions underlying heightened emotional reactivity and difficulties regulating emotion that may in turn put youth at risk for increases in depressive symptoms. Future research using more sophisticated experimental designs that incorporate separable initial/early emotion processing and late cognitive aspects of emotion processing are needed to examine how functional connectivity between neural regions subserving early and late emotion processing are related to social risk factors and depressive symptoms.
It is important to note that DLPFC activity accounted for the relationship between fear of negative evaluation and anxiety symptoms. This result may be because fear of negative evaluation has been frequently associated with both anxiety and depression (Lipton et al., 2016; Nonterah et al., 2015; O’Connor et al., 2002; Weeks et al., 2005). In contrast, findings suggest that peer victimization may have a specific association with depressive symptoms through alteration in prefrontal function. However, future research may be needed to replicate these findings to better understand the extent to which these social risk factors are specific to depressive symptoms versus anxiety through brain function involved in emotion processing.
From a developmental perspective, findings suggest the possibility that, as peer relationships become more important in adolescence, youth with higher levels of social risk may be especially sensitive to social information, and thus more likely to experience heightened distress and difficulty in regulating emotion in response to negative social stimuli. One possibility is that, as these vulnerable youth enter into a developmental period characterized by increased neural plasticity and greater exposure to social stressors, peer victimization and fear of negative evaluation contribute to longer term neural development underlying emotion processing and emotion regulation deficits in social contexts. Alterations in the development of neural function, such as blunted DLPFC function, may in turn increase vulnerability to or worsening of depressive symptoms among adolescent youth. However, more longitudinal research is needed to test the potential effects of social risk factors on the development of neural affective systems over time, and subsequent risk for depressive symptoms.
Surprisingly, affective salience regions did not show increased neural activity in response to negative information compared to positive information. This may be because both negative and positive emotion labeling similarly activate affective salience regions involved in processing emotional stimuli. It is plausible that adolescents could process all social status words as affectively and motivationally salient. One potential way to detect differences in affective salience regions’ response to negative and positive social status words in future research might be to modify the task to increase the personal relevance of the words, such as by explicitly instructing participants to think about how each word applies to their own social status.
There was also no association between age and DLPFC activity. This may have been associated with our small sample and the fact that relatively few subjects were in the early adolescent range. This study presents several other limitations. First, the cross-sectional design limited our ability to make causal inferences about associations among social risk, neural response to social information, and depression. Additionally, we used only adolescents’ self-reported measures of social risk and depressive symptoms. Despite this limitation, there are some benefits of using adolescent-report measures. Evidence suggests that adolescent-report measures of social risk and depressive symptoms are more accurate than measures reported by other informants (e.g., parents and teachers) because adolescents do not always tell parents or teachers about their peer problems (Bowes, Joinson, Wolke, & Lewis, 2015) and they also better know their subjective internal states than parents (Kent, Vostanis, & Feehan, 1997). Third, past work suggested that different types (i.e., overt and relational victimization) of peer victimization have different associations with depressive symptoms (McLaughlin et al., 2009). However, we did not test such differential associations between distinct types of peer victimization and depressive symptoms in this study. More research is needed to better understand associations between types of peer victimization and neural substrates of altered emotion processing. Fourth, although our study found that increased DLPFC activity was associated with self-reported emotion regulation of anger, it still remains unclear whether our participants were actively regulating emotions and, if so, which specific emotion regulation strategies (e.g., reappraisal and distance) our participants used, without the use of specific emotion regulation instructions. Future research using explicit emotion regulation tasks could help to address this question. Fifth, our relatively small sample size, particularly for early adolescents and for male participants, limits our ability to examine the effects of age and gender on our mediation models. Our sensitivity analysis revealed no significant age effect on the models, but it remained unclear whether this finding may be due to no age effect itself or to our relatively small sample size. Sixth, given the higher levels of comorbidity with anxiety disorder in our depressed sample, we cannot conclude that our mediation findings are specific to depression.
Despite these limitations, our findings are consistent with prior studies showing that the link between social risk and depressive symptoms was accounted for through self-report measures of emotion processing (e.g., McLaughlin et al., 2009). Therefore, our study contributes to a growing body of evidence showing that altered emotion processing may serve as an important pathway through which social risk may contribute to increased depressive symptoms in both clinical and nonclinical populations of youth during the transition into and across adolescence. In addition, this is the first study, to the best of our knowledge, to use more objective brain-based measures of altered emotion processing to examine the role of emotion processing in linking social risk with depressive symptoms. Finally, our findings suggest that improving DLPFC function in social contexts may be a neural target for preventions or intervention geared toward breaking the cycle of social difficulties and depressive symptoms in adolescence. Increased DLPFC activation is known to be associated with cognitive control strategies (e.g., acting as a “detached observer” in response to emotional stimuli), or other types of cognitive reappraisal (Frank et al., 2014; Kohn et al., 2014). Interventions that use these emotion regulation strategies and repeated practice/trainings may improve emotion regulation by engaging greater recruitment of the DLPFC. Such interventions hold the potential to alter developmental trajectories toward depression among adolescents with social risk.
Acknowledgments
The authors are grateful to Marcie Walker, Katie Burkhouse, Terri Nicely, and Karen Garelik for their assistance in data acquisition. The authors also thank the participants and their families.
This research was supported by a National Institute of Drug Abuse grant R21DA024144 (J.S.S./R.E.D., PI’s).
Contributor Information
Kyung Hwa Lee, University of Pittsburgh.
Caroline W. Oppenheimer, University of Pittsburgh
Greg J. Siegle, University of Pittsburgh
Cecile D. Ladouceur, University of Pittsburgh
Grace E. Lee, University of Pittsburgh
Ronald E. Dahl, University of California, Berkeley
Jennifer S. Silk, University of Pittsburgh
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th. Washington, DC: Author; 1994. [Google Scholar]
- Angold A, Costello EJ, Messer SC, Pickles A. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International Journal of Methods in Psychiatric Research. 1995;5:237–249. [Google Scholar]
- Barrett LF, Gross JJ, Christensen TC, Benvenuto M. Knowing what you’re feeling and knowing what to do about it: Mapping the relation between emotion differentiation and emotion regulation. Cognition and Emotion. 2001;15:713–724. https://doi.org/10.1080/02699930143000239. [Google Scholar]
- Becker SP, Luebbe AM, Joyce AM. The Child Concentration Inventory (CCI): Initial validation of a child self-report measure of sluggish cognitive tempo. Psychological Assessment. 2015;27:1037–1052. doi: 10.1037/pas0000083. https://doi.org/10.1037/pas0000083. [DOI] [PubMed] [Google Scholar]
- Belden AC, Luby JL, Pagliaccio D, Barch DM. Neural activation associated with the cognitive emotion regulation of sadness in healthy children. Developmental Cognitive Neuroscience. 2014;9:136–147. doi: 10.1016/j.dcn.2014.02.003. https://doi.org/10.1016/j.dcn.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. https://doi.org/10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Bowes L, Joinson C, Wolke D, Lewis G. Peer victimisation during adolescence and its impact on depression in early adulthood: Prospective cohort study in the United Kingdom. British Medical Journal. 2015;350:h2469. doi: 10.1136/bmj.h2469. https://doi.org/10.1136/bmj.h2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Ochsner K. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex. 2014;24:2981–2990. doi: 10.1093/cercor/bht154. https://doi.org/10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burklund LJ, Creswell JD, Irwin MR, Lieberman MD. The common and distinct neural bases of affect labeling and reappraisal in healthy adults. Frontiers in Psychology. 2014;5:221. doi: 10.3389/fpsyg.2014.00221. https://doi.org/10.3389/fpsyg.2014.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau AM, Mezulis AH, Hyde JS. Stress and emotional reactivity as explanations for gender differences in adolescents’ depressive symptoms. Journal of Youth and Adolescence. 2009;38:1050–1058. doi: 10.1007/s10964-009-9398-8. https://doi.org/10.1007/s10964-009-9398-8. [DOI] [PubMed] [Google Scholar]
- Collins KA, Westra HA, Dozois DJ, Stewart SH. The validity of the brief version of the Fear of Negative Evaluation Scale. Journal of Anxiety Disorders. 2005;19:345–359. doi: 10.1016/j.janxdis.2004.02.003. https://doi.org/10.1016/j.janxdis.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Compas BE, Connor-Smith JK, Saltzman H, Thom-sen AH, Wadsworth ME. Coping with stress during childhood and adolescence: Problems, progress, and potential in theory and research. Psychological Bulletin. 2001;127:87–127. https://doi.org/10.1037/0033-2909.127.1.87. [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry. 2003;60:837–844. doi: 10.1001/archpsyc.60.8.837. https://doi.org/10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. https://doi.org/10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–650. doi: 10.1038/nrn3313. https://doi.org/10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dijkstra JK, Cillessen AH, Borch C. Popularity and adolescent friendship networks: Selection and influence dynamics. Developmental Psychology. 2013;49:1242–1252. doi: 10.1037/a0030098. https://doi.org/10.1037/a0030098. [DOI] [PubMed] [Google Scholar]
- Douglas KM, Porter RJ. Recognition of disgusted facial expressions in severe depression. British Journal of Psychiatry. 2010;197:156–157. doi: 10.1192/bjp.bp.110.078113. https://doi.org/10.1192/bjp.bp.110.078113. [DOI] [PubMed] [Google Scholar]
- Doyle ST, Sullivan TN. Longitudinal relations between peer victimization, emotion dysregulation, and internalizing symptoms among early adolescents. Journal of Early Adolescence. 2017;37:165–191. https://doi.org/10.1177/0272431615594458. [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28:409–423. doi: 10.1002/hbm.20291. https://doi.org/10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. https://doi.org/10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emslie GJ, Mayes TL, Ruberu M. Continuation and maintenance therapy of early-onset major depressive disorder. Paediatric Drugs. 2005;7:203–217. doi: 10.2165/00148581-200507040-00001. https://doi.org/10.2165/00148581-200507040-00001. [DOI] [PubMed] [Google Scholar]
- Fissell K, Tseytlin E, Cunningham D, Iyer K, Carter CS, Schneider W, Cohen JD. Fiswidgets: A graphical computing environment for neuroimaging analysis. Neuroinformatics. 2003;1:111–125. doi: 10.1385/ni:1:1:111. https://doi.org/10.1385/ni:1:1:111. [DOI] [PubMed] [Google Scholar]
- Flannery JE, Giuliani NR, Flournoy JC, Pfeifer JH. Neurodevelopmental changes across adolescence in viewing and labeling dynamic peer emotions. Developmental Cognitive Neuroscience. 2017;25:113–127. doi: 10.1016/j.dcn.2017.02.003. https://doi.org/10.1016/j.dcn.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Sabatinelli D. Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neuroscience and Biobehavioral Reviews. 2014;45:202–211. doi: 10.1016/j.neubiorev.2014.06.010. https://doi.org/10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Garber J, Braafladt N, Weiss B. Affect regulation in depressed and nondepressed children and young adolescents. Development and Psychopathology. 1995;7:93–115. https://doi.org/10.1017/S0954579400006362. [Google Scholar]
- Grandjean D, Scherer KR. Unpacking the cognitive architecture of emotion processes. Emotion. 2008;8:341–351. doi: 10.1037/1528-3542.8.3.341. https://doi.org/10.1037/1528-3542.8.3.341. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Sheppes G, Urry HL. Cognition and emotion lecture at the 2010 SPSP emotion preconference. Cognition and Emotion. 2011;25:765–781. doi: 10.1080/02699931.2011.555753. https://doi.org/10.1080/02699931.2011.555753. [DOI] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. https://doi.org/10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience. 2012;7:81–92. doi: 10.1093/scan/nsr043. https://doi.org/10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Silk JS, Nelson EE. The neurobiology of the emotional adolescent: From the inside out. Neuroscience and Biobehavioral Reviews. 2016;70:74–85. doi: 10.1016/j.neubiorev.2016.07.037. https://doi.org/10.1016/j.neubiorev.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen CL, Rudolph KD, Abaied JL. Child and adolescent depression. In: Mash E, Barkley R, editors. Child psychopathology. New York, NY: Guilford Press; 2014. pp. 225–263. [Google Scholar]
- Hankin BL, Mermelstein R, Roesch L. Sex differences in adolescent depression: Stress exposure and reactivity models. Child Development. 2007;78:279–295. doi: 10.1111/j.1467-8624.2007.00997.x. https://doi.org/10.1111/j.1467-8624.2007.00997.x. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Young JF, Abela JR, Smolen A, Jenness JL, Gulley LD, Oppenheimer CW. Depression from childhood into late adolescence: Influence of gender, development, genetic susceptibility, and peer stress. Journal of Abnormal Psychology. 2015;124:803–816. doi: 10.1037/abn0000089. https://doi.org/10.1037/abn0000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63:927–934. doi: 10.1016/j.biopsych.2008.03.015015. https://doi.org/10.1016/j.biopsych.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML, McLaughlin KA, Nolen-Hoeksema S. Emotion regulation and internalizing symptoms in a longitudinal study of sexual minority and heterosexual adolescents. Journal of Child Psychology and Psychiatry. 2008;49:1270–1278. doi: 10.1111/j.1469-7610.2008.01924.x. https://doi.org/10.1111/j.1469-7610.2008.01924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker DS, Boulton MJ. Twenty years’ research on peer victimization and psychosocial maladjustment: A meta-analytic review of cross-sectional studies. Journal of Child Psychology and Psychiatry. 2000;41:441–455. [PubMed] [Google Scholar]
- Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling [White paper] 2012 Retrieved from http://www.afhayes.com/public/process2012,pdf.
- Hazel NA, Oppenheimer CW, Technow JR, Young JF, Hankin BL. Parent relationship quality buffers against the effect of peer stressors on depressive symptoms from middle childhood to adolescence. Developmental Psychology. 2014;50:2115–2123. doi: 10.1037/a0037192. https://doi.org/10.1037/a0037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herts KL, McLaughlin KA, Hatzenbuehler ML. Emotion dysregulation as a mechanism linking stress exposure to adolescent aggressive behavior. Journal of Abnormal Child Psychology. 2012;40:1111–1122. doi: 10.1007/s10802-012-9629-4. https://doi.org/10.1007/s10802-012-9629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner TE, Jr, Coyne JC. The interactional nature of depression: Advances in interpersonal approaches. Washington, DC: American Psychological Association; 1999. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. https://doi.org/10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kent L, Vostanis P, Feehan C. Detection of major and minor depression in children and adolescents: Evaluation of the Mood and Feelings Questionnaire. Journal of Child Psychology and Psychiatry. 1997;38:565–573. doi: 10.1111/j.1469-7610.1997.tb01543.x. https://doi.org/10.1111/j.1469-7610.1997.tb01543.x. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: A meta-analysis of neuroimaging studies. NeuroImage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. https://doi.org/10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer-Ladd B. Peer victimization: The role of emotions in adaptive and maladaptive coping. Social Development. 2004;13:329–349. https://doi.org/10.1111/j.1467-9507.2004.00271.x. [Google Scholar]
- Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation—An ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. https://doi.org/10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koval P, Kuppens P. Changing emotion dynamics: Individual differences in the effect of anticipatory social stress on emotional inertia. Emotion. 2012;12:256–267. doi: 10.1037/a0024756. https://doi.org/10.1037/a0024756. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: The dangers of double dipping. Nature Neuroscience. 2009;12:535–540. doi: 10.1038/nn.2303. https://doi.org/10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD. Neural systems supporting cognitive-affective interactions in adolescence: The role of puberty and implications for affective disorders. Frontiers in Integrative Neuroscience. 2012;6:65. doi: 10.3389/fnint.2012.00065. https://doi.org/10.3389/fnint.2012.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam CB, McHale SM, Crouter AC. Parent-child shared time from middle childhood to late adolescence: Developmental course and adjustment correlates. Child Development. 2012;83:2089–2103. doi: 10.1111/j.1467-8624.2012.01826.x. https://doi.org/10.1111/j.1467-8624.2012.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange K, Williams LM, Young AW, Bullmore ET, Brammer MJ, Williams SC, Phillips ML. Task instructions modulate neural responses to fearful facial expressions. Biological Psychiatry. 2003;53:226–232. doi: 10.1016/s0006-3223(02)01455-5. https://doi.org/10.1016/s0006-3223(02)01455-5. [DOI] [PubMed] [Google Scholar]
- Leary MR. A brief version of the Fear of Negative Evaluation Scale. Personality and Social Psychology Bulletin. 1983;9:371–375. https://doi.org/10.1177/0146167283093007. [Google Scholar]
- Lee KH, Siegle GJ. Common and distinct brain networks underlying explicit emotional evaluation: A meta-analytic study. Social Cognitive and Affective Neuroscience. 2012;7:521–534. doi: 10.1093/scan/nsp001. https://doi.org/10.1093/scan/nsp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Siegle GJ. Different brain activity in response to emotional faces alone and augmented by contextual information. Psychophysiology. 2014;51:1147–1157. doi: 10.1111/psyp.12254. https://doi.org/10.1111/psyp.12254. [DOI] [PubMed] [Google Scholar]
- Lee KH, Siegle GJ, Dahl RE, Hooley JM, Silk JS. Neural responses to maternal criticism in healthy youth. Social Cognitive and Affective Neuroscience. 2015;10:902–912. doi: 10.1093/scan/nsu133. https://doi.org/10.1093/scan/nsu133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppanen JM, Milders M, Bell JS, Terriere E, Hietanen JK. Depression biases the recognition of emotionally neutral faces. Psychiatry Research. 2004;128:123–133. doi: 10.1016/j.psychres.2004.05.020. https://doi.org/10.1016/j.psychres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: Affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. https://doi.org/10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Satpute AB, Wager TD, Weber J, Barrett LF. The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cerebral Cortex. 2016;26:1910–1922. doi: 10.1093/cercor/bhv001. https://doi.org/10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton MF, Weeks JW, De Los Reyes A. Individual differences in fears of negative versus positive evaluation: Frequencies and clinical correlates. Personality and Individual Differences. 2016;98:193–198. https://doi.org/10.1016/j.paid.2016.03.072. [Google Scholar]
- Liu RT, Kraines MA, Massing-Schaffer M, Alloy LB. Rejection sensitivity and depression: Mediation by stress generation. Psychiatry. 2014;77:86–97. doi: 10.1521/psyc.2014.77.1.86. https://doi.org/10.1521/psyc.2014.77.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Marek S, Larsen B, Tervo-Clemmens B, Chahal R. An integrative model of the maturation of cognitive control. Annual Review of Neuroscience. 2015;38:151–170. doi: 10.1146/annurev-neuro-071714-034054. https://doi.org/10.1146/annurev-neuro-071714-034054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston EG, Hare A, Allen JP. Rejection sensitivity in late adolescence: Social and emotional sequelae. Journal of Research on Adolescence. 2010;20:959–982. doi: 10.1111/j.1532-7795.2010.00675.x. https://doi.org/10.1111/j.1532-7795.2010.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4:143–157. doi: 10.1093/scan/nsp007. https://doi.org/10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Hatzenbuehler ML, Hilt LM. Emotion dysregulation as a mechanism linking peer victimization to internalizing symptoms in adolescents. Journal of Consulting and Clinical Psychology. 2009;77:894–904. doi: 10.1037/a0015760. https://doi.org/10.1037/a0015760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Ochsner KN. The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience. 2012;7:11–22. doi: 10.1093/scan/nsr093. https://doi.org/10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonterah CW, Hahn NC, Utsey SO, Hook JN, Abrams JA, Hubbard RR, Opare-Henako A. Fear of negative evaluation as a mediator of the relation between academic stress, anxiety and depression in a sample of Ghanaian college students. Psychology and Developing Societies. 2015;27:125–142. https://doi.org/10.1177/0971333614564747. [Google Scholar]
- O’Connor LE, Berry JW, Weiss J, Gilbert P. Guilt, fear, submission, and empathy in depression. Journal of Affective Disorders. 2002;71:19–27. doi: 10.1016/s0165-0327(01)00408-6. https://doi.org/10.1016/s0165-0327(01)00408-6. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Masten CL, Moore WE, 3rd, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: Resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. https://doi.org/10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:829, 833–857. doi: 10.1038/mp.2008.65. https://doi.org/10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. https://doi.org/10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Prinstein MJ, Cheah CS, Guyer AE. Peer victimization, cue interpretation, and internalizing symptoms: Preliminary concurrent and longitudinal findings for children and adolescents. Journal of Clinical Child and Adolescent Psychology. 2005;34:11–24. doi: 10.1207/s15374424jccp3401_2. https://doi.org/10.1207/s15374424jccp3401_2. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychological Bulletin. 2002;128:330–366. https://doi.org/10.1037//0033-2909.128.2.330. [PubMed] [Google Scholar]
- Rigby K. The Peer Relations Questionnaire (PRQ): Professional reading guide for educational administrators. Point Lonsdale, Vic., Australia: Geelong; 1998. [Google Scholar]
- Rigby K, Slee PT. Dimensions of interpersonal relation among Australian children and implications for psychological well-being. Journal of Social Psychology. 1993;133:33–42. doi: 10.1080/00224545.1993.9712116. https://doi.org/10.1080/00224545.1993.9712116. [DOI] [PubMed] [Google Scholar]
- Rossignol M, Campanella S, Bissot C, Philippot P. Fear of negative evaluation and attentional bias for facial expressions: An event-related study. Brain and Cognition. 2013;82:344–352. doi: 10.1016/j.bandc.2013.05.008. https://doi.org/10.1016/j.bandc.2013.05.008. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Bukowski W, Parker J. Peer interactions, relationships, and groups. In: Eisenberg N, editor. Social, emotional, and personality development. 6th. Vol. 3. New York, NY: Wiley; 2006. pp. 571–645. (Handbook of child psychology). [Google Scholar]
- Rudolph KD, Flynn M, Abaied JL. A developmental perspective on interpersonal theories of youth depression. In: Abela JRZ, Hankin BL, editors. Handbook of depression in children and adolescents. New York, NY: Guilford Press; 2008. pp. 79–102. [Google Scholar]
- Scherer KR. Appraisal considered as a process of multilevel sequential checking. In: Scherer KR, Schorr A, Johnstone T, editors. Appraisal processes in emotion: Theory, methods, research. New York, NY: Oxford University Press; 2001. pp. 92–120. [Google Scholar]
- Schwarz KA, Wieser MJ, Gerdes AB, Muhlberger A, Pauli P. Why are you looking like that? How the context influences evaluation and processing of human faces. Social Cognitive and Affective Neuroscience. 2013;8:438–445. doi: 10.1093/scan/nss013. https://doi.org/10.1093/scan/nss013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeber LB, Allen NB, Leve C, Davis B, Wu Shortt J, Katz LF. Dynamics of affective experience and behavior in depressed adolescents. Journal of Child Psychology and Psychiatry. 2009;50:1419–1427. doi: 10.1111/j.1469-7610.2009.02148.x. https://doi.org/10.1111/j.1469-7610.2009.02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: Event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biological Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. https://doi.org/10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Silk JS, Dahl RE, Ryan ND, Forbes EE, Axelson DA, Birmaher B, Siegle GJ. Pupillary reactivity to emotional information in child and adolescent depression: Links to clinical and ecological measures. American Journal of Psychiatry. 2007;164:1873–1880. doi: 10.1176/appi.ajp.2007.06111816. https://doi.org/10.1176/appi.ajp.2007.06111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Forbes EE, Whalen DJ, Jakubcak JL, Thompson WK, Ryan ND, Dahl RE. Daily emotional dynamics in depressed youth: A cell phone ecological momentary assessment study. Journal of Experimental Child Psychology. 2011;110:241–257. doi: 10.1016/j.jecp.2010.10.007. https://doi.org/10.1016/j.jecp.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience. 2014;9:1798–1807. doi: 10.1093/scan/nst175. https://doi.org/10.1093/scan/nst175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Whalen DJ, Ostapenko LJ, Ladouceur CD, Dahl RE. Pubertal changes in emotional information processing: Pupillary, behavioral, and subjective evidence during emotional word identification. Development and Psychopathology. 2009;21:7–26. doi: 10.1017/S0954579409000029. https://doi.org/10.1017/S0954579409000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents’ emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74:1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. https://doi.org/10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JD, Gross JJ, Remy KA, Ochsner KN. Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion. 2012;12:1235–1247. doi: 10.1037/a0028297. https://doi.org/10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeting H, Young R, West P, Der G. Peer victimization and depression in early-mid adolescence: A longitudinal study. British Journal of Educational Psychology. 2006;76:577–594. doi: 10.1348/000709905X49890. https://doi.org/10.1348/000709905X49890. [DOI] [PubMed] [Google Scholar]
- Telzer EH, Qu Y, Goldenberg D, Fuligni AJ, Galvan A, Lieberman MD. Adolescents’ emotional competence is associated with parents’ neural sensitivity to emotions. Frontiers in Human Neuroscience. 2014;8:558. doi: 10.3389/fnhum.2014.00558. https://doi.org/10.3389/fnhum.2014.00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. https://doi.org/10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Touroutoglou A, Lindquist KA, Dickerson BC, Barrett LF. Intrinsic connectivity in the human brain does not reveal networks for ‘basic’ emotions. Social Cognitive and Affective Neuroscience. 2015;10:1257–1265. doi: 10.1093/scan/nsv013. https://doi.org/10.1093/scan/nsv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troop-Gordon W, Rudolph KD, Sugimura N, Little TD. Peer victimization in middle childhood impedes adaptive responses to stress: A pathway to depressive symptoms. Journal of Clinical Child and Adolescent Psychology. 2015;44:432–445. doi: 10.1080/15374416.2014.891225. https://doi.org/10.1080/15374416.2014.891225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks JW, Heimberg RG, Fresco DM, Hart TA, Turk CL, Schneier FR, Liebowitz MR. Empirical validation and psychometric evaluation of the Brief Fear of Negative Evaluation Scale in patients with social anxiety disorder. Psychological Assessment. 2005;17:179–190. doi: 10.1037/1040-3590.17.2.179. https://doi.org/10.1037/1040-3590.17.2.179. [DOI] [PubMed] [Google Scholar]
- Winton EC, Clark DM, Edelmann RJ. Social anxiety, fear of negative evaluation and the detection of negative emotion in others. Behavior Research and Therapy. 1995;33:193–196. doi: 10.1016/0005-7967(94)e0019-f. https://doi.org/10.1016/0005-7967(94)e0019-f. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Yap MB, Schwartz OS, Byrne ML, Simmons JG, Allen NB. Maternal positive and negative interaction behaviors and early adolescents’ depressive symptoms: Adolescent emotion regulation as a mediator. Journal of Research on Adolescence. 2010;20:1014–1043. https://doi.org/10.1111/j.1532-7795.2010.00665.x. [Google Scholar]
- Zeman J, Shipman K, Penza-Clyve S. Development and initial validation of the Children’s Sadness Management Scale. Journal of Nonverbal Behavior. 2001;25:187–205. https://doi.org/10.1023/A:1010623226626. [Google Scholar]


