Abstract
Purpose of review
Obesity is a major risk factor for the development of de novo chronic kidney disease (CKD). However, once kidney disease is acquired, obesity is paradoxically linked with greater survival, especially in those with advanced CKD. This review examines current evidence for obesity as a risk factor for incident CKD, studies of obesity and mortality across various CKD populations, and potential mechanisms underlying the ‘obesity paradox’ in kidney disease.
Recent findings
Large cohort studies show that overweight body habitus, especially in the context of metabolic syndrome, is associated with higher risk of incident CKD. Emerging data also suggest weight-loss interventions retard or reverse early CKD progression, whereas in hemodialysis patients weight-loss paradoxically heralds poor outcomes. Although the pathogenesis of CKD in obesity remains unclear, studies indicate that excess body fat leads to kidney disease via indirect and direct mechanisms. Meta-analyses suggest that overweight and obese BMI ranges are counterintuitively associated with lower mortality in advanced predialysis and dialysis-dependent CKD patients, whereas a pooled analysis observed that higher pretransplantation BMI was associated with higher mortality in kidney transplantation recipients.
Summary
In addition to its role as a risk factor for de novo CKD, there appears to be a consistent association between obesity and lower mortality in those with established CKD, particularly among hemodialysis patients, suggesting that the reverse epidemiology of obesity is biologically plausible.
Keywords: excess fat, kidney disease, mortality, obesity paradox, reverse epidemiology
Introduction
In the United States, obesity affects more than one-third (35% or 78.6 million) of the adult population and accounts for over $140 billion in annual medical costs [1,2▪]. In the general population, obesity accelerates death risk and has been linked with many comorbidities, such as cardiovascular disease, type 2 diabetes, hypertension, dyslipidemia, obstructive sleep apnea, fatty liver and biliary disease, osteoarthritis, various malignancies, including kidney cancer, neuropsychiatric complications, and impaired health-related quality of life [3–6,7▪▪,8,9].
Obesity is also a major risk factor for the development and progression of chronic kidney disease (CKD) [10–12]. However, past studies examining the association between obesity and outcomes in the CKD population, including those receiving dialysis and kidney transplantation, are complex and somewhat controversial [7▪▪,13]. The current body of evidence suggests that once CKD is established, obesity is paradoxically associated with greater survival, particularly among those receiving hemodialysis, a phenomenon which has been coined the ‘obesity paradox’ or ‘reverse epidemiology’ [6]. Although the underlying mechanisms of the obesity paradox remain unclear, these observations have been markedly consistent across numerous studies, suggesting scientific plausibility. In this review, we will summarize existing evidence of obesity as a risk factor for incident CKD, including underlying pathophysiologic mechanisms; provide an overview of studies examining obesity and CKD-related complications across the nondialysis dependent CKD (NDD-CKD), hemodialysis, peritoneal dialysis, and kidney transplantation populations; examine putative causes by which obesity may counterintuitively promote greater survival in the CKD population; and discuss the clinical implications of the dual roles of obesity, including the obesity paradox, upon the management of CKD patients and future areas of research.
Obesity and Risk of Incident Chronic Kidney Disease
Epidemiologic studies
A growing body of evidence indicates that obesity is a potent risk factor for the development of de novo CKD and end-stage renal disease (ESRD) (Table 1 and Fig. 1) [10–12,14–18]. In a longitudinal analysis of 2585 participants in the Framingham Offspring Study cohort without preexisting kidney disease, incrementally higher BMI was associated with higher risk of developing CKD [defined as an estimated glomerular filtration rate (eGFR) <59 and <64ml/min/1.73m2 in men and women, respectively, based on the ‘Modification of Diet in Renal Disease formula’] over a mean follow-up of 19 years [adjusted odds ratio (95% confidence interval) 1.23 (1.08–1.41) per +1 SD of BMI] [10]. Subsequently, Hsu et al. [14] examined the association between BMI and risk of incident ESRD in one of the largest US studies of a multiracial population (among whom some had underlying CKD). Among more than 300000 patients in the Northern California Kaiser Permanente system whose medical information were linked with the United States Renal Data System (USRDS), the investigators found that those who were overweight or had class I, II, and extreme obesity (defined as BMI ranges of 25.0–29.9, 30.0–34.9, 35.0–39.9, and ≥40.0kg/ m2, respectively) had a 1.9, 3.6, 6.1, and 7.1-fold higher risk of developing ESRD compared with those of normal weight (BMI 18.0–24.9 kg/m2) independent of socio-demographics, comorbidities, and laboratory tests, including proteinuria. In a recent epidemiologic study of 3.4 million veterans with normal baseline kidney function [eGFR >60ml/min/1.73m2 based on the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula] stratified by age and BMI, patients with obesity (defined by BMI > 30 kg/m2) experienced a faster decline in kidney function, particularly among older age groups, and those with BMI ranges more than 35 kg/m2 also had higher mortality risk. Nevertheless, the most favorable kidney health outcomes were observed with overweight BMI ranges (25–30kg/m2) in this study [19▪].
Table 1. Selected studies of the association between higher BMI and incident chronic kidney disease and end-stage renal disease.
| Author (year) | Cohort (N) | Exposure definition | Results | Comments |
|---|---|---|---|---|
| Fox et al. [10] (2004) | Framingham Offspring cohort (2585) | BMI examined in 1-SD increments | Incrementally higher BMI associated with ↑ risk of incident CKD | N/A |
| Tsujimoto et al. [11] (2014) | Japanese participants (105 611) | Fine gradations of BMI: <18.5kg/m2 18.5–20.9 kg/m2 21.0–22.9 kg/m2 23.0–24.9 kg/m2 25.0–26.9 kg/m2 27.0-29.9 kg/m2 ≥30.0kg/m2 |
Graded association between higher BMI and risk of incident CKD in analyses stratified by sex | BMI threshold for ↑ CKD risk: Men: 23.0 kg/m2 Women: 27.0kg/m2 |
| Hsu et al. [14] (2006) | Northern California Kaiser Permanente patients (320 252) | Overweight: 25.0–29.9 kg/m2 Class I obese: 30.0–34.9 kg/m2 Class II obese: 35.0–39.9 kg/m2 Morbidly obese: ≥40.0kg/m2 |
BMI levels >25.0kg/m2 associated with ↑ risk of ESRD | Some patients had underlying CKD at study entry |
| Munkhaugen et al. [15] (2009) | Norwegian participants (74986) | Overweight: 25.0–29.9 kg/m2 Class I obese: 30.0–34.9 kg/m2 Class II/III obese: ≥35.0kg/m2 |
Among normotensive participants, those who had overweight, class I, and class II/III obesity did not have higher risk of ESRD and CKD-related death compared with those of normal weight (BMI 18.5–25.0 kg/m2) In contrast, among prehypertensive participants, class I and II/III obesity were each associated with a higher risk of ESRD and CKD-related death compared with normal weight individuals |
Blood pressure modifies the BMI – ESRD and death association |
| Panwar et al. [16] (2015) | REGARDS cohort (21840) | Overweight: 25.0–29.9 kg/m2 Obese: ≥30.0kg/m2 |
Analyses stratified by weight and metabolic health Overweight and obese individuals with the metabolic syndrome had a two and 2.3-fold higher risk of developing ESRD, respectively, compared with those of normal weight (BMI 18.5–24.9 kg/m2) without the metabolic syndrome |
Presence of the metabolic syndrome modifies the BMI – ESRD association |
| Pscheidt et al. [17] (2015) | Participants in an Austrian health monitoring program (185 341) | Overweight: 25–<30kg/m2 Obese: ≥30 kg/m2 |
No association between overweight and obese habitus with CKD | N/A |
| Vivante et al. [18] (2012) | Israeli adolescents examined for military service fitness (1194 704) | Overweight: 85th–95th BMI percentile Obese: ≥95th BMI percentile |
Overweight and obese habitus associated with ↑ risk of ESRD | N/A |
CKD, chronic kidney disease; ESRD, end-stage renal disease; REGARDS, Reasons for Geographic and Racial Differences in Stroke study.
Figure 1.
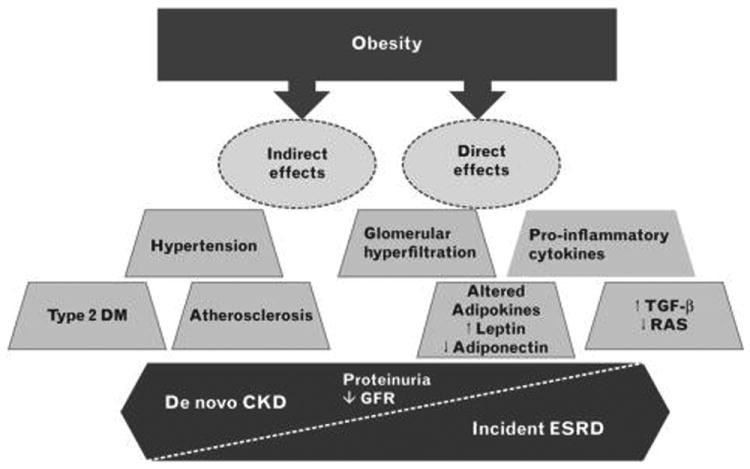
Potential pathways by which obesity leads to the development of chronic kidney disease. CKD, chronic kidney disease; DM, diabetes mellitus; ESRD, end-stage renal disease; GFR, glomerular filtration rate; RAS, renin–angiotensin– aldosterone system; TGF-β, transforming growth factor beta.
Similar findings have been observed in large international cohorts with extended follow-up intervals. For example, among 105 611 Japanese adults with normal underlying kidney function who were stratified by sex, a graded association between higher BMI levels and risk of incident CKD was observed in both men and women (albeit at different BMI thresholds of more than 23.0 and 27.0 kg/m2, respectively) after a mean follow-up of 5 years [11]. In addition, Vivante et al. [18] examined ∼1.2 million Israeli adolescents (i.e., age 17 years) examined for military service fitness whose medical data were linked to the national ESRD registry, and after a mean follow-up of 26 years, those who were overweight and obese (85th–95th and ≥95th percentiles of BMI, respectively) had a three and seven-fold higher risk of incident all-cause ESRD, independent of sex, country of origin, blood pressure, and enrollment period. However, these findings stand in contrast to a study of 185 341 participants in an Austrian health monitoring program whose data were linked to the national dialysis and transplantation registry [17]. In this study, neither overweight nor obese body habitus (BMI levels 25–<30kg/m2 and ≥30kg/m2, respectively) were associated with a higher risk of incident ESRD after a mean follow-up interval of 18 years (reference: BMI 18.5–<25.0kg/m2). Yet in a meta-analysis of 25 cohorts, three cross-sectional studies, and 19 case-control studies by Wang et al. [12], pooled analyses showed that individuals who were overweight and obese (BMI 25.0–29.9 and ≥30.0kg/m2, respectively) had a higher risk of developing kidney disease ascertained by International Classification of Disease codes [hazard ratio (95% confidence interval) 1.40 (1.30–1.50) and 1.83 (1.57–2.13), respectively], with stronger associations observed in women vs. men. Finally, a recent meta-analysis by Ahmadi et al. [20▪] showed that, among patients with stages 3–5 CKD, higher BMI classes were associated with incrementally higher risk of progression to ESRD (Fig. 2) [21].
Figure 2.
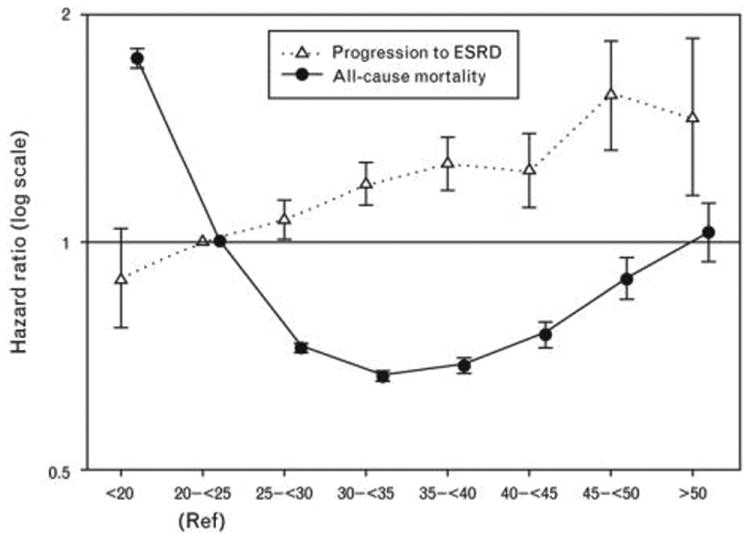
Reanalysis of the largest study of BMI, progression to ESRD, and mortality in nondialysis dependent CKD patients by Lu et al. [21] Among patients with stages 3–5 CKD, higher BMI was associated with higher risk of incident ESRD, but obesity was paradoxically associated with survival advantage. Adapted from [20▪]. CKD, chronic kidney disease; ESRD, end-stage renal disease.
Emerging data also suggest that interventions targeted at reducing obesity may reverse or retard CKD progression. For example, in a large case series of 233 severely obese patients who underwent bariatric surgery, there was a statistically significant rise in mean eGFR 12 months after surgery among those with obesity and CKD [22]. In another case series of 255 morbidly obese patients with type 2 diabetes, reductions in BMI were associated with normalization of urine albumin-to-creatinine ratio levels 12 months following bariatric surgery [23].
Pathophysiologic mechanisms
Despite compelling epidemiologic evidence that excess body fat is a potent risk factor for kidney disease, the mechanisms involved in the pathogenesis of CKD in obesity have not been fully elucidated (Fig. 1). By increasing the risk of type 2 diabetes, hypertension, and atherosclerosis, excess fat mass may ‘indirectly’ lead to CKD [9,24]. Obesity may also have ‘direct’ pathophysiological effects on the kidney via alterations in renal hemodynamics, inflammatory milieu, and growth factor and adipokine production [9,25]. For example, obesity may lead to mesangial expansion of the kidneys and increased renal metabolic demand, resulting in glomerular hyperfiltration, hypertrophy, and hypertension, leading to increased glomerular filtration fraction, and subsequent glomerulosclerosis and proteinuria [9,25,26]. Indeed, focal segmental glomerulosclerosis has long been recognized as the hallmark lesion of obesity-related CKD [27].
There may be additional mechanisms by which obesity leads to de novo CKD. Adipocytes may directly synthesize proinflammatory and potentially proatherogenic cytokines such as TNF-α and IL-6 that may be implicated in the development of CKD [28,29]. Obesity also leads to greater production of the adipokine leptin, which has been shown to increase oxidative stress, sympathetic nervous system activity, glomerulosclerosis, renal fibrosis, and proteinuria [30]. Conversely, adiponectin has been shown to reduce podocyte dysfunction and permeability to albumin, and lower levels of this adipokine are typically observed in obesity [31]. However, as recent data suggest that higher adiponectin levels are paradoxically associated with a three-fold higher death risk in hemodialysis patients, independent of body composition and lipid levels, further study dissecting the impact of adipokines upon kidney health outcomes are needed [32]. In addition, excess fat may stimulate production of growth and hormonal factors that promote kidney damage, such as transforming growth factor-β and renin–angiotensin [9].
Obesity and Outcomes In The Chronic Kidney Disease Population
Whereas excess body fat appears to be a strong risk factor for the development and progression of kidney disease, some, but not all, studies suggest that obesity is associated with favorable outcomes (e.g., lower death risk) once CKD is acquired, particularly among those receiving hemodialysis (Fig. 3).
Figure 3.
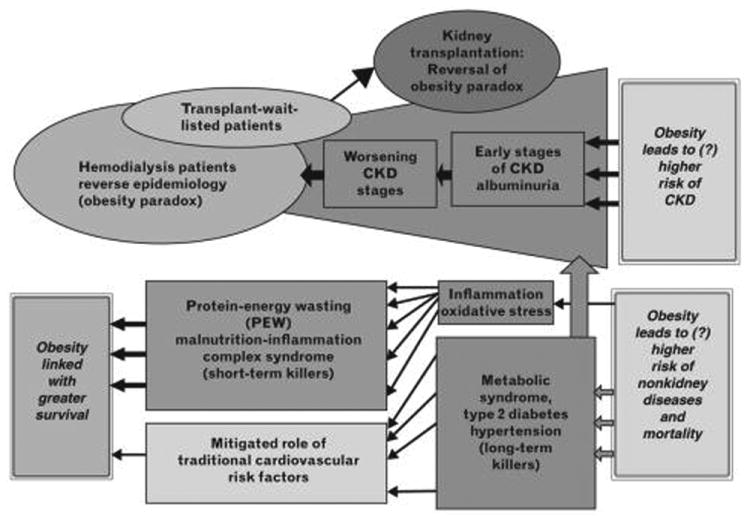
Dual roles of obesity as a risk factor for the development and progression of chronic kidney disease (CKD), as well as a predictor of greater survival among those with established kidney disease.
Nondialysis dependent chronic kidney disease patients
Although studies of BMI and mortality in NDD-CKD patients have shown mixed results, recent data in populations at high-risk for CKD suggest that these associations may be modified by age. In a study that examined the impact of age and BMI upon kidney function decline and mortality among a large cohort of US veterans with an eGFR greater than 60ml/ min/1.73m2 based on the CKD-EPI formula, primary analyses demonstrated a U-shaped association between BMI and mortality risk (i.e., BMI<25.0 and ≥30.0kg/m2 associated with higher mortality); however, upon adjustment for urine albumin-to-creatinine ratio, the higher BMI – higher mortality association was attenuated in older age groups but persisted in those of younger age [19▪].
When taken together, it also appears that the BMI-mortality association may vary according to underlying severity of CKD. In a study of 920 Swedish patients with advanced kidney dysfunction (i.e., stages 4–5 CKD), obesity (BMI > 30kg/m2) was associated with lower death risk [33]. Similarly in a study of 521 US veterans largely comprised of stages 3–5 CKD patients, BMI levels exceeding the 50th percentile (BMI ≥ 28.1 kg/m2) predicted greater survival [34]. However, in studies of patients with moderate CKD (i.e., predominantly stages 3–4 CKD), Dalrymple et al. [35], Madero et al. [36], and Weiner et al. [37] did not observe a significant association between higher BMI and lower death risk. Yet in a study inclusive of stage 1–2 CKD patients, Hsu et al. [14] found that higher BMI levels portended higher mortality risk, similar to that of the non-CKD population.
In light of these disparate findings, Ahmadi et al. [20▪] conducted a meta-analysis comprised of four studies of BMI and mortality in patients with stages 3-5 CKD. Pooled analyses, as well as reanalysis of the largest included study, demonstrated a reverse J-shaped association with death, such that those who were underweight had higher death risk and those of overweight and class I obesity body habitus had lower mortality (Fig. 2) [20▪,21]. In contrast, those with class II and III obesity had no association with death.
Maintenance hemodialysis patients
In contrast to the NDD-CKD population with mixed obesity – mortality findings, a consistent association between obesity and greater survival or lack of higher mortality has been observed in the hemodialysis population. The Diaphane collaborative study was the first to report that higher BMI was not associated with higher death risk among 1453 nondiabetic hemodialysis patients recruited across 33 French dialysis clinics, which was confirmed in a study of 3607 USRDS patients by Leavey et al. [38,39]. Shortly thereafter, Fleischmann et al. [40] published the first study showing that overweight and obese hemodialysis patients had lower mortality risk, propelling the concept of the obesity paradox in the kidney disease population.
Following these studies, a number of large national and international hemodialysis cohort studies have similarly observed robust associations between higher BMI and lower mortality risk [41–44]. Although these prior studies have largely examined BMI at a single point-in-time (i.e., baseline BMI ascertained at study entry), hemodialysis patients may frequently experience fluctuations in BMI resulting from changes in dietary intake, dialysis prescriptions, and comorbidity status. To account for these dynamic changes in BMI, in a recent study of more than 120 000 hemodialysis patients from a large national dialysis organization, Doshi et al. [45▪] examined the association between BMI as a time-varying exposure with all-cause mortality. Using a marginal structural model analysis to account for time-varying covariates (i.e., covariate that simultaneously functions as a confounder and intermediate of the BMI–mortality association), the investigators confirmed an inverse association between higher BMI and lower mortality risk, such that the lowest BMI category (BMI<18.0 kg/m2) was associated with a 3.2-fold higher death risk and the BMI category of 40.0 to less than 45.0 kg/m2 was associated with the greatest survival (i.e., 31% lower death risk) as compared with the BMI reference of 25.0–27.5kg/m2. Finally, in a historic cohort study of 5904 incident hemodialysis patients recruited from 312 facilities across 15 European countries over 2007–2009, inflammation ascertained by C-reactive protein levels was observed to an important modifier of the BMI–mortality association, such that higher BMI was associated with higher death risk among inflamed patients, whereas this association was mitigated in noninflamed patients [46▪].
Peritoneal dialysis patients
Studies of BMI and mortality in peritoneal dialysis patients have shown somewhat heterogeneous results. In a study of more than 40000 US incident peritoneal dialysis patients by Snyder et al. [47], those of overweight and obese body habitus (BMI 25.0–29.9 and >30.0kg/m2) had greater survival compared with those of lower BMI. However, in a study of 1675 hemodialysis and 1662 peritoneal dialysis patients from the USRDS Dialysis Morbidity and Mortality Wave II study conducted by Abbott et al. [48], BMI levels of at least 30.0 kg/m2 were associated with lower mortality in the hemodialysis cohort, but not in those receiving peritoneal dialysis. Similarly, in a study of 134 728 incident dialysis patients using the USRDS database, the lowest BMI quintile was associated with higher death risk in both hemodialysis and peritoneal dialysis patients; in contrast, the highest BMI quintile was associated with lower risk of death in hemodialysis patients but did not demonstrate a survival advantage in the peritoneal dialysis cohort [49].
A recent meta-analysis pooled four studies examining BMI and mortality in peritoneal dialysis patients, in which results were stratified according follow-up interval [50▪]. When follow-up was restricted to 1 year, investigators found that underweight body habitus was associated with higher death risk, whereas overweight and obese body habitus were associated with lower death risk; when follow-up intervals were extended to 2 and 3–5 years, no association between BMI and mortality was observed.
Kidney transplantation recipients
There are considerable knowledge gaps surrounding the optimal BMI threshold for kidney transplantation eligibility, particularly in regards to avoidance of peri and postoperative complications. Although ‘posttransplantation obesity’ is known to be associated with higher risk of mortality and allograft failure [51], Molnar et al. [52] showed that, among 14 632 wait-listed hemodialysis patients awaiting transplantation, each unit of higher ‘pretransplantation BMI’ was associated with lower mortality, whereas weight loss increments of 3 to less than 5 kg and 5 kg or greater over 6 months were associated with higher mortality risk. Furthermore, past studies of pretransplantation obesity and posttransplantation mortality have shown mixed findings [53,54]. To address these uncertainties, a meta-analysis of four studies examining pretransplantation BMI upon posttransplantation outcomes, including mortality and allograft failure was conducted [55▪]. This study found that those of underweight, overweight, and obese body habitus had higher risk of death (reference: normal BMI class). Although underweight and obese body habitus were also associated with higher risk of graft failure, those who were overweight had similar risk as those of normal BMI. These findings did not support the presence of an obesity paradox in kidney transplantation recipients whose longevity is substantially increased compared with their hemodialysis counterparts. Hence, the investigators concluded that, in contrast to hemodialysis patients in whom the short-term benefits of obesity may outweigh its long-term risks, in kidney transplant recipients there may be sufficient time for obesity to lead to long-term adverse effects.
Other Metrics of Obesity
Although BMI is accepted as one of the most reliable anthropometric measures for obesity and is widely usedin research and clinical practice guidelines, it has limited ability in distinguishing between fat vs. muscle mass [7▪▪,56]. Although direct measurement of fat vs. muscle mass in large epidemiologic studies may not be feasible, more accurate estimation of body composition and fat distribution in clinical practice and research are needed. For example, waist circumference and waist-to-hip ratio are known to be better indicators of visceral obesity in both the general and CKD populations, and emerging data indicate that these metrics are also potent predictors of adverse outcomes, including de novo CKD in those without underlying kidney disease, as well as incident ESRD and death risk in CKD patients [7▪▪,9,56–59]. Further research is needed to identify and refine practical methods of body composition assessment, to better ascertain the relationship between fat mass and outcomes in the CKD population.
Potential Mechanisms Underlying The Obesity Paradox
This paradoxically inverse association between higher BMI and lower mortality in CKD patients has also been observed in other chronic disease populations, including those with malignancy, HIV, congestive heart failure, type 2 diabetes, and emphysema [7▪▪,13,60,61]. Although there has been rather extensive scrutiny of the obesity paradox as being an artefact of survivor bias, reverse causation, or residual confounding, the strikingly robust associations across many studies of CKD and other chronic illness states and emerging data highlighting the putative beneficial effects of increased fat mass support this as a biologically plausible phenomenon (Table 2) [7▪▪,13,61–73].
Table 2. Proposed explanatory factors underlying the obesity paradox.
| Mechanism | Pathways by which obesity may reduce mortality risk |
|---|---|
| Time-discrepancy between competing risk factors for death (undernutrition vs. overnutrition) [7▪▪,13] | Given shortened lifespan of dialysis patients, short-term benefits of overnutrition overrides long-term risks of obesity |
| Reverse causation [7▪▪,13] | Lower BMI is not the cause but rather the sequelae of comorbidities/conditions leading to higher mortality in CKD patients |
| Survivor bias [7▪▪,13] | Majority of CKD patients will die prior to developing ESRD, and the resultant ESRD population represents a selected cohort Observed associations between mortality predictors in ESRD survivors may not be generalizable to the greater CKD population |
| PEW [7▪▪,13,61] | Greater metabolic reserve during periods of deficient energy and protein intake reduce risk of developing PEW Excess fat and protein stores may reduce susceptibility to infection and other inflammatory processes Weight loss may lead to reduced skeletal muscle oxidative metabolism and mitigated antioxidant defense |
| Alteration of circulating cytokines [62–65] | Favorable alterations in TNF-α system: In ESRD patients TNF-α is elevated and contributes to cardiac injury via proapoptotic and negative inotropic effects Adipose tissue produces soluble TNF-α receptors that neutralize adverse biologic effects of TNF-α |
| Favorable genetic variants/ expression [66–68] | Genetic variant of a-2-Heremans-Schmid glycoprotein that is associated with lower fetuin-A levels is more common in lean vs. obese patients Obese patients have higher levels of fetuin-A, reducing their genetic predisposition to vascular calcification In obese patients with renal cell cancer, there is differential gene expression of metabolic and fatty acid genes vs. normal weight patients that may contribute to the obesity paradox in malignancy |
| More stable hemodynamic status [7▪▪,13,63,69] | Overweight/obese dialysis patients with heart failure have higher systolic blood pressure values (even in the context of similar pulmonary capillary wedge pressures and cardiac indices) Higher tolerance of large ultrafiltration volumes, less intra and postdialytic hypotension, and myocardial stunning |
| Endotoxin–lipoprotein interaction [70] | Obese patients have higher lipid and lipoprotein concentrations Lipoproteins can bind and remove circulating endotoxins, subsequently reducing effects of inflammation, atherosclerosis, etc. |
| Uremic toxin sequestration [7▪▪,71] | Adipose tissue sequesters uremic toxins Weight loss and loss of fat mass may result in release of lipophilic hexachlorobenzene and other chlorinated hydrocarbons |
| Neurohormonal alterations [72,73] | Lean patients may have heightened sympathetic nervous system and renin-angiotensin activity vs. obese patients |
CKD, chronic kidney disease; ESRD, end-stage renal disease; PEW, protein-energy wasting.
Conclusion
Despite extensive past studies, there are substantial uncertainties regarding the clinical implications of obesity in the management of CKD patients [74]. For example, further research is needed to determine the causal factors engendering the obesity paradox, which will provide greater insight into its pathogenesis and consequences in other chronic disease populations. Given the heterogeneous associations between BMI and mortality in NDD-CKD studies, future investigations are also needed to determine at which stage of kidney disease the obesity paradox develops, and which subpopulations of patients may be particularly susceptible. The therapeutic targets for BMI and body composition warrant more precise definition in NDD-CKD and dialysis patients, and in particular those participating in kidney transplantation (recipients and living donors). Lastly, given the compelling evidence for obesity as risk factor for incident CKD and ESRD, further studies are needed to determine the safety and effectiveness of weight loss interventions (e.g., pharmacologic, surgical, and physical activity) upon the development and progression of kidney disease.
Supplementary Material
Key Points.
Obesity is highly prevalent among US adults, and has been associated with various comorbidities, including CKD.
Obesity may lead to CKD via both indirect (type 2 diabetes, hypertension, metabolic syndrome, atherosclerosis) and direct mechanisms (glomerular hyperfiltration, inflammatory milieu, and altered growth factor and adipokine production).
Once CKD is acquired, obesity is paradoxically associated with improved survival, particularly among those receiving hemodialysis, a phenomenon described as the ‘obesity paradox,’ in which short-term benefits of obesity may outweigh its long-term risks.
However, recent pooled data do not confirm the presence of an obesity paradox in kidney transplantation recipients, whose longevity is comparatively longer than dialysis patients, and hence may have sufficient time for obesity to lead to long-term adverse effects.
Acknowledgments
None.
Financial support and sponsorship: The authors are supported by the research grants from the NIH/NIDDK including K23-DK102903 (CMR), K24-DK091419 (KKZ), and philanthropist grants from AVEO Inc., Harold Simmons, Louis Chang, and Dr Joseph Lee.
Footnotes
Conflicts of interest: There are no conflicts of interest.
References and Recommended Reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–w831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 2▪.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. Study of childhood and adult obesity trends in the United States using National Health and Nutrition Examination Survey data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Rhee CM, Amin AN. To legitimize the contentious obesity paradox. Mayo Clin Proc. 2014;89:1033–1035. doi: 10.1016/j.mayocp.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪▪.Park J, Ahmadi SF, Streja E, et al. Obesity paradox in end-stage kidney disease patients. Prog Cardiovasc Dis. 2014;56:415–425. doi: 10.1016/j.pcad.2013.10.005. Comprehensive review of studies of BMI and body composition and mortality indialysis patients, as well as mechanisms underlying the obesity paradox. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma A, Vallakati A, Einstein AJ, et al. Relationship of body mass index with total mortality, cardiovascular mortality, and myocardial infarction after coronary revascularization: evidence from a meta-analysis. Mayo Clin Proc. 2014;89:1080–1100. doi: 10.1016/j.mayocp.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 9.Stenvinkel P, Zoccali C, Ikizler TA. Obesity in CKD: what should nephrologists know? J Am Soc Nephrol. 2013;24:1727–1736. doi: 10.1681/ASN.2013040330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox CS, Larson MG, Leip EP, et al. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 11.Tsujimoto T, Sairenchi T, Iso H, et al. The dose-response relationship between body mass index and the risk of incident stage ≥3 chronic kidney disease in a general Japanese population: the Ibaraki prefectural health study (IPHS) J Epidemiol. 2014;24:444–451. doi: 10.2188/jea.JE20140028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Chen X, Song Y, et al. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int. 2008;73:19–33. doi: 10.1038/sj.ki.5002586. [DOI] [PubMed] [Google Scholar]
- 13.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, et al. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81:543–554. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 14.Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Inter Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 15.Munkhaugen J, Lydersen S, Wideroe TE, Hallan S. Prehypertension, obesity, and risk of kidney disease: 20-year follow-up of the HUNT I study in Norway. Am J Kidney Dis. 2009;54:638–646. doi: 10.1053/j.ajkd.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 16.Panwar B, Hanks LJ, Tanner RM, et al. Obesity, metabolic health, and the risk of end-stage renal disease. Kidney Int. 2015;87:1216–1222. doi: 10.1038/ki.2014.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pscheidt C, Nagel G, Zitt E, et al. Sex- and time-dependent patterns in risk factors of end-stage renal disease: a large Austrian cohort with up to 20 years of follow-up. PLoS One. 2015;10:e0135052. doi: 10.1371/journal.pone.0135052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vivante A, Golan E, Tzur D, et al. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch Intern Med. 2012;172:1644–1650. doi: 10.1001/2013.jamainternmed.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪.Lu JL, Molnar MZ, Naseer A, et al. Association of age and BMI with kidney function and mortality: a cohort study. Lancet Diabetes Endocrinol. 2015;3:704–714. doi: 10.1016/S2213-8587(15)00128-X. Rigorous study examining the association between age, BMI, kidney function decline, and mortality in more than 3 million US veterans with CKD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪.Ahmadi SF, Zahmatkesh G, Ahmadi E, et al. Association of Body Mass Index with Clinical Outcomes in Non-Dialysis-Dependent Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2016;6:37–49. doi: 10.1159/000437277. Rigorous systematic review and meta-analysis of studies of BMI, kidney disease progression, and mortality in predialysis CKD patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu JL, Kalantar-Zadeh K, Ma JZ, et al. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol. 2014;25:2088–2096. doi: 10.1681/ASN.2013070754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou CC, Shyu RS, Lee WJ, et al. Improved renal function 12 months after bariatric surgery. Surg Obes Relat Dis. 2013;9:202–206. doi: 10.1016/j.soard.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Amor A, Jimenez A, Moize V, et al. Weight loss independently predicts urinary albumin excretion normalization in morbidly obese type 2 diabetic patients undergoing bariatric surgery. Surg Endosc. 2013;27:2046–2051. doi: 10.1007/s00464-012-2708-3. [DOI] [PubMed] [Google Scholar]
- 24.Wanner C, Jager KJ. Kidneys do not like excess body fat. Lancet Diabetes Endocrinol. 2015;3:669–671. doi: 10.1016/S2213-8587(15)00235-1. [DOI] [PubMed] [Google Scholar]
- 25.Mallamaci F, Tripepi G. Obesity and CKD progression: hard facts on fat CKD patients. Nephrol Dial Transplant. 2013;28(Suppl 4):iv105–iv108. doi: 10.1093/ndt/gft391. [DOI] [PubMed] [Google Scholar]
- 26.Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. 2013;33:14–22. doi: 10.1016/j.semnephrol.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Warnke RA, Kempson RL. The nephrotic syndrome in massive obesity: a study by light, immunofluorescence, and electron microscopy. Arch Pathol Lab Med. 1978;102:431–438. [PubMed] [Google Scholar]
- 28.Spoto B, Zoccali C. Spleen IL-10, a key player in obesity-driven renal risk. Nephrol Dial Transplant. 2013;28:1061–1064. doi: 10.1093/ndt/gft094. [DOI] [PubMed] [Google Scholar]
- 29.Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia: the good, the bad, and the ugly. Kidney Int. 2005;67:1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- 30.Wolf G, Ziyadeh FN. Leptin and renal fibrosis. Contrib Nephrol. 2006;151:175–183. doi: 10.1159/000095328. [DOI] [PubMed] [Google Scholar]
- 31.Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee CM, Nguyen DV, Moradi H, et al. Association of adiponectin with body composition and mortality in hemodialysis patients. Am J Kidney Dis. 2015;66:313–321. doi: 10.1053/j.ajkd.2015.02.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans M, Fryzek JP, Elinder CG, et al. The natural history of chronic renal failure: results from an unselected, population-based, inception cohort in Sweden. Am J Kidney Dis. 2005;46:863–870. doi: 10.1053/j.ajkd.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 34.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. AmJ Kidney Dis. 2007;49:581–591. doi: 10.1053/j.ajkd.2007.02.277. [DOI] [PubMed] [Google Scholar]
- 35.Dalrymple LS, Katz R, Kestenbaum B, et al. Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med. 2011;26:379–385. doi: 10.1007/s11606-010-1511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madero M, Sarnak MJ, Wang X, et al. Body mass index and mortality in CKD. Am J Kidney Dis. 2007;50:404–411. doi: 10.1053/j.ajkd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Weiner DE, Tighiouart H, Elsayed EF, et al. The relationship between non-traditional risk factors and outcomes in individuals with stage 3 to 4 CKD. Am J Kidney Dis. 2008;51:212–223. doi: 10.1053/j.ajkd.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Degoulet P, Legrain M, Reach I, et al. Mortality risk factors in patients treated by chronic hemodialysis. Report of the Diaphane collaborative study. Nephron. 1982;31:103–110. doi: 10.1159/000182627. [DOI] [PubMed] [Google Scholar]
- 39.Leavey SF, Strawderman RL, Jones CA, et al. Simple nutritional indicators as independent predictors of mortality in hemodialysis patients. Am J Kidney Dis. 1998;31:997–1006. doi: 10.1053/ajkd.1998.v31.pm9631845. [DOI] [PubMed] [Google Scholar]
- 40.Fleischmann E, Teal N, Dudley J, et al. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999;55:1560–1567. doi: 10.1046/j.1523-1755.1999.00389.x. [DOI] [PubMed] [Google Scholar]
- 41.Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr. 2004;80:324–332. doi: 10.1093/ajcn/80.2.324. [DOI] [PubMed] [Google Scholar]
- 42.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005;46:489–500. doi: 10.1053/j.ajkd.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Leavey SF, McCullough K, Hecking E, et al. Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2001;16:2386–2394. doi: 10.1093/ndt/16.12.2386. [DOI] [PubMed] [Google Scholar]
- 44.Port FK, Ashby VB, Dhingra RK, et al. Dialysis dose and body mass index are strongly associated with survival in hemodialysis patients. J Am Soc Nephrol. 2002;13:1061–1066. doi: 10.1681/ASN.V1341061. [DOI] [PubMed] [Google Scholar]
- 45▪.Doshi M, Streja E, Rhee CM, et al. Nephrol Dial Transplant. 2015. Examining the robustness of the obesity paradox in maintenance hemodialysis patients: a marginal structural model analysis. [Epub ahead of print] First study to examine the association between time-varying BMI and mortality in a large national cohort of hemodialysis patients using marginal structural modeling to account for time-varying confounders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪.Stenvinkel P, Gillespie IA, Tunks J, et al. J AmSocNephrol. 2015. Inflammation modifies the paradoxical association between body mass index and mortality in hemodialysis patients. [Epub aheadofprint] Rigorous large-scale study showing that inflammation is an important modifier of the association between BMI and mortality in a large hemodialysis cohort across many European nations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Snyder JJ, Foley RN, Gilbertson DT, et al. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int. 2003;64:1838–1844. doi: 10.1046/j.1523-1755.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 48.Abbott KC, Glanton CW, Trespalacios FC, et al. Body mass index, dialysis modality, and survival: analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int. 2004;65:597–605. doi: 10.1111/j.1523-1755.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- 49.Stack AG, Murthy BV, Molony DA. Survival differences between peritoneal dialysis and hemodialysis among ‘large’ ESRD patients in the United States. Kidney Int. 2004;65:2398–2408. doi: 10.1111/j.1523-1755.2004.00654.x. [DOI] [PubMed] [Google Scholar]
- 50▪.Ahmadi SF, Zahmatkesh G, Streja E, et al. Association of body mass index with mortality in peritoneal dialysis patients: a systematic review and meta-analysis. Perit Dial Int. 2015 doi: 10.3747/pdi.2015.00052. [Epub ahead of print] Comprehensive systematic review and meta-analysis of studies of BMI, mortality, and technique failure in peritoneal dialysis patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan W, Bosch JA, Jones D, et al. Obesity in kidney transplantation. J Ren Nutr. 2014;24:1–12. doi: 10.1053/j.jrn.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Molnar MZ, Streja E, Kovesdy CP, et al. Associations of body mass index and weight loss with mortality in transplant-waitlisted maintenance hemodialysis patients. Am J Transplant. 2011;11:725–736. doi: 10.1111/j.1600-6143.2011.03468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cannon RM, Jones CM, Hughes MG, et al. The impact of recipient obesity on outcomes after renal transplantation. Ann Surg. 2013;257:978–984. doi: 10.1097/SLA.0b013e318275a6cb. [DOI] [PubMed] [Google Scholar]
- 54.Hatamizadeh P, Molnar MZ, Streja E, et al. Recipient-related predictors of kidney transplantation outcomes in the elderly. Clin Transplant. 2013;27:436–443. doi: 10.1111/ctr.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪.Ahmadi SF, Zahmatkesh G, Streja E, et al. Body mass index and mortality in kidney transplant recipients: a systematic review and meta-analysis. Am J Nephrol. 2014;40:315–324. doi: 10.1159/000367812. Excellent systematic review and meta-analysis of studies of pretransplantation BMI and posttransplantation mortality among kidney transplantation recipients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Postorino M, Marino C, Tripepi G, Zoccali C. Abdominal obesity and all-cause and cardiovascular mortality in end-stage renal disease. J Am Coll Cardiol. 2009;53:1265–1272. doi: 10.1016/j.jacc.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 57.Elsayed EF, Tighiouart H, Weiner DE, et al. Waist-to-hip ratio and body mass index as risk factors for cardiovascular events in CKD. Am J Kidney Dis. 2008;52:49–57. doi: 10.1053/j.ajkd.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Foster MC, Hwang SJ, Larson MG, et al. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis. 2008;52:39–48. doi: 10.1053/j.ajkd.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kramer H, Shoham D, McClure LA, et al. Association of waist circumference and body mass index with all-cause mortality in CKD: The REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. Am J Kidney Dis. 2011;58:177–185. doi: 10.1053/j.ajkd.2011.02.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chlebowski RT, Grosvenor M, Lillington L, et al. Dietary intake and counseling, weight maintenance, and the course of HIV infection. J Am Diet Assoc. 1995;95:428–432. doi: 10.1016/S0002-8223(95)00115-8. [DOI] [PubMed] [Google Scholar]
- 61.Hughes V. The big fat truth. Nature. 2013;497:428–430. doi: 10.1038/497428a. [DOI] [PubMed] [Google Scholar]
- 62.Feldman AM, Combes A, Wagner D, et al. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. 2000;35:537–544. doi: 10.1016/s0735-1097(99)00600-2. [DOI] [PubMed] [Google Scholar]
- 63.Horwich TB, Fonarow GC, Hamilton MA, et al. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–795. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 64.Kalantar-Zadeh K, Kopple JD, Humphreys MH, Block G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1507–1519. doi: 10.1093/ndt/gfh143. [DOI] [PubMed] [Google Scholar]
- 65.Mohamed-Ali V, Goodrick S, Bulmer K, et al. Production of soluble tumor necrosis factor receptors by human subcutaneous adipose tissue in vivo. Am J Physiol. 1999;277:E971–E975. doi: 10.1152/ajpendo.1999.277.6.E971. [DOI] [PubMed] [Google Scholar]
- 66.Hakimi AA, Furberg H, Zabor EC, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst. 2013;105:1862–1870. doi: 10.1093/jnci/djt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lavebratt C, Dungner E, Hoffstedt J. Polymorphism of the AHSG gene is associated with increased adipocyte beta2-adrenoceptor function. J Lipid Res. 2005;46:2278–2281. doi: 10.1194/jlr.M500201-JLR200. [DOI] [PubMed] [Google Scholar]
- 68.Li L, Kalantar-Zadeh K. Obesity that makes kidney cancer more likely but helps fight it more strongly. J Natl Cancer Inst. 2013;105:1848–1849. doi: 10.1093/jnci/djt348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McIntyre CW, Burton JO, Selby NM, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19–26. doi: 10.2215/CJN.03170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rauchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;356:930–933. doi: 10.1016/S0140-6736(00)02690-8. [DOI] [PubMed] [Google Scholar]
- 71.Jandacek RJ, Anderson N, Liu M, et al. Effects of yo-yo diet, caloric restriction, and olestra on tissue distribution of hexachlorobenzene. Am J Physiol Gastrointest Liver Physiol. 2005;288:G292–G299. doi: 10.1152/ajpgi.00285.2004. [DOI] [PubMed] [Google Scholar]
- 72.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341:577–585. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 73.Weber MA, Neutel JM, Smith DH. Contrasting clinical properties and exercise responses in obese and lean hypertensive patients. J Am Coll Cardiol. 2001;37:169–174. doi: 10.1016/s0735-1097(00)01103-7. [DOI] [PubMed] [Google Scholar]
- 74.Kalantar-Zadeh K, Kopple JD. Obesity paradox in patients on maintenance dialysis. Contrib Nephrol. 2006;151:57–69. doi: 10.1159/000095319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


