Abstract
Although breast cancer is becoming more prevalent in Africa, few epidemiologic studies have been undertaken and appropriate methodologic approaches remain uncertain. We therefore conducted a population-based case-control study in Accra and Kumasi, Ghana, enrolling 2,202 women with lesions suspicious for breast cancer and 2,161 population controls. Biopsy tissue for cases prior to neoadjuvant therapy (if given), blood, saliva and fecal samples were sought for study subjects. Response rates, risk factor prevalences, and odds ratios (OR) for established breast cancer risk factors were calculated. A total of 54.5% of the recruited cases were diagnosed with malignancies, 36.0% with benign conditions and 9.5% with indeterminate diagnoses. Response rates to interviews were 99.2% in cases and 91.9% in controls, with the vast majority (82-99%) of interviewed subjects providing blood and saliva samples; lower proportions (46-58%) provided fecal samples. While risk factor prevalences were quite unique as compared to women in other countries (e.g., less education, higher parity), cancer risk factors resembled patterns identified elsewhere (elevated risks associated with higher levels of education, familial histories of breast cancer, low parity, and larger body sizes). Subjects with benign conditions were younger and exhibited higher socioeconomic profiles (e.g., higher education, lower parity) than those with malignancies, suggesting selective referral influences. While further defining breast cancer risk factors in Africa, this study showed that successful population-based interdisciplinary studies of cancer in Africa are possible but require close attention to diagnostic referral biases and standardized and documented approaches for high-quality data collection, including biospecimens.
Keywords: breast cancer, epidemiology, risk factors, Africa
INTRODUCTION
Although historically low, breast cancer incidence is rapidly rising throughout Africa (1). Well-designed molecular epidemiologic studies are critical to enable public health systems to prepare and develop strategies to tackle this growing burden of cancer. Some of the increased incidence likely reflects that women are living longer and adopting lifestyles that favor higher incidence rates (e.g., decreased fecundity, more obesity) (2), but there could also be unique risk factors. Only a few well-designed epidemiologic studies of breast cancer have been conducted in Africa and the potential risk factors that have been examined have been limited mainly to reproductive and anthropometric variables (3–9). Further, although a few studies have examined biologic factors that might affect some of these risk factors (10–12), there are many more biomarkers worthy of consideration, including a wide variety of genetic, hormonal and immunologic markers that are increasingly enhancing our understanding of the biologic underpinnings of breast cancer.
Breast tumors occurring among African women tend to be diagnosed approximately a decade earlier than among Caucasian women in westernized countries [reviewed in (13)]. Although this may reflect the truncated age distributions in Africa (14), data consistently show that African-American women are also diagnosed with earlier onset breast cancer. This suggests that there may be distinctive genetic or environmental factors involved (15). In addition, African women tend to present with advanced diseases and high prevalences of estrogen receptor negative and basal-like cancers (13). The extent to which this may reflect tissue fixation or molecular subtyping classification problems has yet to be determined. Thus, acquisition of tumor material for accurate immuno-histochemical classification, and assessment of risk factors according to molecular tumor characteristics is essential for furthering our understanding of etiologic determinants (16).
To assess factors underlying breast cancer increases in Africa, etiologic heterogeneity of diagnosed tumors, and reasons for the common presentation of poor prognostic tumors, we launched the Ghana Breast Health Study (GBHS). We describe herein the design components of our study with the hopes that some of our challenges and successes may be useful in guiding others who wish to undertake population-based studies in African or other developing countries.
METHODS
Ghana was chosen as the site for study given its political stability, that English is the primary language for professional communications, and that many African-Americans originated from West Africa, prompting interest in comparability of genetic markers with African-American women (17–19). Furthermore, the National Cancer Institute (NCI) previously conducted a study of prostate cancer in Accra (20), which established certain aspects of the infrastructure for this study.
The lack of opportunities for systematic follow-up prompted use of a case-control rather than a cohort design. The case-control design was also better suited given the multidisciplinary orientation of the investigation and the desire to collect biologic samples from a large number of both diseased and non-diseased women.
Source of Breast Cancer Patients
The source of breast cancer cases included the two largest teaching/cancer treatment hospitals in the country, Korle Bu Teaching Hospital in Accra and Komfo Anoyke Teaching Hospital in Kumasi, as well as Peace and Love Hospital, a private hospital in Kumasi that specializes in breast cancer care and diagnoses and treats a large number of breast cancer patients. Although a few private facilities in Accra diagnose breast cancer, at the beginning of our study these accounted for only a minority of breast cancer cases in the metropolitan area and were not included in our study.
Eligibility criteria for study cases included being recommended for a biopsy given lesions suspicious of malignancy or presenting at a study hospital for treatment of pathologically-documented breast cancers diagnosed within the preceding year. Given that there are no organized mammographic screening programs in Ghana, most women were recommended for biopsy on the basis of clinical symptoms, with many women presenting with large tumor masses (e.g., 62.4% presented with tumors >5 cm) (21). Subjects also had to be between 18-74 years of age and have lived in defined study catchment areas for at least a year’s time. The catchment areas were based on their proximity to the study hospitals (generally ≤30 minutes of travel time) and the number of patients from these areas that had recently sought care at the study hospitals. The catchment area initially included 13 districts in Accra and 7 in Kumasi, but were expanded to 2 additional districts near Kumasi after the first six months to capture additional cases.
Source of Controls
Considerable deliberation was spent determining the most appropriate controls for the study. We decided against hospital controls given they would likely be ill and have unusual prevalences of risk factors of interest. Regional health clinic controls were also considered sub-optimal given uncertain referral patterns and the potential for deriving comparison subjects from different catchment areas than the cases. Given that we anticipated that the vast majority of breast cancer cases would be residents of Accra and Kumasi, population-based controls seemed the most desirable option. We therefore developed procedures for identifying a source of population controls, within the two study areas (Accra and Kumasi), who could be frequency matched to the cases on age with similar restrictions regarding catchment areas and at least one year of residence in these areas.
A national census conducted in Ghana in 2010 was the basis for defining the catchment areas. Since we were unable to obtain names and addresses of potential study subjects through the census, we launched our own survey research, which built upon the census by using randomly selected census enumeration areas (EA) from the districts from which cases were expected to derive. In brief, all residences in selected EAs—geographic areas generally comprised of approximately 750 residents–were enumerated by trained census workers with respect to the sexes and ages of the residents. This information was facilitated by the fact that individuals live in close proximity to their neighbors in compound housing units (a collection of bedrooms clustered around common areas). When households were enumerated, a brochure was left explaining the study and encouraging participation should an individual be selected for inclusion. After selected EAs had been enumerated, individuals were randomly selected to approximate the age distribution of female breast cancers expected to be diagnosed during the study. Similar age and duration of residency criteria were used for controls as were used for cases. Study personnel visited subjects’ homes to determine eligibility, inform them of study selection, and invite them for a hospital visit.
Developing Infrastructure for the Study
The success of the study was dependent on an emphasis on leadership and coordination of activities, which was achieved by having well-coordinated teams at each of the study hospitals. Full-time study managers oversaw all activities and assured coordination across the many individuals who contributed to the success of the investigation, including interviewers, nurses, phlebotomists, and laboratory technicians. In addition, activities were regularly monitored by a central coordinating center in the U.S.
To enable collection of high-quality biological samples, dedicated study equipment such as centrifuges, tissue embedders, microtome processors, freezers, and backup generators were purchased. Most of these were purchased outside of the country and shipped to Ghana, oftentimes with complicated customs clearance procedures. Establishing proper electrical connections often posed technical challenges, resulting in delays in processing and storage of samples.
Each individual chosen for the study was assigned a unique participant identification number and all study forms were linked via this number, using pre-labelled stickers affixed to the forms. To assist in keeping track of samples, a bar coding system, implemented by the NCI Biospecimen Inventory (BSI) system (http://www.bsisystems.com/) was utilized, along with assembled kits that contained all collection devices, each of which was pre-labelled with the appropriate bar code for accurate specimen tracking.
Data Collection
The primary data for the study were collected via a standardized interview-based questionnaire, which focused on breast cancer risk factors identified in non-African settings, as well as on a number of novel exposures that have been described as possibly especially pertinent to Africa (1). The questionnaire also incorporated a number of questions to address reasons for delays in seeking hospital treatment for signs/symptoms of breast cancer, including visits to traditional healers.
Prior to finalizing the questionnaire, focus groups were held among Ghanaian women in the U.S. and in Ghana to determine relevant etiologic hypotheses and appropriate questions. The instrument was also pilot tested in Ghana among women of appropriate ages. The questionnaire was designed to obtain information on both established and speculative breast cancer risk factors. Although both Twi and Ga are common languages in Ghana, they are generally spoken rather than written languages, necessitating use of an English questionnaire with interviewers translating questions into Twi as needed (periodic discussions between interviewers involved sharing information regarding the best Twi words to address certain concepts). A limited number of controls (n=10) who could only respond to the questionnaire in Ga or other dialects were ineligible for study inclusion. All questionnaires were administered after obtaining written informed consent on forms approved by institutional review boards in the U.S. and Ghana.
Interviewers were uniformly trained in a week-long intensive training session conducted by personnel from the NCI and the study’s coordinating center, Westat, Inc. prior to study initiation. Detailed manuals with instructions for administering questionnaires, completing case abstract forms, and taking anthropometric measurements were reviewed. These sessions emphasized the importance of asking questions exactly as written, of not leading to the respondents to answers questions in any specific way, and in documenting unusual situations through detailed written comments. Most questions required recording pre-categorized responses, although a few asked for additional information to be specified. Although the goal was to administer the entire questionnaire to all eligible subjects, a limited number of essential questions were deemed ‘critical questions’ to ask of subjects who were reluctant to complete the entire questionnaire.
Attempts were made to interview all subjects at the hospital in order to have a private setting for data collection and facilitate collection of biologic samples. The study also involved the collection of various anthropometric measurements, for which a private setting was desirable. To assist with control subject visits to the hospitals, either transportation (via hospital vehicles) or transportation costs were provided; at the hospital a snack was provided.
After administration, all questionnaires were checked by a study manager for completeness and readability of the responses. The questionnaires were then double key punched, scanned, and transmitted electronically to the U.S. study coordinating center. This enabled ‘real-time’ review of materials, with immediate correction of any problems encountered.
Collection of Biologic Samples
Saliva samples for DNA were collected via Oragene DNA OG-500 kits (DNA Genotek Inc., Ottawa, Canada). The study also involved collection of 20 mL of blood, which was subsequently processed into aliquots of plasma, serum, buffy coat, red blood cells, and clot. Collection procedures were approached similarly for cases and controls in terms of time of day of collection (which impacted whether samples were fasting or not), as well as with respect to time to processing after sample collection. Approximately nine months after the study began, we implemented fecal sample collection. We used vials containing RNALater to collect fecal samples, which for various logistical concerns were collected when subjects visited the hospitals.
Given that many Ghanaian women present with advanced breast cancers and are given neoadjuvant therapy, we focused our efforts on enrolling potential cases at the time of their biopsies, prior to an assignment of a pathologic diagnosis. This resulted in the inclusion of both malignant and non-malignant (benign) cases in the study. Since women commonly present with large tumors, core biopsies were predominantly used to obtain tissue samples. At one of the facilities, a U.S. surgeon provided specialized training prior to study initiation since some of the surgeons there did not have experience in taking core biopsies. Between 4-8 cores were taken for diagnostic purposes and processed into formalin-fixed paraffin-embedded blocks; blocks not needed for diagnoses were sent to NCI for research purposes.
Handling, Processing and Shipment of Biologic Specimens
Although most of the technicians for the study were already familiar with standard laboratory procedures, to assure standardization across all sties we conducted training related to specific procedures for handling, processing and storing study samples. Given that most assays of collected samples were to be performed in the U.S., the study also entailed periodic shipments of materials. Most of the samples to be shipped needed to remain frozen at −80° C. Shipments were made every 3-4 months, with extensive oversight as to when the samples left Ghana and were received at the U.S. biorepository. The shipments were accompanied by detailed sample manifestos, and there was careful accounting of the numbers and types of vials sent and received.
Community Support
Field efforts were strengthened by assuring that there was support for the investigation from community, church and tribal leaders. This included legitimizing the activities of the field staff by providing them with study uniforms. Interviews were occasionally given to the press in order to garner additional support for its implementation.
Collaborative Arrangements
A major emphasis throughout the investigation was on having well-defined collaborative arrangements in terms of responsibilities and expectations and for there to be frequent communications between all team members. This was facilitated by frequent communications between the U.S. and Ghanaian investigators, especially at the beginning phases of the study, and for there to be written documentation of agreed-upon decisions. Principal investigator meetings and site visits were held annually and a steering committee was developed to oversee study arrangements and plans for analysis and publications.
Preliminary Analyses of Data
In initial analyses, we focused on breast cancer risk factors that have been well established in other populations and that were sufficiently prevalent in our study, calculating odds ratios (OR) and 95% confidence intervals (CI) via logistic regression.
RESULTS
Of the 2,676 cases initially approached for study, 458 (17.1%) were deemed ineligible, primarily because of inadequate suspicion of cancer or they did not live in the catchment area (Table 1). 6.9% of the controls approached for study inclusion were found ineligible, primarily due to wrong or non-locatable addresses or the subject having moved.
Table 1.
Number of Subjects Approached and Eligible for Study and Response Rates to Interview Among the Eligible Subjects, Ghana Breast Health Study
|
|
||||
|---|---|---|---|---|
| Cases | Controls | |||
| Number | Percent | Number | Percent | |
| Approached | 2,676 | 2,528 | ||
| Not eligible | 458 | 176 | ||
| Inadequate grounds for suspicion of breast cancer | 34 | |||
| Not biopsied Unknown reason for no biopsy | 9 | |||
| Not age 18-74 years | 52 | 9 | ||
| Does not live in catchment area | 261 | 4 | ||
| Not lived in catchment area ≥ 1 year | 16 | 3 | ||
| Diagnosed > 1 year ago | 72 | |||
| Wrong address or could not be located | 0 | 70 | ||
| Relocated/moved from the area | 0 | 54 | ||
| Wrong person, male, duplicate | 2 | 9 | ||
| Deceased | 0 | 5 | ||
| Not on EA list | 0 | 3 | ||
| Language, hearing, speaking problem | 5 | 10 | ||
| Mentally incompetent | 6 | 2 | ||
| Comorbidity issue | 1 | 7 | ||
|
| ||||
| Eligible (Approached minus not eligible) | 2,218 | 2,352 | ||
|
| ||||
| Non-response | 1 | 0.1 | 17 | 0.7 |
| Consented by study but not completed | 1 | 0 | ||
| Could not be located after ≥5 attempts | 0 | 12 | ||
| Subject unavailable | 0 | 5 | ||
| Refused | 15 | 0.7 | 174 | 7.4 |
| Enrolled | 2,202 | 99.2 | 2,161 | 91.9 |
Response rates achieved to the various components of the study among eligible study subjects are shown in Table 1. The questionnaire response rates were high, especially among the cases—where we were able to interview 2,202 women and achieve a 99.2% response rate. Among the controls, there were some initial refusals, primarily reflecting subjects having insufficient time to travel to the hospital. Thus, allowing occasional interviews to be conducted in the field helped to improve response rates, although it did present challenges for collection and processing of biologic samples. After refusal conversion techniques, we were able to successfully interview 2,161 women, (91.9% of eligible controls), with the interview refusal rate being 6.5%.
The mean time to complete the questionnaire was 41 minutes among cases and 38 among controls. A total of 93.9% of respondents completed all questions, with 5.5% agreeing to answer only the critical questions. A total of 96.0% of the subjects were judged by the interviewers to be cooperative and 99.5% of the interviews were noted as generally reliable or of high quality.
Table 2 presents collection rates among interviewed subjects of anthropometric measurements and various biologic samples. Nearly all of the participants agreed to have anthropometric measurements taken (96.0% for cases, 99.5% of controls) and to provide saliva samples (97.9% for cases, 98.8% for controls). Lower response rates were seen for the collection of blood samples (91.8% for cases, 82.5% for controls). The lower rate among controls reflected to some extent the use of conversion refusal techniques, with phlebotomists not always accompanying the interviewers to the field.
Table 2.
Participation Rates to Various Ancillary Components of the Ghana Breast Health Study Among Enrolled Subjects
|
|
||||
|---|---|---|---|---|
| Cases | Controls | |||
| Number | Percent | Number | Percent | |
| Enrolled Subjects | 2,202 | 100.0 | 2,161 | 100.0 |
| Anthropometric measurements | ||||
| Yes | 2,115 | 96.0 | 2,151 | 99.5 |
| No | 87 | 4.0 | 10 | 0.5 |
| Saliva samples | ||||
| Yes | 2,155 | 97.9 | 2,134 | 98.8 |
| No | 47 | 2.1 | 27 | 1.2 |
| Blood samples | ||||
| Yes | 2,021 | 91.8 | 1,782 | 82.5 |
| No | 181 | 8.2 | 379 | 17.5 |
| Stool samples | ||||
| Yes | 968 | 58.11 | 645 | 46.12 |
| No | 699 | 41.9 | 754 | 53.9 |
| N/A (protocol not initiated) | 535 | 762 | ||
| Biopsy tissue | ||||
| Yes, pathologic diagnosis rendered | 1873 | 85.1 | ||
| Yes, inadequate for pathology3 | 145 | 6.6 | ||
| Yes, missing pathology report | 29 | 1.3 | ||
| Diagnosis confirmed in other ways4 | 81 | 3.7 | ||
| No biopsy attempted5 | 53 | 2.4 | ||
| Unknown biopsy status | 21 | 0.9 | ||
Used denominator of 1,667 (sample collection after protocol was initiated) for calculation of response rate.
Used denominator of 1,399 (sample collection after protocol was initiated) for calculation of response rate.
39 cases had inadequate tissue for pathologic diagnosis but were considered malignant on basis of clinical manifestations.
Diagnosed at a non-study facility (n=60) or pathology obtained from procedure other than study intake core biopsy (e.g., fine needle aspirate, excisional biopsy, mastectomy) (n=21)
Includes 33 patients whose lump was impalpable or too small to biopsy (n=33), 1 patient with a mass too tender to biopsy, and 19 patients who refused a biopsy.
The collection of stool samples showed response rates, among those approached for these specimens, of 58.1% among the cases and 46.1% among the controls. The response rates were particularly low at the beginning phases of collection but improved over time as staff became more familiar with successful collection techniques. Tissue samples were acquired from the vast majority of cases approached for biopsies (93.0%), with the reasons for non-collection being that they had a biopsy before coming to the study hospitals and/or were on chemotherapy, they did not have a palpable lump, their lesion was too small, or they refused the biopsy.
Table 3 shows that 52.8% of the cases were pathologically confirmed as malignant, with an additional 1.7% showing clinical manifestations indicative of malignancy. A total of 36.0% were pathologically confirmed as benign, and 9.5% having indeterminate diagnoses, with the vast majority of these due to inadequate tissue. The proportion of malignant diagnoses increased with age, whereas benign diagnoses declined.
Table 3.
Case Status Based on Attempted Pathologic Review of Tissue Samples, Ghana Breast Health Study
| All patients N=2,202 | Percent | 18–39 yrs N = 7681 |
Percent | 40–49 yrs N = 6051 |
Percent | ≥50 yrs N = 8231 |
Percent | |
|---|---|---|---|---|---|---|---|---|
| Malignancy | 1162 | 52.8 | 240 | 31.3 | 340 | 56.2 | 578 | 70.2 |
| Malignancy without pathologic confirmation2 | 39 | 1.7 | 8 | 1.0 | 13 | 2.1 | 18 | 2.2 |
| Benign conditions | 792 | 36.0 | 440 | 57.3 | 198 | 32.7 | 152 | 18.5 |
| Indeterminate pathology | 209 | 9.5 | 80 | 10.4 | 54 | 8.9 | 75 | 9.1 |
| Inadequate tissue | 106 | 4.8 | 37 | 4.8 | 24 | 4.0 | 45 | 5.5 |
| Missing or conflicting information | 50 | 2.3 | 23 | 3.0 | 13 | 2.1 | 14 | 1.7 |
| No biopsy attempted | 53 | 2.4 | 20 | 2.6 | 17 | 2.8 | 16 | 1.9 |
Excluded from the age-specific columns were 6 patients with unknown ages.
Considered malignant on the basis of clinical manifestations, but tissue samples were inadequate for pathology.
The distribution of established breast cancer risk factors according to case status is shown in Table 4. The majority of the controls had limited education, multiple births that began at early ages, and late ages at menarche. Relatively small proportions of controls reported a family history of breast cancer. Patients who were diagnosed with benign breast conditions or had indeterminate pathology showed some distinctions from the malignant cancer cases in terms of being younger, more educated, nulliparous, and having earlier ages at menarche.
Table 4.
Percentage Distributions of Demographic and Risk Factors Among Controls and Women Biopsied on Suspicion of Breast Cancer in the Ghana Breast Health Study
| Study Characteristics | Controls (N=2,161) |
Malignancies (N=1,201) |
Benign Conditions (N=792) |
Indeterminate Pathology (N=209) |
|---|---|---|---|---|
| Age (years) | ||||
| < 35 | 20.5 | 9.7 | 41.9 | 27.3 |
| 35-44 | 26.9 | 24.5 | 26.8 | 24.4 |
| 45-54 | 26.1 | 30.0 | 18.7 | 23.0 |
| ≥ 55 | 26.1 | 35.6 | 12.4 | 25.4 |
| Unknown | 0.5 | 0.3 | 0.3 | 0.0 |
| Medians | 45 | 49 | 38 | 44 |
| Education | ||||
| No formal education | 23.9 | 22.7 | 12.2 | 12.4 |
| Primary school | 17.4 | 13.7 | 7.8 | 11.0 |
| Junior secondary school | 30.7 | 23.5 | 23.7 | 26.3 |
| ≥ Senior secondary school | 24.6 | 33.3 | 52.9 | 44.0 |
| Unknown | 3.4 | 6.7 | 3.3 | 6.2 |
| Family history of breast cancer | ||||
| No | 96.7 | 91.8 | 91.2 | 91.4 |
| Yes | 2.2 | 7.0 | 7.4 | 7.7 |
| Unknown | 1.1 | 1.2 | 1.4 | 1.0 |
| Parity | ||||
| Nulliparous | 10.7 | 9.2 | 30.3 | 23.4 |
| 1-2 | 26.1 | 28.5 | 30.6 | 29.2 |
| 3-4 | 32.3 | 32.6 | 24.6 | 26.8 |
| ≥ 5 | 30.4 | 29.2 | 14.3 | 20.1 |
| Unknown | 0.4 | 0.4 | 0.3 | 0.5 |
| Age at menarche (years) | ||||
| < 15 | 27.9 | 23.8 | 38.3 | 32.1 |
| 15 | 25.7 | 22.4 | 24.0 | 21.1 |
| 16 | 18.0 | 19.7 | 16.9 | 16.7 |
| ≥ 17 | 18.5 | 20.6 | 13.8 | 18.2 |
| Unknown | 9.9 | 13.4 | 7.1 | 12.0 |
| Age at menopause (years) | ||||
| Premenopausal | 57.6 | 42.2 | 75.8 | 61.7 |
| < 45 | 7.9 | 11.4 | 6.8 | 8.6 |
| 45-49 | 12.4 | 16.4 | 6.8 | 9.1 |
| 50-54 | 13.7 | 18.2 | 7.2 | 12.9 |
| ≥ 55 | 3.2 | 5.1 | 1.0 | 1.4 |
| Unknown | 5.2 | 6.7 | 2.4 | 6.2 |
| Body size1 | ||||
| Slight | 27.3 | 22.7 | 36.0 | 28.2 |
| Average | 39.8 | 38.2 | 38.6 | 39.2 |
| Slightly heavy | 22.5 | 22.8 | 17.4 | 18.7 |
| Very heavy | 7.6 | 9.3 | 4.5 | 6.2 |
| Unknown | 2.8 | 6.9 | 3.4 | 7.7 |
Body shapes classified according to 9-scale pictograms, with 1 corresponding to the slimmest figure and 9 to the heaviest figure. For purposes of this analysis pictograms were classified as follows: Slight = 1 and 2, average = 3 and 4, slightly heavy = 5 and 6, and heavy = 7, 8 and 9.
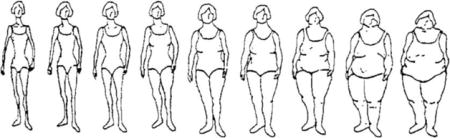
Preliminary analyses of risk factors (Table 5) demonstrated significantly elevated risks associated with high levels of education (OR=1.50, 95% CI 1.21–1.87 for ≥senior secondary school vs. no formal education; a family history of breast cancer (2.97, 2.04–4.33), and heavy vs. slight body sizes (1.50, 1.11–2.02). Women who had 5 or more births were at a significantly reduced risk compared with nulliparous women (OR=0.71, 95% CI 0.52–0.97). Age at menarche was not significantly associated with risk, although a large number of women were unable to recall this information.
Table 5.
Role of Established Breast Cancer Risk Cancers for Malignancies, Ghana Breast Health Study
| Study Characteristics | Controls (N=2,161) |
Malignancies (N=1,201) |
OR1 | 95% CI |
|---|---|---|---|---|
| Education | ||||
| No formal education | 517 | 273 | 1.00 | Referent |
| Primary school | 375 | 165 | 0.84 | 0.66-1.08 |
| Junior secondary school | 664 | 282 | 0.99 | 0.80-1.23 |
| ≥ Senior secondary school | 532 | 400 | 1.50 | 1.21-1.87 |
| Unknown | 73 | 81 | ||
| P-trend | <0.01 | |||
| Family history of breast cancer | ||||
| No | 2089 | 1102 | 1.00 | Referent |
| Yes | 48 | 84 | 2.97 | 2.04-4.33 |
| Unknown | 24 | 15 | ||
| Parity | ||||
| Nulliparous | 232 | 111 | 1.00 | Referent |
| 1-2 | 565 | 342 | 1.05 | 0.79-1.40 |
| 3-4 | 698 | 392 | 0.81 | 0.60-1.08 |
| ≥ 5 | 658 | 351 | 0.71 | 0.52-0.97 |
| Unknown | 8 | 5 | ||
| P-trend | <0.01 | |||
| Age at menarche (years) | ||||
| < 15 | 603 | 286 | 1.00 | Referent |
| 15 | 555 | 269 | 0.93 | 0.75-1.15 |
| 16 | 390 | 237 | 1.21 | 0.96-1.52 |
| ≥ 17 | 400 | 248 | 1.24 | 0.99-1.55 |
| Unknown | 213 | 161 | ||
| P-trend | 0.02 | |||
| Body size | ||||
| Slight | 590 | 273 | 1.00 | Referent |
| Average | 859 | 459 | 1.15 | 0.95-1.40 |
| Slightly heavy | 487 | 274 | 1.30 | 1.04-1.62 |
| Heavy | 164 | 112 | 1.50 | 1.11-2.02 |
| Unknown | 61 | 83 | ||
| P-trend | 0.01 |
ORs adjusted for study site and categorical ages as well as all factors in table.
DISCUSSION
In the GBHS, we demonstrated that it is possible to conduct a successful interdisciplinary population-based case-control study in an African setting. Facilitated by a recent census, we were able to identify well-defined geographic areas that could be enumerated for household listings to randomly select population controls. In contrast to challenges encountered with poor participation within epidemiologic studies in high-income countries (22, 23), we found that we could achieve excellent response rates to a detailed interview-based questionnaire, with only slightly lower response rates for controls than cases.
Despite the excellent interview response rates, there were challenges involved in identifying appropriate patients to approach for recruitment. This reflected that medical referral patterns in Africa are not well defined and that many women with breast cancer may receive care from traditional healers (24, 25) and/or experience significant delays in seeking medical assistance at a hospital (26). Although we believe that the vast majority of women with breast cancer will eventually make it to hospital, we could not verify this. It is thus possible that there were some referral biases inherent in the cases included; for instance, women of the lowest social classes might not have preferentially been excluded, which could have affected our derived risk factors. In addition, given other factors which could have prevented all cases in the two study sites from having been identified (e.g., some patients in Accra might not have been ascertained if they sought private care), it would have been desirable to compare our included cases with cancer registry records. However, cancer registration is in its infancy in most African countries (27), including Ghana, although efforts are underway to overcome this limitation (28, 29).
Determining the most appropriate controls to use in a case-control investigation in a low-resource setting is always challenging (30, 31), and was no exception in this study. Although hospitals controls have been successfully used in other investigations in Africa, given selected referral patterns in our investigation (including patients with non-malignant conditions presenting at our study hospitals being from different geographic areas than those with maligiancies), we did not feel that they would be optimal. We were thus fortunate to be able to utilize population controls given that they avoid the inherent biases associated with other types of controls (including hospital controls, who may have unusual prevalences of certain risk factors). However, identification of population controls was quite labor intensive, even with the availability of recent census data.
One of the more challenging aspects of the study centered around the desire to obtain breast tissue samples from cases prior to neoadjuvant therapy, which was quite common given that many women presented with advanced diseases. As documented in many African settings (32), pathologic diagnoses were often delayed, necessitating that patients be enrolled prior to the assignment of a diagnosis. This resulted in a number of recruited study subjects not being confirmed with malignancies. In addition, the benign cases diagnosed at our hospitals specializing in cancer care were younger than the women with malignancies and demonstrated high socioeconomic risk profiles (e.g., higher levels of education, more nulliparity or late ages at first birth), likely reflecting the influence of a combination of selection and referral biases.
As documented throughout Africa (33), an additional complexity in terms of defining case status centered around the unavailability of mammographic and ultrasound-guided imaging techniques, which affected biopsy accuracy. This sometimes presented difficulties for localizing the area of the breast to biopsy, possibly leading some of the biopsied lesions to be incorrectly labelled as non-malignant. Further, some biopsy samples were deemed inadequate for diagnoses, either because of small tissue amounts or specimens being necrotic. Although we attempted to determine if subsequent surgeries (e.g., excisional biopsies, mastectomies) might provide more definitive diagnoses, many women declined further treatment, which has been shown in other investigations to reflect both financial and social factors (34, 35).
Various challenges are associated with developing a comprehensive questionnaire to evaluate breast cancers in sub-Saharan Africa (36). We expended considerable effort to determine the best approaches for collecting valid information, including consulting with individuals in Ghana who identified impediments to collecting data, as well as opportunities for assessing unique exposures. Some exposures readily evaluated in westernized populations were not possible to collect. For instance, the lack of in-home scales prevented weight recall, leading us to ask subjects to characterize their somatotype using a 9-level pictogram (37) that has previously been related to breast cancer risk (38). In-country cultural expertise also led to the incorporation of questions about novel exposures among Ghanaian women that could potentially affect breast cancer risk, including skin lighteners (39, 40), hair straighteners (41, 42), and DDT exposures (43, 44).
Our initial analyses regarding established breast cancer risk factors among the women diagnosed with malignant conditions demonstrated quite different prevalences than are normally encountered in high-resource countries, including subjects having higher fecundity, later ages at menarche, and less obesity. However, the magnitude of risk associated with most of these factors was remarkably similar to what has been encountered elsewhere, with elevated breast cancer risks associated with higher levels of education, a family history of breast cancer, limited parity, and larger body sizes. These findings generally agree with the few other epidemiologic investigations that have been undertaken in Africa (1, 45, 46). One established risk factor that did not prevail was age at menarche, which has been inversely related to risk in other populations (47); however, the lack of association in our study may have reflected the inability of many women to recall such information. Given that breast cancer is now widely recognized as a heterogeneous condition, with risk factor associations differing according to molecular markers (48), future analyses which focus on risk relations according to breast cancer subtypes should enhance our understanding of breast carcinogenesis among African women. These analyses will enable more in-depth exploration of intermingled risk factors, including parity and breastfeeding, which have been suggested to play especially important etiologic roles for women of African descent (49).
Throughout the study, we placed a major emphasis on capacity building and knowledge transfer to assure that efforts would have long-standing in-country influences (50). Embedded were opportunities for Ghanaian investigators, oftentimes through visitor exchanges, to enhance their knowledge of epidemiology, surgery, pathology, and molecular biology. Since a major emphasis was on accurate pathologic classification and subdivision of breast cancers, we employed U.S. laboratory training to ensure proper methods of obtaining tissue samples and classifying histopathology and tissue marker expression status. Nonetheless, we did face major challenges centered around the provision and maintenance of equipment, including problems with customs clearances, establishing electrical connections, and maintaining the integrity of equipment through frequent electricity outages. Internet connection problems were also a major hindrance, affecting the ability to keypunch data and transfer electronic material back to the U.S. We provided generators for the freezers to assure consistent storage conditions, but the absence of dry ice in Ghana presented challenges for U.S. shipments, requiring liquid nitrogen dewars being sent from other locales. A problem with the maintenance of consistent temperature of samples during one shipment emphasized the importance of not including all vials from a given subject in the same shipment.
The conduct of this study provided a number of valuable lessons regarding issues to consider in launching a large-scale investigation in Africa. Our experience supports that it is possible to successfully conduct a population-based multidisciplinary cancer study in sub-Saharan Africa. Essential study components included standardized approaches documented in manuals, data collection coordinated through detailed inventories, and real-time review of study data and biospecimens to immediately correct problems. Given the extensive amount of data collected, the investigation should provide important insights regarding breast cancer pathogenesis in Africa. Further, given the emphasis on capacity building and knowledge transfer, this investigation should have long-lasting effects, including serving as a model for others who wish to undertake similar investigations under challenging field conditions.
Novelty and Impact.
Breast cancer is becoming more prevalent in Africa, but few epidemiologic studies have been undertaken and appropriate methodologic approaches remain unclear. A novel population-based multidisciplinary case-control study undertaken in Ghana discusses important methodologic considerations, including proper recruitment approaches and means of collecting data. While demonstrating different prevalences of risk factors, the magnitude of associations were similar to those observed elsewhere. The methods outlined indicate that successful studies in Africa are possible, despite many challenges.
Acknowledgments
Funding
This work was supported in part by funding from the intramural research program of the National Cancer Institute, National Institutes of Health. The success of this investigation would not have been possible without exceptional teamwork and the diligence of the field staff who oversaw the recruitment, interviewing and collection of data from study subjects. Special thanks are due to the following individuals: Korle Bu Teaching Hospital, Accra – Dr. Adu-Aryee, Angela Kenu, Obed Ekpedzor, Evelyn Tay; Komfo Anoyke Teaching Hospital, Kumasi – Emmanuel Asiamah, Samuel Ka-chungu; Peace and Love Hospital, Kumasi – Bridget Nortey Mensah, Samuel Amanama, Prince Agyapong, Debora Boateng-Ansong, Thomas Agyei, Richard Opoku, and Kofi Owusu Gyimah. The study was further enhanced by surgical expertise provided by Dr. Lisa Newman of the University of Michigan and by pathological expertise provided by Dr. Stephen Hewitt of the National Cancer Institute. Study management assistance was received from Shelley Niwa, Ricardo Diaz, and Usha Singh at Westat, Inc., and data analysis support from Shannon Wood, Maya Palakal, and Jake Thistle at NCI. Appreciation is also expressed to the many women who agreed to participate in the study and to provide information and biospecimens.
References
- 1.Brinton LA, Figueroa JD, Awuah B, et al. Breast cancer in Sub-Saharan Africa: opportunities for prevention. Breast Cancer Res Treat. 2014;144:467–478. doi: 10.1007/s10549-014-2868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Nambooze S, Wabwire-Mangen F, et al. Changing cancer incidence in Kampala, Uganda, 1991–2006. Int J Cancer. 2010;126:1187–1195. doi: 10.1002/ijc.24838. [DOI] [PubMed] [Google Scholar]
- 3.Coogan PF, Rosenberg L, Shapiro S, et al. Lactation and breast carcinoma risk in a South African population. Cancer. 1999;86:982–989. doi: 10.1002/(sici)1097-0142(19990915)86:6<982::aid-cncr13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Hou N, Ndom P, Jombwe J, et al. An epidemiologic investigation of physical activity and breast cancer risk in Africa. Cancer Epidemiol Biomarkers Prev. 2014;23:2748–2756. doi: 10.1158/1055-9965.EPI-14-0675. [DOI] [PubMed] [Google Scholar]
- 5.Hou N, Ogundiran T, Ojengbede O, et al. Risk factors for pregnancy-associated breast cancer: a report from the Nigerian Breast Cancer Study. Ann Epidemiol. 2013;23:551–557. doi: 10.1016/j.annepidem.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huo D, Adebamowo CA, Ogundiran TO, et al. Parity and breastfeeding are protective against breast cancer in Nigerian women. Br J Cancer. 2008;98:992–996. doi: 10.1038/sj.bjc.6604275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogundiran TO, Huo D, Adenipekun A, et al. Case-control study of body size and breast cancer risk in Nigerian women. Am J Epidemiol. 2010;172:682–690. doi: 10.1093/aje/kwq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okobia M, Bunker C, Zmuda J, et al. Case-control study of risk factors for breast cancer in Nigerian women. Int J Cancer. 2006;119:2179–2185. doi: 10.1002/ijc.22102. [DOI] [PubMed] [Google Scholar]
- 9.Qian F, Ogundiran T, Hou N, et al. Alcohol consumption and breast cancer risk among women in three sub-Saharan African countries. PLoS One. 2014;9:e106908. doi: 10.1371/journal.pone.0106908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fackenthal JD, Zhang J, Zhang B, et al. High prevalence of BRCA1 and BRCA2 mutations in unselected Nigerian breast cancer patients. Int J Cancer. 2012;131:1114–1123. doi: 10.1002/ijc.27326. [DOI] [PubMed] [Google Scholar]
- 11.Okobia MN, Bunker CH, Garte SJ, et al. Leptin receptor Gln223Arg polymorphism and breast cancer risk in Nigerian women: a case control study. BMC Cancer. 2008;8:338. doi: 10.1186/1471-2407-8-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okobia MN, Bunker CH, Garte SJ, et al. Cytochrome P450 1B1 Val432Leu polymorphism and breast cancer risk in Nigerian women: a case control study. Infect Agent Cancer. 2009;4(Suppl 1):S12. doi: 10.1186/1750-9378-4-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eng A, McCormack V, dos-Santos-Silva I. Receptor-defined subtypes of breast cancer in indigenous populations in Africa: a systematic review and meta-analysis. PLoS Med. 2014;11:e1001720. doi: 10.1371/journal.pmed.1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohammed SI, Harford JB. Sorting reality from what we think we know about breast cancer in Africa. PLoS Med. 2014;11:e1001721. doi: 10.1371/journal.pmed.1001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newman LA. Disparities in breast cancer and african ancestry: a global perspective. Breast J. 2015;21:133–139. doi: 10.1111/tbj.12369. [DOI] [PubMed] [Google Scholar]
- 16.Akarolo-Anthony SN, Ogundiran TO, Adebamowo CA. Emerging breast cancer epidemic: evidence from Africa. Breast Cancer Res. 2010;12(Suppl 4):S8. doi: 10.1186/bcr2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnholtz-Sloan JS, Raska P, Rebbeck TR, et al. Replication of GWAS “Hits” by Race for Breast and Prostate Cancers in European Americans and African Americans. Front Genet. 2011;2:37. doi: 10.3389/fgene.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen F, Chen GK, Stram DO, et al. A genome-wide association study of breast cancer in women of African ancestry. Hum Genet. 2013;132:39–48. doi: 10.1007/s00439-012-1214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Narvaez EA, Rosenberg L, Yao S, et al. Fine-mapping of the 6q25 locus identifies a novel SNP associated with breast cancer risk in African-American women. Carcinogenesis. 2013;34:287–291. doi: 10.1093/carcin/bgs334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsing AW, Yeboah E, Biritwum R, et al. High prevalence of screen detected prostate cancer in West Africans: implications for racial disparity of prostate cancer. J Urol. 2014;192:730–735. doi: 10.1016/j.juro.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinton L, Figueroa J, Adjei E, et al. Factors contributing to delays in diagnosis of breast cancers in Ghana, West Africa. Breast Cancer Res Treat. 2017;162:105–114. doi: 10.1007/s10549-016-4088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17:643–653. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Moorman PG, Newman B, Millikan RC, et al. Participation rates in a case-control study: the impact of age, race, and race of interviewer. Ann Epidemiol. 1999;9:188–195. doi: 10.1016/s1047-2797(98)00057-x. [DOI] [PubMed] [Google Scholar]
- 24.Clegg-Lamptey JN, Dakubo JC, Attobra YN. Psychosocial aspects of breast cancer treatment in Accra, Ghana. East Afr Med J. 2009;86:348–353. doi: 10.4314/eamj.v86i7.54152. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien KS, Soliman AS, Annan K, et al. Traditional herbalists and cancer management in Kumasi, Ghana. J Cancer Educ. 2012;27:573–579. doi: 10.1007/s13187-012-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donkor AL, Lathlean J, Wiafe S, et al. Factors contributing to late presentation of breast cancer in Africa: A systematic literature review. Arch Med. 2015;8:2. [Google Scholar]
- 27.Crocker-Buque T, Pollock AM. Appraising the quality of sub-Saharan African cancer registration systems that contributed to GLOBOCAN 2008: a review of the literature and critical appraisal. J R Soc Med. 2015;108:57–67. doi: 10.1177/0141076814554671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien KS, Soliman AS, Awuah B, et al. Establishing effective registration systems in resource-limited settings: cancer registration in Kumasi, Ghana. J Registry Manag. 2013;40:70–77. [PMC free article] [PubMed] [Google Scholar]
- 29.Laryea DO, Awuah B, Amoako YA, et al. Cancer incidence in Ghana, 2012: evidence from a population-based cancer registry. BMC Cancer. 2014;14:362. doi: 10.1186/1471-2407-14-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinton LA, Herrero R, Brenes M, et al. Considerations for conducting epidemiologic case-control studies of cancer in developing countries. Bull Pan Am Health Organ. 1991;25:1–15. [PubMed] [Google Scholar]
- 31.Soliman AS, Schairer C. Considerations in setting up and conducting epidemiologic studies of cancer in middle- and low-income countries: the experience of a case-control study of inflammatory breast cancer in North Africa in the past 10 years. Cancer Med. 2012;1:338–349. doi: 10.1002/cam4.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adesina A, Chumba D, Nelson AM, et al. Improvement of pathology in sub-Saharan Africa. Lancet Oncol. 2013;14:e152–157. doi: 10.1016/S1470-2045(12)70598-3. [DOI] [PubMed] [Google Scholar]
- 33.Pace LE, Shulman LN. Breast Cancer in Sub-Saharan Africa: Challenges and Opportunities to Reduce Mortality. Oncologist. 2016;21:739–744. doi: 10.1634/theoncologist.2015-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clegg-Lamptey J, Dakubo J, Attobra YN. Why do breast cancer patients report late or abscond during treatment in ghana? A pilot study. Ghana Med J. 2009;43:127–131. [PMC free article] [PubMed] [Google Scholar]
- 35.Obrist M, Osei-Bonsu E, Awuah B, et al. Factors related to incomplete treatment of breast cancer in Kumasi, Ghana. Breast. 2014;23:821–828. doi: 10.1016/j.breast.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deming SL, Egbuji A, Smith J, et al. Challenges unique to the design of a comprehensive questionnaire assessing breast cancer risk factors among women in sub-Saharan Africa. J Health Care Poor Underserved. 2010;21(1 Suppl):11–6. doi: 10.1353/hpu.0.0274. [DOI] [PubMed] [Google Scholar]
- 37.Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis. 1983;60:115–120. [PubMed] [Google Scholar]
- 38.Baer HJ, Tworoger SS, Hankinson SE, et al. Body fatness at young ages and risk of breast cancer throughout life. Am J Epidemiol. 2010;171:1183–194. doi: 10.1093/aje/kwq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benn EK, Alexis A, Mohamed N, et al. Skin bleaching and dermatologic health of African and Afro-Caribbean populations in the US: new directions for methodologically rigorous, multidisciplinary, and culturally sensitive research. Dermatol Ther (Heidelb) 2016;6:453–459. doi: 10.1007/s13555-016-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dlova NC, Hamed SH, Tsoka-Gwegweni J, et al. Skin lightening practices: an epidemiological study of South African women of African and Indian ancestries. Br J Dermatol. 2015;173(Suppl 2):2–9. doi: 10.1111/bjd.13556. [DOI] [PubMed] [Google Scholar]
- 41.Aryiku SA, Salam A, Dadzie OE, et al. Clinical and anthropological perspectives on chemical relaxing of afro-textured hair. J Eur Acad Dermatol Venereol. 2015;29:1689–1695. doi: 10.1111/jdv.13028. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg L, Boggs DA, Adams-Campbell LL, et al. Hair relaxers not associated with breast cancer risk: evidence from the black women’s health study. Cancer Epidemiol Biomarkers Prev. 2007;16:1035–1037. doi: 10.1158/1055-9965.EPI-06-0946. [DOI] [PubMed] [Google Scholar]
- 43.Dey S, Soliman AS, Merajver SD. Xenoestrogens may be the cause of high and increasing rates of hormone receptor positive breast cancer in the world. Med Hypotheses. 2009;72:652–656. doi: 10.1016/j.mehy.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 44.Kortenkamp A. Breast cancer, oestrogens and environmental pollutants: a re-evaluation from a mixture perspective. Int J Androl. 2006;29:193–198. doi: 10.1111/j.1365-2605.2005.00613.x. [DOI] [PubMed] [Google Scholar]
- 45.Ogundiran TO, Huo D, Adenipekun A, et al. Body fat distribution and breast cancer risk: findings from the Nigerian breast cancer study. Cancer Causes Control. 2012;23:565–574. doi: 10.1007/s10552-012-9916-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sighoko D, Ogundiran T, Ademola A, et al. Breast cancer risk after full-term pregnancies among African women from Nigeria, Cameroon, and Uganda. Cancer. 2015;121:2237–2243. doi: 10.1002/cncr.29305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collaborative Group on Hormonal Factors in Breast C. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. The Lancet Oncology. 2012;13:1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barnard ME, Boeke CE, Tamimi RM. Established breast cancer risk factors and risk of intrinsic tumor subtypes. Biochim Biophys Acta. 2015;1856:73–85. doi: 10.1016/j.bbcan.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Palmer JR, Boggs DA, Wise LA, et al. Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol Biomarkers Prev. 2011;20:1883–1891. doi: 10.1158/1055-9965.EPI-11-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adewole I, Martin DN, Williams MJ, et al. Building capacity for sustainable research programmes for cancer in Africa. Nat Rev Clin Oncol. 2014;11:251–259. doi: 10.1038/nrclinonc.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]


