Abstract
Noxious input can sensitize pain (nociceptive) circuits within the spinal cord, inducing a lasting increase in spinal cord neural excitability (central sensitization) that is thought to contribute to chronic pain. The development of spinally-mediated central sensitization is regulated by descending fibers and GABAergic interneurons. The current study provides evidence that spinal cord injury (SCI) transforms how GABA affects nociceptive transmission within the spinal cord, recapitulating an earlier developmental state wherein GABA has an excitatory effect. In spinally transected rats, noxious electrical stimulation and inflammation induce enhanced mechanical reactivity (EMR), a behavioral index of nociceptive sensitization. Pretreatment with the GABAA receptor antagonist bicuculline blocked these effects. Peripheral application of an irritant (capsaicin) also induced EMR. Both the induction and maintenance of this effect were blocked by bicuculline. Cellular indices of central sensitization [c-fos expression and ERK phosphorylation (pERK)] were also attenuated. In intact (sham operated) rats, bicuculline had the opposite effect. Pretreatment with a GABA agonist (muscimol) attenuated nociceptive sensitization in intact, but not spinally injured, rats. The effect of SCI on GABA function was linked to a reduction in the Cl− transporter, KCC2, leading to a reduction in intracellular Cl− that would attenuate GABA-mediated inhibition. Pharmacologically blocking the KCC2 channel (with i.t. DIOA) in intact rats mimicked the effect of SCI. Conversely, a pharmacological treatment (bumetanide) that should increase intracellular Cl− levels blocked the effect of SCI. The results suggest that GABAergic neurons drive, rather than inhibit, the development of nociceptive sensitization after spinal injury.
Keywords: GABA, GABAA receptor, KCC2, Central sensitization, allodynia, pain, SCI
Introduction
Afferent fibers that transmit signals related to noxious stimulation project to the dorsal horn of the spinal cord and engage pain (nociceptive) circuits that are regulated by descending fibers and intraspinal biological processes (Woolf, 2004). Of particular concern are processes that foster neural excitability within the dorsal horn, generating a sensitized state that increases behavioral reactivity and enhanced pain. Research has shown that prolonged activation of unmyelinated (C) fibers can induce a lasting central sensitization that is mediated by molecular signaling pathways related to those implicated in brain-dependent learning and memory (Ji et al., 2003; Woolf and Thompson, 1991). Central sensitization can be induced by electrical stimulation of nociceptive fibers, peripheral injury, or the application of an irritant (e.g., formalin, capsaicin). At a behavioral level, the sensitization of nociceptive circuits is associated with increased reactivity to mechanical stimulation (Woolf, 2011). At a cellular level, central sensitization within the spinal cord is correlated with increased expression of the immediate early proto-oncogene c-fos and the phosphorylation of the protein extracellular-signal-regulated kinase (pERK; (Gao and Ji, 2009).
The development of nociceptive sensitization is regulated within the dorsal horn by interneurons that release the neurotransmitter GABA (Gwak and Hulsebosch, 2011; Sandkuhler, 2009; Woolf and Salter, 2000). The release of GABA can engage the ionotropic GABAA receptor that functions as a Cl− channel. Under normal conditions, neurons maintain a low intracellular concentration of Cl− ([Cl−]i). As a result, engaging the GABAA receptor allows Cl− to flow into the cell, which has a hyperpolarizing effect that diminishes neural excitability. Research has shown that blocking the GABAA receptor with the antagonist bicuculline increases neural excitability and behavioral reactivity (Baba et al., 2003; Dougherty and Hochman, 2008; Roberts et al., 1986; Sivilotti and Woolf, 1994; Sorkin et al., 1998; Zhang et al., 2001). Conversely, microinjecting a GABAA agonist (e.g., muscimol) into the spinal cord reduces neural excitability and responsiveness to stimulation (Hwang and Yaksh, 1997; Kaneko and Hammond, 1997). Likewise, enhancing GABA release by means of neural progenitors attenuates behavioral and cellular signs of central sensitization (Jergova et al., 2012).
Central sensitization within the spinal cord is also modulated by descending fibers that can moderate neural excitability. Supporting this, research has shown that peripheral stimulation at an intensity that engages C-fibers induces a lasting NMDAR-dependent modification of spinal circuits [long-term potentiation (LTP)], central sensitization, and an inhibition of adaptive plasticity in spinally transected subjects (Sandkuhler, 2000). This same stimulation has little effect on spinal circuits in uninjured subjects (Gjerstad et al., 2001; Washburn et al., 2007), implying that the loss of descending fibers places the caudal tissue in a vulnerable state that fosters the development of central sensitization.
Recent evidence suggests spinal injury enhances neural excitability within the spinal cord, in part, because it modifies GABA function. Intracellular Cl− concentrations are regulated by the co-transporters NKCC1 and KCC2 (Cramer et al., 2008). Early in development, the inward flow of Cl− through NKCC1 outweighs the outward flow through KCC2 and, as a result, intracellular Cl− concentrations are high (Ben-Ari, 2002). Under these conditions, engaging the GABAA receptor promotes the outward flow of Cl−, which has a depolarizing effect. Later in development, KCC2 expression is up-regulated and there is a corresponding reduction in intracellular Cl− level. Now, engaging the GABAA receptor allows Cl− to enter the cell, yielding its usual hyperpolarizing effect. Evidence suggests that spinal injury can reduce membrane-bound KCC2 at, and caudal, to the site of injury. This would reduce GABAergic inhibition, fostering the development of chronic pain and spasticity after SCI (Boulenguez et al., 2010; Dougherty and Hochman, 2008; Drew et al., 2004; Lu et al., 2008).
Past studies exploring the role of GABA after SCI have focused on behavioral effects (chronic pain, spasticity) observed weeks after injury (Boulenguez et al., 2010; Cramer et al., 2008; Lu et al., 2008; Medina et al., 2014). Here we examine a different issue: whether the disruption in ascending/descending fibers induces a change in GABA function that affects the development of spinally-mediated central sensitization soon after injury. Our hypothesis is that spinal injury brings about a down-regulation in membrane-bound KCC2 that switches how GABA affects nociceptive circuits, creating a state wherein GABAergic input fuels the development of central sensitization. We explore this possibility by cutting communication with the brain (using a thoracic transection) and evaluating the impact of blocking GABA transmission using the GABAA antagonist bicuculline. In intact rats, bicuculline induces a state akin to central sensitization. An injury-induced reduction in membrane-bound KCC2 should attenuate GABAergic inhibition and eliminate this sensitizing effect of bicuculline. We suggest further that the GABA input may drive the development of central sensitization after spinal injury and, for this reason, administration of bicuculline will block the behavioral and cellular effects of treatments known to induce central sensitization. We test this using three treatments (electrical stimulation, inflammation, and capsaicin) that induce a lasting increase in mechanical reactivity after spinal injury. In all cases, pretreatment with bicuculline blocked the development of enhanced mechanical reactivity (EMR). We reinforce these data using cellular indices of central sensitization (c-fos and ERK expression within the dorsal horn) and confirm that a qualitatively distinct pattern of results is obtained in sham-operated (uninjured) rats. The shift in GABA function is related to an injury-induced down-regulation in membrane-bound KCC2.
Materials and methods
Subjects
Male Sprague-Dawley rats were obtained from Harlan (Houston, TX). Rats were 100–120 days old and weighed 350–400g at the time of surgery. Subjects were housed in pairs and maintained on a 12 hour light-dark cycle. Food and water were available ad libitum. All experiments were carried out in accordance with National Institutes of Health (NIH) standards for the care and use of laboratory animals (NIH publications No. 80-23) and were approved by the University Laboratory Animal Care Committee at Texas A&M University. Every effort was made to minimize suffering and limit the number of animals used.
Surgery and intrathecal cannulization
Subjects were anesthetized with isoflurane gas, induced at 5%, and maintained at 2–3%. Each subject’s head was rendered immobile in a stereotaxic apparatus with a small (5 × 4 × 2.5 cm) gauze pillow under the subject’s chest to provide support for respiration. An anterior to posterior incision over the second thoracic vertebrae (T2) was made and the tissue just rostral to T2 was cleared using rongeurs until the cord was exposed and cauterized. A 25-cm polyethylene cannula (PE-10, VWR International, Bristol, CT, USA) for intrathecal (i.t.) drug administration was subsequently threaded 7.5 cm down the vertebral column, into the subarachnoid space between the dura and the white matter, and placed over the lumbar enlargement so that it laid on the dorsal surface of the spinal cord. After surgery, the wound was closed with Michel clips (Fisher Scientific, Waltham, MA). The subjects were then treated with an intraperitoneal (i.p.) injection of 0.9% saline solution (3 mL) to prevent dehydration. Following surgery, rats were placed in a temperature-controlled environment (25.5 °C) and monitored until awake. All rats were checked every six to eight hours during the 18–24 hr post-surgical period. During this time, hydration was maintained with supplemental injections of saline, and the rats’ bladders and colons were expressed as necessary. All experimental treatments were initiated approximately 24 hrs after surgery.
Noxious stimulation
Variable intermittent leg shock was applied while spinalized rats were loosely restrained in Plexiglas tubes as previously described (Crown et al., 2002). Leg shock was delivered using a BRS/LVE (Laurel, MD) constant current (60 Hz, AC) shock generator (Model SG-903). Electrical stimulation was applied by attaching one lead from the shock generator to a 2.5 cm stainless steel pin that was inserted 0.4 cm into the tibialis anterior muscles. The other lead was inserted through the skin over the tibia, 1.5 cm from the tarsals. Rats treated with intermittent nociceptive stimulation received 900, 80-ms leg shocks on a variable time schedule with a mean inter-stimulus interval (ISI) of 2 s (range 0.2 –3.8 s). Unshocked subjects were placed in the restraining tubes for an equal amount of time as the shocked subjects with the electrodes attached, but did not receive the electrical stimuli.
Noxious stimulation was also induced using the irritant capsaicin. Three percent capsaicin (Sigma-Aldrich, St. Louis, MO) was dissolved in 50 µL of vehicle [Tween 20 (7%) and saline (93%)] and was injected subcutaneously into the dorsal surface of the hindpaw while loosely restrained in Plexiglas tubes.
Drug Administration
Bicuculline (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% saline (0.3% in 1 µL). One hundred µg of lipopolysaccharide (LPS; Sigma-Aldrich, St. Louis, MO) was dissolved in 10 µL of 0.9% saline. Gabazine (Sigma-Aldrich, St. Louis, MO) was dissolved in 10 µL saline. Muscimol (Sigma-Aldrich, St. Louis, MO) was dissolved in 0.9% saline (0.1% in 1 µL). Twenty µg of DIOA (Santa-Cruz Biotech, Dallas, TX) was dissolved in 2 µL vehicle [DMSO (1%) and saline (99%)]. One mM of bumetanide (Santa-Cruz Biotech, Dallas, TX) was dissolved in 10 µL vehicle [Tween20 (2%) and saline (98%)]. All the drugs were administered i.t. and followed by a 20 µL saline (0.9%) flush that was slowly infused over a period of 2 min.
Mechanical testing
Mechanical reactivity was assessed using von Frey filaments (Stoelting, Wood Dale, IL) that were applied while rats were loosely restrained in Plexiglas tubes. Sensitivity was determined by stimulating the mid-plantar surface of each hindpaw by an ascending order until a flexion response is elicited. Stimuli were presented twice to each paw in an ABBA counterbalanced fashion (A = left, B = right), with testing on the same leg separated by a 2 min interval. Filament thickness/force is related to behavior using the transformation provided by the manufacturer: Intensity = log10 (10,000g). This transformation yields a scale that is approximately linear and amenable to parametric analyses. Data were converted to change from baseline scores for purposes of analysis. The experimenter performing the behavioral tests was unaware of the subject’s treatment condition.
RNA extraction and RT-PCR
Subjects were deeply anesthetized with a lethal dosage of pentobarbital (50mg/kg) and 1 centimeter of spinal cord around the lumbar enlargement (L3-L5) was rapidly removed within 3 minute after confirmation of death. The spinal cord was further subdivided into dorsal and ventral portions and then processed for extracting both total RNA (RNeasy Mini Kit; Qiagen, Valencia, CA) and protein (see below). Total RNA (100 ng) was converted into cDNA by TaqMan EZ RT-PCR Core reagents (Applied Biosystems, Carlsbad, CA) and the mRNA levels of all targets were measured by TaqMan quantitative real-time (RT)-PCR using a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Carlsbad, CA.). The sequences of probes, forward and reverse primers for β-actin, c-fos, and c-jun, were obtained from Applied Biosystems, Carlsbad, CA. The mRNA expression for each gene of interest was normalized to β-actin expression, and is presented as a fold change increase or decrease in experimental groups relative to controls.
Protein extraction and western blotting
After RNA extraction, total protein was extracted from the organic layer, using the QIAzol™ lysis reagent protocol (Hummon et al., 2007) for isolation of genomic DNA and/or proteins from fatty tissue, as described in a previous report from our laboratory (Garraway et al., 2014; Qiagen, Valencia, CA). After determining the protein concentration by Bradford Assay (BioRad, Hercules, CA), protein samples were diluted in Laemmli sample buffer and were stored at −80 °C at known concentrations (usually 2–5µg/µl). Western blotting was used for the protein quantification of ERK1/2 and pERK1/2 (~ 42/44 kDa). Equal amounts (30µg) of total protein were subjected to SDS-PAGE with 12% Tris-HEPES precast gels (Pierce, Rockford, IL). After transferring onto PVDF membranes (Millipore, Bedford, MA) by Bio-Rad Semi-dry transfer apparatus, the blots for ERK1/2 and other non-phosphorylated proteins (see below) were blocked for one hour in 5% blotting grade milk (BioRad, Hercules, CA) in Tris-Buffered Saline Tween-20 (TBST), while blots for pERK1/2 were blocked in 5% BSA in TBST. After blocking, the PVDF membranes were incubated overnight at 4 °C in one of the following primary antibodies generated in rabbit: ERK1/2 (1:2000; #06-182 - Millipore, Temecula, CA), pERK1/2 (1:500; #07-467 - Millipore, Temecula, CA). β- actin (1:2500; #Ab8227 - Abcam, Cambridge, MA) served as the control. All primary antibodies were diluted in blocking solution. The following day, PVDF membranes were washed in TBST (3 × 5 min) at room temperature and incubated in HRP-conjugated goat anti-rabbit or anti-mouse secondary antibodies (1:5,000; #31460 or 31430, respectively; Pierce, Rockford, IL) for 1 hour at room temperature. After another 3 × 5 min series of washes, the blots were incubated with ECL (Pierce, Rockford, IL) and were imaged with Fluorchem HD2 (ProteinSimple, Santa Clara, CA). The protein expression for each gene of interest was normalized to β-actin expression and presented as a fold change relative to the sham controls. Other targets of interest including KCC2, phospho-Ser940KCC2, N-cadherin and NKCC1 were assessed in the same fashion.
Fractionation
To assess KCC2 (1:500; #07-432 - Millipore, Temecula, CA) and phospho-Ser940KCC2 (1:1000; PhosphoSolutions, Aurora, CO, USA) expression, spinal cord specimens were homogenized with dounce homogenizer (Kontes), followed by 5 passes through a 22 gauge needle in ice-cold buffer, pH 7.5, containing 10 mm Tris, 300 mm sucrose, and a complete mini protease inhibitor mixture (Roche). Crude homogenates were centrifuged at 5000 RCF for 5 min at 4°C. Supernatant was further fractionated at 13,000 RCF for 30 min. After centrifugation, supernatant was collected as cytoplasmic fraction. A membrane rich fraction was then obtained by resuspending the pellet in PBS (50 µl) containing protease inhibitor. All samples were sonicated and stored at −80°C for later processing (Western blotting). N-cadherin (1:1000; Cell Signaling - Danvers, MA) was used to confirm plasma membrane enrichment. NKCC1 (1:500; #sc-21545 – Santa Cruz, CA) was also tested.
Statistics
All data were analyzed using an analysis of variance (ANOVA) or an analysis of covariance (ANCOVA). Individual variability in mechanical reactivity was controlled for by: (1) Analyzing the test data using an ANCOVA, entering the baseline score as a covariate; and (2) Computing a change from baseline score and analyzing the data using ANOVA. Both sets of analyses yielded similar patterns of statistical significance. Differences between group means were assessed using Duncan’s New Multiple Range post hoc test as needed. In all cases, the criterion for statistical significance was p < 0.05.
Results
Bicuculline blocks shock-induced EMR in spinally transected rats
Prior studies have shown that noxious electrical stimulation induces both a learning impairment and EMR in spinally transected rats (Crown et al., 2002; Ferguson et al., 2006). Further, the learning impairment lasts 24–48 hrs. We have shown that intrathecal administration of bicuculline prior to initial shock exposure (induction), or prior to testing (expression), restores the capacity to learn (Ferguson et al., 2003). Given this, we predicted that bicuculline would also block the shock induced EMR.
A day after surgery, spinally transected and cannulized rats were randomly assigned to one of four treatment conditions (n=8 per group). Subjects were microinjected i.t. with either saline or 0.3µg bicuculline. This dose was selected on the basis of past work demonstrating that it blocks the learning impairment induced by nociceptive stimulation (Ferguson et al., 2003). Fifteen minutes after drug delivery, half the subjects in each drug condition were given 30 min of intermittent shock while other subjects remained unshocked. This yielded a 2 (bicuculline vs. vehicle) × 2 (variable shock vs. unshock) factorial design (illustrated at the top of Fig. 1). Mechanical reactivity was assessed on each paw prior to drug delivery (baseline), prior to leg shock, and again immediately after shock treatment and 1, 2, and 3 hrs later.
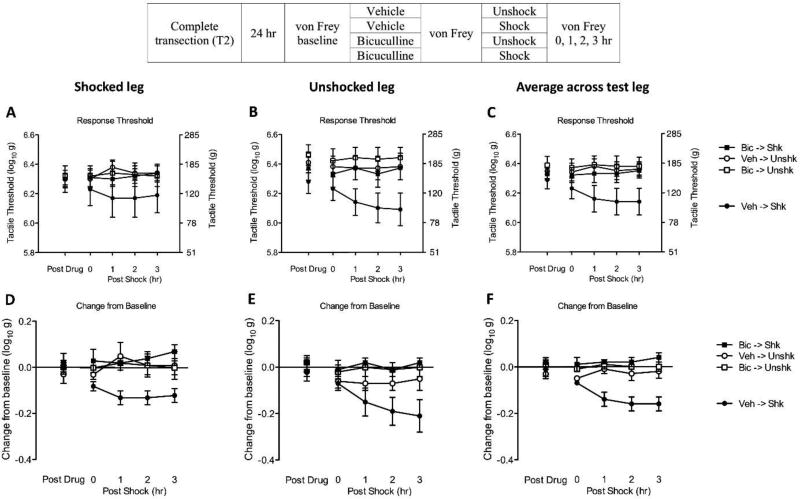
Fig. 1. Bicuculline blocks shock-induced EMR in spinally transected rats.
The experimental design is illustrated at the top of the figure. Subjects that received bicuculline (Bic) or its vehicle (Veh) are depicted as squares and circles, respectively. Groups given intermittent shock (Shk) or nothing (Unshk) are shown in black and white, respectively. (A) Mechanical thresholds on the treated leg after bicuculline administration (Post Drug) and 0, 1, 2, 3 hr after shock treatment (Post Shock). The left y-axis depicts linearized mechanical scores based on a transformation [log 10 (10,000g)] of the force required to bend the thinnest filament that elicited a paw withdraw and the right axis depicts the gram force equivalents. (B) Mechanical thresholds on the untreated leg. (C) Mechanical thresholds averaged across test leg. (D) The change from baseline scores for the treated leg. (E) The change from baseline scores for the untreated leg. (F) The change from baseline scores of averaged across test leg. The error bars depict ± SEM.
Prior to drug treatment, mechanical reactivity scores ranged from 6.27 ± 0.06 to 6.33 ± 0.06 [mean ± standard error of the mean (SE)] across groups. These differences were not statistically significant (p > 0.05). Before shock treatment (Fig. 1), bicuculline administration (Post Drug) did not have a significant effect (p > 0.05).
As in previous studies (Ferguson et al., 2006), shock (Post Shock) induced EMR in vehicle treated rats (Veh->Veh). Pretreatment with bicuculline blocked the development of EMR (Bic->Shk). Statistical analyses of the after shock data were first performed on the raw data (Figs. 1A, 1B, and 1C). To control for variation in baseline reactivity, baseline scores were entered as a covariate using an analysis of covariance (ANCOVA). The main effect of shock and bicuculline treatment, as well as their interaction, were statistically significant (all p < 0.05). The main effect of time, as well as its interactions with shock and bicuculline treatment, were also significant (all p < 0.05). Post hoc comparisons of the group means confirmed that the vehicle treated group that received shock (Veh->Shk) differed from the other groups (p < 0.05). No other group comparison was significant, (p > 0.05).
Variation in baseline reactivity can also be addressed by computing a change from baseline score. An analysis of variance (ANOVA) performed on these scores (Figs. 1D, 1E, and 1F) revealed a significant main effect of bicuculline treatment and a Bicuculline by Shock interaction (both p < 0.05). The Time by Shock interaction was also statistically significant (p < 0.05). Post hoc comparisons confirmed that the vehicle treated group that received shock (Veh-> Shk) differed from the other groups (p < 0.05). No other group comparisons were significant (p > 0.05).
An advantage of the change from baseline score is that our index of variability [the standard error of the mean (SE)] is computed after we adjust for individual differences. Because this simplifies the assessment of group differences, and because an ANCOVA performed on the raw scores and an ANOVA conducted on the change from baseline values yielded the same pattern of statistical significance in subsequent experiments, we present just the change from baseline scores in subsequent experiments.
Bicuculline blocks LPS-induced EMR in spinally transected rats
To explore the generality of our results, we tested whether bicuculline treatment affects the development of the EMR observed after the application of the endotoxin lipopolysaccharide (LPS) to the central nervous system (CNS). LPS induces an immune response, learning impairment, and EMR in spinally transected rats (Reeve et al., 2000; Vichaya et al., 2009; Young et al., 2007). Both the learning impairment and EMR have been linked to an up-regulation of proinflammatory cytokines [e.g., tumor necrosis factor (TNF)] and the development of spinally-mediated central sensitization (Huie et al., 2012; Kanaan et al., 2000; Watkins et al., 1995). Again, we predict that blocking GABA’s action at the GABAA receptor with bicuculline will attenuate, rather than enhance, central sensitization.
A day after surgery, spinally-transected and cannulized rats were microinjected with either vehicle or 0.3µg bicuculline i.t. Fifteen minutes after drug delivery, subjects received either 100 µg LPS or vehicle i.t. This yielded a 2 (bicuculline vs. vehicle) × 2 (LPS vs. vehicle) factorial design (illustrated at the top of Fig. 2) with 8 subjects per group. Mechanical reactivity was assessed on each paw prior to drug delivery (baseline), prior to LPS treatment, and again 0, 1, 2, 3 hr after LPS injection.
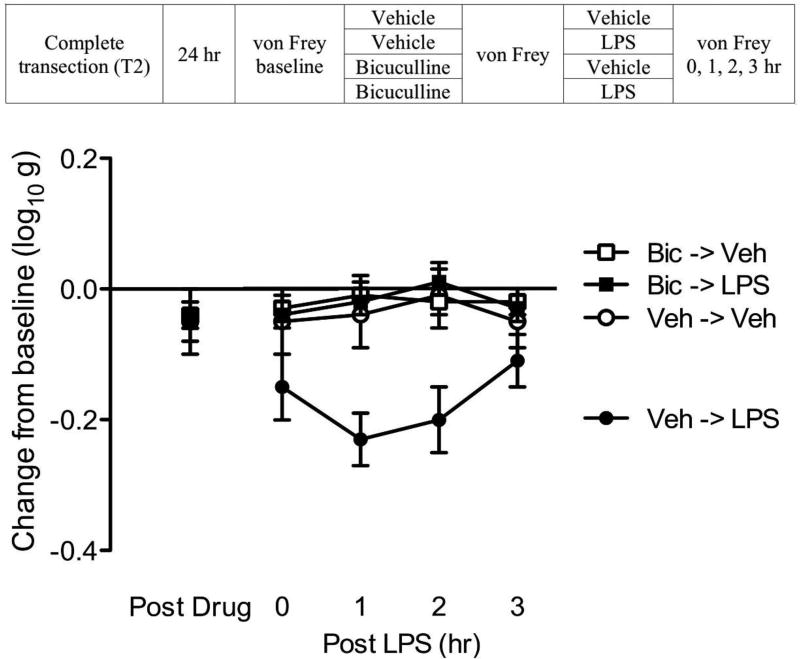
Fig. 2. Bicuculline blocks LPS-induced EMR in spinally transected rats.
The experimental design is illustrated at the top of the figure. Subjects that received bicuculline (Bic) or its vehicle (Veh) are depicted as squares and circles, respectively. Groups given LPS or nothing (Veh) are shown in black and white, respectively. The y-axis depicts the change from baseline scores averaged across test leg after bicuculline (Post Drug), and 0, 1, 2, 3 hr after LPS (Post LPS), treatment. The error bars depict ± SEM.
Mean baseline scores ranged from 6.26 ± 0.05 to 6.36 ± 0.62 (mean ± SE) across groups. These differences were not statistically significant (p > 0.05). Before LPS treatment (Fig. 2), bicuculline administration (Post Drug) had no effect on mechanical reactivity (p > 0.05). LPS treatment (Post LPS) induced EMR (Veh->LPS) and this effect was blocked by pretreatment with bicuculline (Bic->LPS). An ANOVA performed on the change from baseline scores revealed a significant main effect of LPS and bicuculline treatment (both p < 0.05). Also, the LPS × Bicuculline interaction was significant (p < 0.05). Post hoc comparison confirmed that the group that received LPS alone (Veh->LPS) differed from the other groups (p < 0.05). No other group comparison was significant (p > 0.05).
Bicuculline blocks capsaicin-induced EMR in spinally transected rats
We have suggested that the application of shock interferes with spinally-mediated learning, and produces EMR, because it produces a diffuse state of over-excitation within the spinal cord akin to central sensitization (Ferguson et al., 2006; Grau et al., 2006; Grau et al., 2012). Supporting this, we have shown that treatments known to sensitize spinal nociceptive circuits (e.g., peripheral application of carrageenan, formalin, or the TRPV1 receptor agonist capsaicin) produce EMR and inhibit instrumental learning in spinally transected rats (Ferguson et al., 2006; Ferguson et al., 2012; Hook et al., 2008). When coupled with the results reported above, we are led to an odd prediction—that pretreatment with the GABAA antagonist bicuculline should block central sensitization in spinally transected rats. This appears counter-intuitive given the wealth of data demonstrating that treatment with bicuculline sensitizes spinal nociceptive circuits (Baba et al., 2003; Dougherty and Hochman, 2008; Roberts et al., 1986; Sivilotti and Woolf, 1994; Sorkin et al., 1998; Zhang et al., 2001).
A day after surgery, 24 transected and cannulized rats were microinjected with either vehicle or 0.3µg bicuculline i.t. Fifteen minutes after drug delivery, subjects received an intradermal injection of 3% capsaicin or its vehicle in the left or right hind paw (counter-balanced across subjects). This yielded a 2 (bicuculline vs. vehicle) × 2 (capsaicin vs. vehicle) factorial design (illustrated at the top of Fig. 3; n=6 per group). Mechanical reactivity was assessed on each paw prior to drug delivery (baseline), prior to capsaicin injection, and again 0, 1, 2, 3 hr after capsaicin treatment. After the last behavior test (3 hr), subjects were sacrificed. A 1 centimeter section of spinal cord around the lumbar enlargement (L3–L5) region was rapidly removed for subsequent cellular assays.
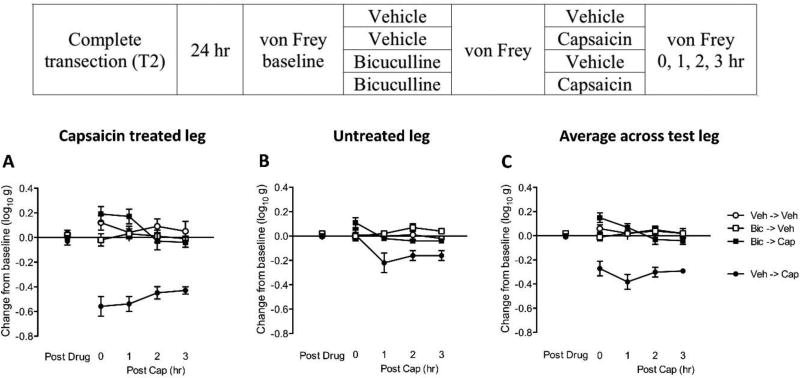
Fig. 3. Bicuculline blocks capsaicin-induced EMR in spinally transected rats.
The experimental design is illustrated at the top of the figure. Subjects that received bicuculline (Bic) or its vehicle (Veh) are depicted as squares and circles, respectively. Groups treated with capsaicin (Cap) or its vehicle (Veh) are shown in black and white, respectively. (A) The y-axis depicts the change from baseline scores for the capsaicin-treated leg after bicuculline (Post Drug), and 0, 1, 2, 3 hr after capsaicin (Post Cap), treatment. (B) The change from baseline scores for the untreated leg. (C) The change from baseline scores averaged over test leg. The error bars depict ± SEM.
Before capsaicin treatment (Fig. 3), bicuculline administration (Post Drug) had no effect on mechanical reactivity (p > 0.05). As expected, capsaicin treatment (Post Cap) induced a weaker EMR on the contralateral (untreated) leg [−0.13 ± 0.03 (Figs. 3B)] relative to the treated leg [−0.5 ± 0.03 (Figs. 3A); p < 0.001]. Nevertheless, the overall pattern of results was similar on both legs: Capsaicin induced a lasting EMR (Veh-> Cap) and this effect was blocked by pretreatment with bicuculline (Bic->Cap). Because similar results were obtained across legs (Figs. 3A & B) in this, and subsequent experiments, we collapsed the data across test leg (Figs. 3C). An ANOVA performed on the mean change from baseline scores showed that the main effect of capsaicin and bicuculline, as well as their interaction, were significant (all p < 0.0001). The Time × Capsaicin interaction, and the three-way interaction of Time × Capsaicin × Bicuculline were also statistically significant (both p < 0.05). Post hoc comparison confirmed that the group that received capsaicin alone (Veh->Cap) differed from the other groups (p < 0.05). No other group comparison was significant, (p > 0.05).
We recognized that that bicuculline might attenuate the sensitization of noiceptive neurons because it affects the development of primary afferent depolarization (PAD), which can have a peripheral effect through antidromic neural activity [dorsal root reflex (DRR); Willis, 1999]. In response to capsaicin treatment, this GABA-dependent effect can induce an increase in peripheral blood flow at the affected site, leading to edema and an increase in skin temperature. To examine whether PAD contributes to the effects reported above, we assessed both paw width and temperature after capsaicin treatment (Data Not Shown). As expected, capsaicin induced an increase on both measures, but neither was affected by i.t. bicuculline. Further, the bilateral nature of our effects suggests that a central (spinal) mechanism is involved.
Across three experimental treatments (shock stimulation, i.t. LPS, and peripheral application of capsaicin) we obtained a similar pattern of results. In all cases, an EMR was induced and this effect was attenuated by pretreatment with the GABAA antagonist bicuculline. While others have noted a reduction in bicuculline-induced EMR in contused rats (Drew et al., 2004), to our knowledge this is the first demonstration that pretreatment with a GABAA antagonist blocks central sensitization after SCI. Interestingly, bicuculline also blocks formalin-induced EMR in diabetic rats (Jolivalt et al., 2008). As will be shown below, these effects appear to be mediated by common neurochemical events.
Bicuculline reverses capsaicin-induced EMR
We have shown that administration of bicuculline prior to capsaicin treatment blocks the induction of EMR in spinally transected rats. For a treatment to be clinically relevant, we also need to establish whether it can reverse the spinally-mediated central sensitization after it has been induced. Prior work has shown that this is not always the case (e.g., Torebjork et al., 1992). The present experiment addresses this issue by comparing the effect of bicuculline treatment given prior to, versus 1 hr after, the induction of capsaicin-induced central sensitization.
Spinally transected and cannulized rats were placed in the restraining tubes and baseline mechanical reactivity was assessed as described above. Subjects were then randomly assigned to one of three conditions (n=6 per group). The first (Bic->Cap) was treated with bicuculline and 15 min later received an injection of capsaicin into the dorsum of one hind paw. To examine whether bicuculline can reverse the EMR after it is induced, the second group (Cap->Bic) received bicuculline 1 hr after capsaicin treatment. A third group (Veh->Cap) received capsaicin alone. Mechanical reactivity was re-tested prior to capsaicin treatment and 0, 1, 2, and 3 hrs after. The test at 1 hr occurred immediately before the Cap->Bic group was administered bicuculline.
Before capsaicin treatment (Fig. 4), mean mechanical reactivity ranged from 6.12 ± 0.07 to 6.19 ± 0.02 (mean ± SE). These group differences did not approach significance (p > 0.05). In the first hour (0–1 hr) after capsaicin treatment (Post Cap), capsaicin induced a significant EMR in the groups that had not received bicuculline (Veh->Cap and Cap->Bic). This effect was blocked by bicuculline pretreatment (Bic->Cap), replicating the results reported above. An ANOVA showed that the groups were significantly different (p < 0.01). Also, the main effect of time, and its interaction with groups, were statistically significant (both p < 0.01). Post hoc comparisons of the 0–1 hr group means confirmed that subjects given bicuculline prior to capsaicin (Bic->Cap) differed from the other groups (p < 0.05). No other group comparison was significant (p > 0.05).
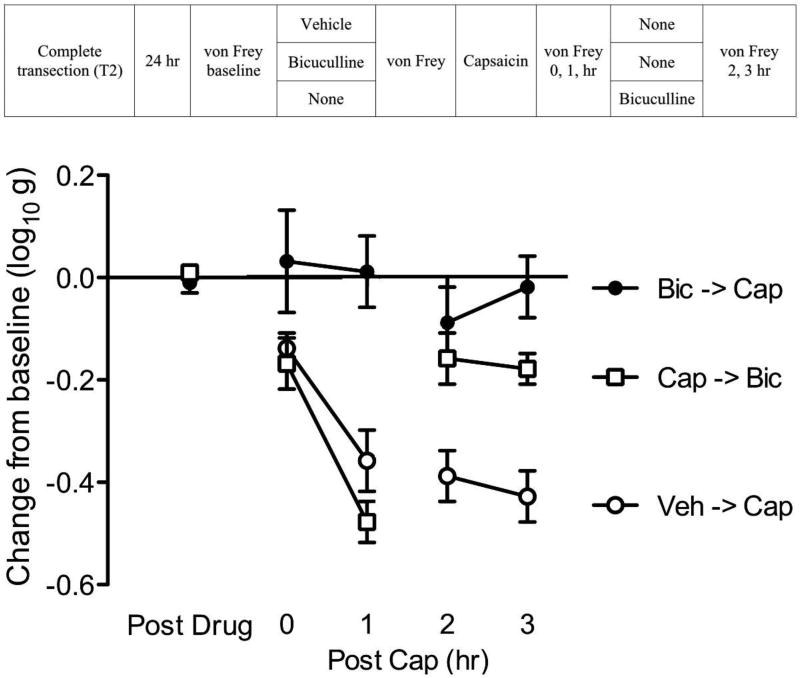
Fig. 4. Bicuculline reverses capsaicin-induced EMR.
The experimental design is illustrated at the top of the figure. Subjects that received vehicle and capsaicin (Veh->Cap) are depicted as open circles, subjects that received bicuculline before capsaicin (Bic->Cap) are depicted as black circles, and subjects that received bicuculline 1hr after capsaicin are depicted as open squares (Cap->Bic). The y-axis depicts the change from baseline scores. Post drug illustrates the thresholds observed after initial drug treatment. The second injection was made 1 hour after the application of capsaicin (Post cap). The error bars depict ± SEM.
During the second and third hour of testing, rats that received bicuculline after capsaicin treatment (Cap->Bic) exhibited a loss of EMR. An ANOVA revealed that the groups were significantly different (p < 0.001). Post hoc comparisons of the 2–3 hr means showed that the group that received capsaicin alone (Veh->Cap) differed from the other groups (p < 0.05). No other group comparison was significant (p > 0.05).
The results imply that the maintenance of capsaicin-induced EMR depends upon activation of the GABAA receptor. EMR is routinely used as a behavioral index of central sensitization. This implies that GABA activity is essential to maintaining a state of over-excitation.
Across experiments, the EMR observed on the untreated leg was somewhat weaker. Nonetheless, the effect of drug treatment was unchanged. Because this remained true in all of the subsequent experiments, we focus on the averaged data (collapsed across test legs) and only present the data for each leg when we introduce a new surgical procedure (examining the effect of capsaicin in sham operated rats).
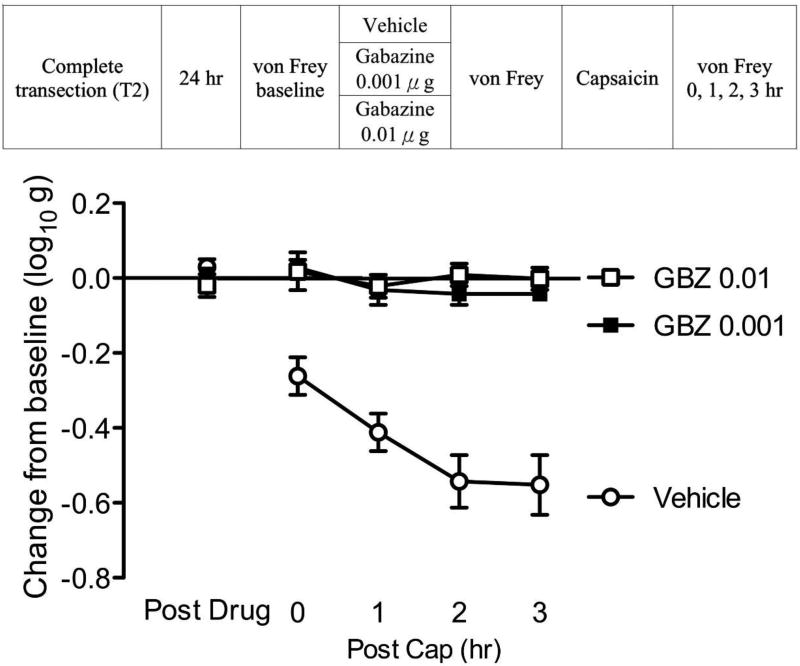
Gabazine blocks capsaicin induced EMR
The present results show that the GABAA receptor antagonist bicuculline blocks capsaicin-induced EMR in spinally transected rats. However, bicuculline also affects Ca2+-activated potassium channels (Khawaled et al., 1999). Given this, we sought further evidence that the GABAA receptor plays a critical role. This was accomplished by assessing the impact of another GABAA receptor antagonist (gabazine) on capsaicin-induced EMR.
Spinally-transected and cannulized rats were microinjected i.t. with one of three doses of gabazine [0.0 (vehicle), 0.001, 0.01 µg; see Fig. 5]. Fifteen minutes after drug delivery, subjects in each group (n=6) received intradermal capsaicin to the left or right hind paw (counter-balanced across subjects). Mechanical reactivity was assessed on each paw prior to drug delivery (baseline), prior to capsaicin injection, and again 0, 1, 2, 3 hr following capsaicin treatment.
Fig. 5. Gabazine blocks capsaicin induced EMR.
The experimental design is illustrated at the top of the figure. Subjects that received gabazine (GBZ) are shown in squares and subjects that received vehicle are shown in triangles. The y-axis depicts the change from baseline after drug injection (Post Drug), and 0, 1, 2, 3 hr after capsaicin treatment (Post Cap). The error bars depict ± SEM.
Before capsaicin treatment (Fig. 5), drug administration (Post Drug) had no effect on mechanical reactivity (p > 0.05). Capsaicin treatment (Post Cap) induced a lasting EMR (Vehicle). This effect was blocked by pretreatment with gabazine (GBZ 0.01, GBZ 0.001). An ANOVA showed that the main effect of drug and time, as well as their interaction, were statistically significant (all p < 0.0001). Post hoc comparisons confirmed that the group that received capsaicin alone (Vehicle) differed from the other groups (p < 0.05). These data reinforce our conclusion that the GABAA receptor plays an essential role in central sensitization after spinal injury.
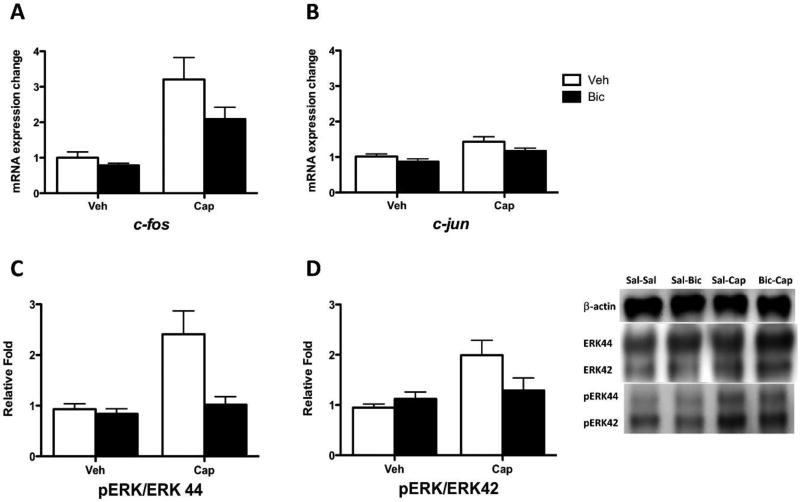
Bicuculline blocks cellular indices of central sensitization in spinally transected rats
Prior work has shown that central sensitization is associated with the expression of c-fos and pERK within the dorsal horn of the spinal cord (Gao and Ji, 2009). To reinforce our behavioral results, the present experiment examined whether pretreatment with bicuculline blocks the expression of these cellular indices of central sensitization. For comparison, another transcription factor (c-jun) was also assessed. For ERK, two isoforms (42 and 44) were evaluated and, in both cases, ERK activation was derived by computing the ratio of pERK to ERK.
Twenty-four spinally transected rats were treated with bicuculline and capsaicin (or their vehicle) as described in the third experiment. All details were the same, except that behavioral testing was ended 2 hr after capsaicin treatment. After behavioral testing, the spinal cord samples (24, n=8) were hemi-dissected into dorsal and ventral halves, and the dorsal halves were subjected to RNA extraction for qRT-PCR. The mRNA expression for each gene of interest was normalized to β-actin expression level, and is presented as a fold change relative to the sham controls. Total protein was extracted from the organic layer of the samples and was used for Western blotting. The protein expression for each target was normalized to β-actin expression level and calculated as a fold change relative to the sham controls. Subsequently, the protein expression of pERK1/2 was normalized to ERK1/2 expression yielding a pERK/ERK ratio.
As reported above, subjects that did not receive capsaicin (Veh->Veh, Bic->Veh) exhibited little change in mechanical reactivity (0.00 ± 0.03 to 0.02 ± 0.01). Capsaicin treatment (Veh->Cap) induced a robust EMR (−0.47 ± 0.07). This effect was blocked (0.15 ± 0.05) in subjects pretreated with bicuculline (Bic->Cap). An ANOVA confirmed that these behavioral effects were statistically significant (all p < 0.05). Capsaicin induced an increase in c-fos mRNA expression level in the dorsal region and this effect was attenuated by pretreatment with bicuculline (Fig. 6A; p < 0.0001). Post hoc comparisons confirmed that the group that received bicuculline before capsaicin (Bic->Cap) differed from the other groups (p < 0.05). Also, the group that received capsaicin alone (Veh->Cap) differed from the other groups (p < 0.05). No other group comparison was significant (p > 0.05).
Fig. 6. Bicuculline blocks cellular indices of central sensitization in spinally transected rats.
(A) mRNA c-fos expression in subjects that had previously received vehicle (Veh) or capsaicin (Cap). Subjects that received bicuculline (Bic) or saline (Veh) are shown in black and white bars, respectively. (B) c-jun expression. (C) pERK 44 ratio expression. (D) pERK 42 ratio expression. Representative Western blots for ERK and pERK expression are provided to the right of panel D. The error bars depict ± SEM.
Capsaicin treatment also induced an increase in c-jun mRNA expression level within the dorsal horn (Fig. 6B; p < 0.001). However, bicuculline did not significantly reduce c-jun expression (p > 0.05).
Capsaicin treatment generally increased ERK and pERK expression [2.46 ± 0.01 to 5.7 ± 0.64 (mean ± SEM) fold; both p < 0.001]. Most importantly, it increased the relative phosphorylation of ERK, yielding an increase in the ratio of pERK to ERK in the dorsal region for both isoforms (ERK44 and ERK42; both p < 0.05; Fig. 6C & D). Pretreatment with bicuculline attenuated this effect. For ERK44, the ANOVA also revealed a significant main effect of bicuculline and its interaction with capsaicin treatment (both p < 0.05). For both isoforms, post hoc comparisons confirmed that the group that received capsaicin alone (Veh->Cap) differed from the other groups (p < 0.05). No other group comparison was significant (p > 0.05).
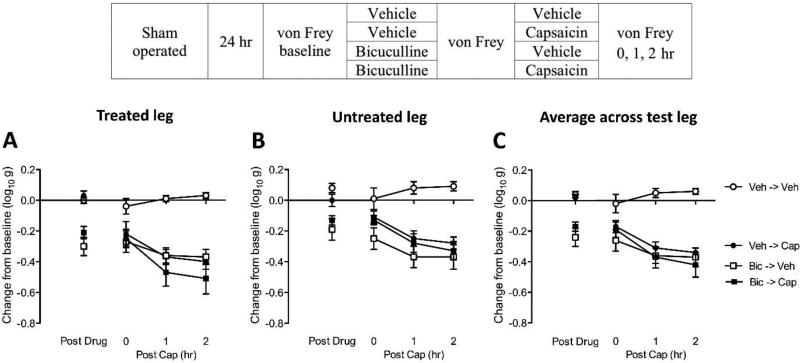
Bicuculline enhances central sensitization in sham-operated rats
Our cellular assays reinforce our behavioral data and suggest that the GABAA receptor antagonist bicuculline blocks capsaicin-induced central sensitization in spinally transected rats. Because this pattern of results stands in contrast to what has been found in intact rats (Baba et al., 2003; Sorkin et al., 1998; Zhang et al., 2001), we examined whether a different pattern of results would be observed in the absence of SCI.
To facilitate comparison across experiments, all details (including surgery) were the same as reported above, except the spinal cord was not transected. Sham-operated and cannulized rat subjects (n=8 per group) were microinjected with either vehicle or 0.3µg bicuculline i.t. (see Fig. 7). Subjects in each group then received either intradermal 3% capsaicin or its vehicle in the left or right hind paw (counter-balanced across subjects), which yielded a 2 (bicuculline vs. saline) × 2 (capsaicin vs. vehicle) factorial design. Mechanical reactivity was assessed on each paw prior to drug delivery (baseline), prior to capsaicin injection, and again 0, 1, 2 hr after capsaicin treatment. Immediately after the last behavior test, subjects were sacrificed. One centimeter of spinal cord around the lumbar enlargement (L3–L5) region was rapidly removed for cellular assay. c-fos and c-jun expression were assessed using qRT-PCR, as described above. ERK and pERK were measured using Western blotting.
Fig. 7. Bicuculline enhances nociceptive reactivity, and fails to block capsaicin induced EMR, in sham-operated rats.
The experimental design is illustrated at the top of the figure. Subjects that received bicuculline (Bic) and vehicle (Veh) are depicted as squares and circles, respectively. Groups treated with capsaicin (Cap) or its vehicle (Veh) are shown in black and white, respectively. (A) The y-axis depicts the change from baseline scores for the capsaicin-treated leg after bicuculline (Post Drug), and 0, 1, 2, 3 hr after capsaicin (Post Cap), treatment. (B) The change from baseline scores for the untreated leg. (C) The change from baseline scores averaged over test leg. The error bars depict ± SEM.
In sham operated rats, bicuculline per se (Post Drug) induced EMR on both legs (p < 0.0001; Fig. 7). Post hoc comparisons confirmed that the groups that received bicuculline (Bic->Veh, Bic->Cap) differed from the other groups (p < 0.05). Over the subsequent 2 hrs of testing (Post Cap), bicuculline treated rats (Bic->Veh) remained more responsive to mechanical stimulation, relative to the untreated controls (Veh->Veh). Subjects that received capsaicin alone (Veh->Cap) exhibited a robust EMR relative to the controls (Veh->Veh) and this effect was not attenuated by pretreatment with bicuculline (Bic->Cap). A similar pattern of results was observed on both the treated (Fig. 7A) and untreated (Fig. 7B) legs. An ANOVA confirmed that the main effects of bicuculline and capsaicin treatment, as well as their interaction, were significant (both p > 0.05). For both the treated and untreated leg, post hoc comparisons confirmed that the group that received capsaicin alone (Veh->Cap) differed from the other groups (p < 0.05).
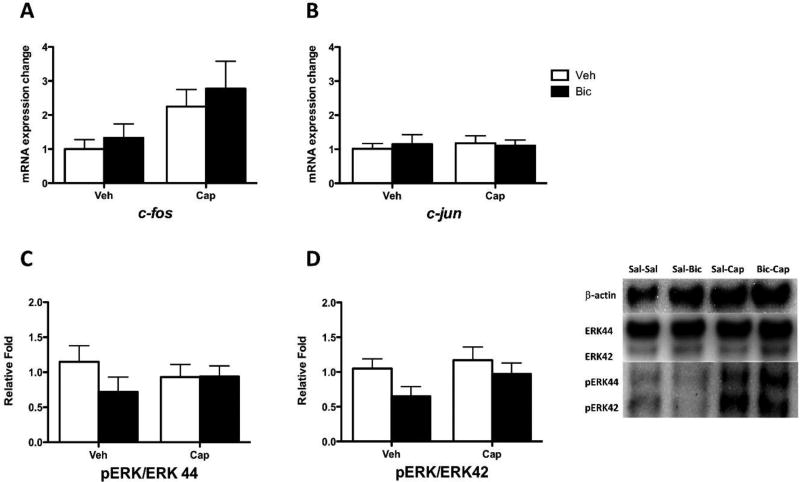
We then evaluated the effect of bicuculline on cellular indices of central sensitization. Treatment with capsaicin induced an increase in c-fos mRNA expression level in dorsal horn (p < 0.05; Fig. 8A) that was not affected by bicuculline (p > .05). For c-jun mRNA expression (Fig. 8B), neither capsaicin nor bicuculline treatment had a statistically significant effect (p > 0.05).
Fig. 8. Bicuculline does not affect cellular indices of central sensitization in sham-operated rats.
(A) mRNA c-fos expression in subjects that had previously received vehicle (Veh) or capsaicin (Cap). Subjects that received bicuculline (Bic) or saline (Veh) are shown in black and white bars, respectively. (B) c-jun expression. (C) pERK 44 ratio expression. (D) pERK 42 ratio expression. Representative Western blots for ERK and pERK expression are provided to the right of panel D. The error bars depict ± SEM.
With the exception of ERK42 (p > 0.05), capsaicin increased ERK and pERK expression in the dorsal region (1.59 ± 0.35 to 1.88 ± 0.34; all p < 0.05). Capsaicin did not, however, increase the ratio of pERK to ERK (Fig. 8C & D; p > 0.05).
Our results suggest that bicuculline attenuates capsaicin-induced central sensitization after SCI, but not in uninjured subjects. Interestingly, our cellular assays suggest that spinal injury amplified capsaicin-induced ERK and pERK expression [SCI: 4.03 ± 0.67; Sham: 1.71 ± 0.12 (mean ± SEM, fold change collapsed across isoforms); p < 0.05]. Furthermore, capsaicin only increased the ratio of pERK to ERK in injured rats [SCI: 2.34 ± 0.38; Sham: 0.96 ± 0.18 (mean ± SEM) ; p < 0.05]. These observations are consistent with prior work demonstrating that brain systems can quell the development of spinally-mediated central sensitization (Heinricher et al., 2009; Liu et al., 2010; Millan, 2002).
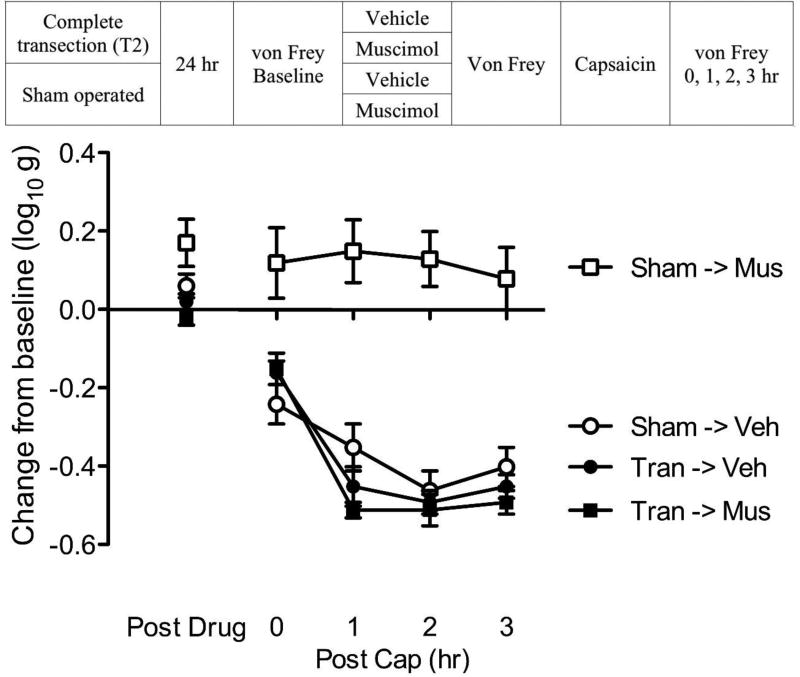
SCI alters how a GABAAR agonist (muscimol) affects capsaicin induced EMR
If SCI impacts GABA-mediated inhibition, it should also affect the impact of a receptor agonist. We explored this possibility by testing the effect of the GABAA receptor agonist muscimol on capsaicin-induced EMR in sham-operated and transected rats.
Thirty-two rats were randomly assigned to receive a spinal transection at T2 or sham-surgery (see Fig. 9). Baseline behavioral reactivity was tested using von Frey stimuli 24 hr later. Half of the rats from each group (n=8 per group) were then microinjected with 0.1% muscimol or vehicle i.t. The dose used was based on prior work (Ferguson et al., 2003). Fifteen minutes after drug delivery, all subjects were treated with capsaicin applied to the left or right hind paw (counter-balanced across subjects). Mechanical reactivity was assessed on each paw prior to drug delivery (baseline), prior to capsaicin injection, and again 0, 1, 2, 3 hr following capsaicin treatment.
Fig. 9. SCI alters how a GABAAR agonist (muscimol) affects capsaicin induced EMR.
The experimental design is illustrated at the top of the figure. Subjects that received muscimol (Mus) or its vehicle (Veh) are depicted as squares and circles, respectively. Groups that are transected (Tran) or sham-operated (Sham) are shown in black and white, respectively. The left y-axis depicts the change from baseline scores after muscimol treatment (Post Drug) and 0, 1, 2, 3 hr after the application of capsaicin (Post Cap). The error bars depict ± SEM.
As expected (Fig. 9), intact rats were more responsive than spinally transected subjects (Group means ± SE: 5.69 ± 0.06 and 6.05 ± 0.02; p < 0.0001). Prior to capsaicin treatment (Post Drug), muscimol reduced mechanical reactivity in sham operated, but not transected, rats. An ANOVA confirmed that the impact of surgery, and its interaction with muscimol, were statistically significant (both p < 0.05). Post hoc comparisons showed that sham-operated rats that received muscimol (Sham->Mus) differed from other groups (p < 0.05). No other comparisons were significant (p > 0.05)
After capsaicin treatment (Post Cap), muscimol inhibited capsaicin-induced EMR in intact, but not transected rats. An ANOVA revealed that the main effect of surgery and muscimol, as well as their interaction, were statistically significant (all p < 0.0001). Also, the main effect of time, and its interaction with surgery were significant (both p < 0.0001). Post hoc comparisons of the group means confirmed that sham-operated intact rats that received muscimol (Tran->Mus) differed from the other groups (p < 0.05). No other comparison was significant (p > 0.05).
As reported by others (Hwang and Yaksh, 1997; Kaneko and Hammond, 1997), pretreatment with a GABAA agonist inhibited mechanical reactivity, and capsaicin-induced EMR, in uninjured rats. SCI eliminated this inhibitory effect.
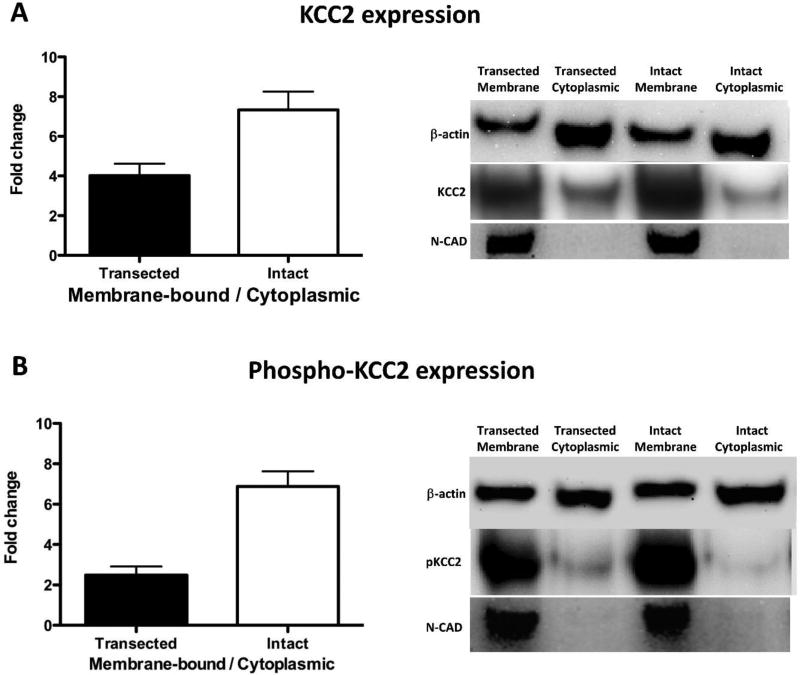
Spinal transection reduces membrane-bound KCC2 expression
The results show that SCI transforms how GABA affects nociceptive processes, which reduces its inhibitory effect and initiates a modification that causes GABA to play an essential role in the development and maintenance of spinally-mediated central sensitization. These changes could be due to an injury-induced down-regulation of membrane-bound KCC2 (Ben-Ari, 2002; Bos et al., 2013; Boulenguez et al., 2010; Jolivalt et al., 2008) which would increase intracellular chloride concentrations ([Cl−]i) and reduce GABA-dependent neural inhibition (Cramer et al., 2008; Hasbargen et al., 2010). To investigate this possibility, we examined cytoplasmic and membrane-bound KCC2 and NKCC1 protein levels in sham operated and injured rats using Western blotting.
Twelve rats were randomly assigned to receive a spinal transection at T2 or sham-operation. Baseline behavioral reactivity was tested using von Frey stimuli 24 hr later. Subjects were then sacrificed and a one-centimeter section of the spinal cord containing the lumbar enlargement (L3–L5) region was rapidly removed. Samples were hemi-dissected and the dorsal halves were subject to homogenization, protein extraction, and fractionation (membrane-bound and cytoplasmic fraction) for Western blotting. We also evaluated N-cadherin (N-CAD) level with Western blotting to verify our fractionation procedure (Fig. 9). KCC2 [inactive and phosphorylated (pKCC2) form] and NKCC1 protein expression were normalized to β-actin expression level, and are presented as a fold change in the transected group relative to the sham group. The protein expression of membrane-bound fraction was then normalized to the cytoplasmic fraction yielding a membrane-bound/cytoplasmic ratio.
At the behavioral level, intact rats were more responsive than spinally transected subjects (sham= 5.52 ± 0.09, injured= 6.11 ± 0.02; p < 0.001). At the protein expression level, spinal transection reduced the ratio of membrane to cytoplasmic KCC2 by 42% (p < 0.05; Fig. 10A). For phospho-KCC2, the ratio was reduced by 63% (Fig. 10B; p < 0.05). Spinal transection did not have a significant effect on NKCC1 expression (p > 0.05) (data not shown).
Fig. 10. Spinal transection reduces membrane-bound KCC2 expression.
(A) The fold change of membrane-bound/cytoplasmic KCC2 ratio. (B) The fold change of membrane-bound/cytoplasmic phospho-KCC2 ratio. Representative Western blots are provided to the right of each panel. The error bars depict ± SEM.
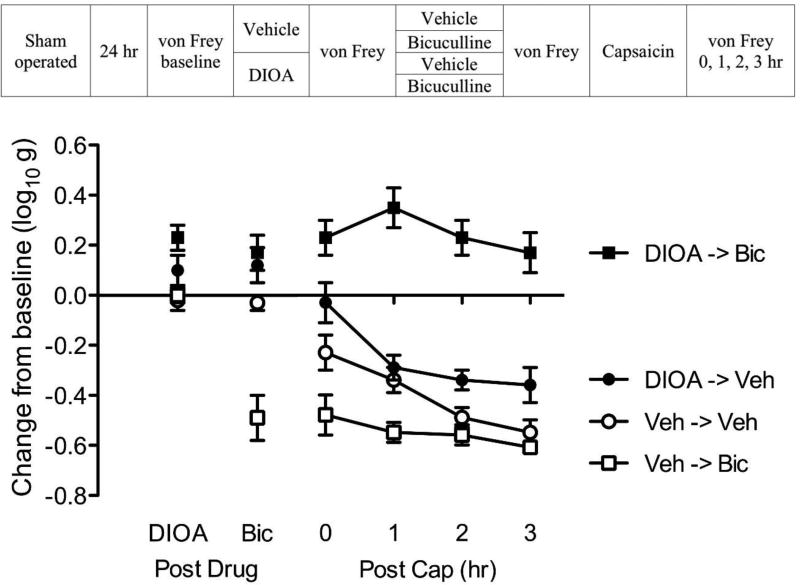
Blocking KCC2 with DIOA transforms GABA function in intact rats
Our results suggest that SCI alters GABA function because it leads to a reduction in membrane-bound KCC2. This suggests that we should be able to emulate the effect of SCI in uninjured (intact) rats by pharmacologically blocking the KCC2 channel with the drug DIOA. We predict that DIOA will transform how GABA affects nociceptive transmission, causing GABA to have a depolarizing effect (Jolivalt et al., 2008; Rheims et al., 2009). Under these conditions, bicuculline should attenuate capsaicin-induced EMR in uninjured rats.
To test this hypothesis, sham operated rats underwent a laminectomy, but the spinal cord was not injured. An intrathecal catheter was inserted and, 24 hrs later (see Fig. 11), subjects were microinjected with 20 µg DIOA i.t. or its vehicle (n=8 per group). Fifteen minutes after drug delivery, subjects received an i.t. injection of 0.3% bicuculline or its vehicle. Fifteen minutes later, all subjects received an intradermal injection of capsaicin on one hindlimb (counter-balanced across subjects). This yielded a 2 (DIOA vs. vehicle) × 2 (bicuculline vs. vehicle) factorial design. Mechanical reactivity was assessed on each paw prior to drug delivery (baseline), after drug treatments, and again 0, 1, 2, 3 hr following capsaicin treatment.
Fig. 11. Blocking KCC2 with DIOA transforms GABA function in intact rats.
The experimental design is illustrated at the top of the figure. Subjects that received DIOA or its vehicle (Veh) are shown in black and white, respectively. Groups given bicuculline (Bic) or its vehicle (Veh) are depicted with squares and circles, respectively. The y-axis depicts the change from baseline after DIOA and bicuculline treatment (Post Drug), and 0, 1, 2, 3 hr after capsaicin treatment (Post Cap). The error bars depict ± SEM.
DIOA treatment (Post Drug, Fig. 11) produced a slight reduction in mechanical reactivity, which mirrored the effect of spinal transection (p < 0.05). Administration of bicuculline induced EMR in the vehicle treated (Veh->Bic), but not DIOA treated (DIOA->Bic), rats. An ANOVA confirmed that the main effect of DIOA and bicuculline, as well as their interaction, were statistically significant (all p < 0.05). Post hoc comparisons confirmed that the group that received bicuculline alone (Veh->Bic) differed from the other groups (p < 0.05). No other group comparison was significant (p > 0.05).
Capsaicin treatment (Post Cap) induced a lasting EMR (Veh->Veh) that was enhanced by bicuculline treatment (Veh->Bic). Pretreatment with DIOA transformed how bicuculline affected capsaicin-induced EMR (DIOA->Bic), unveiling an antinociceptive-like effect that attenuated capsaicin-induced EMR. An ANOVA showed that the main effect of DIOA and bicuculline, as well as their interaction, were statistically significant (all p < 0.0001). Also, the main effect of time, and its interaction with bicuculline, were significant (both p < 0.05). Post hoc comparisons of the group means confirmed that the group that received DIOA and bicuculline (DIOA->Bic) differed from the other groups (p < 0.05). In addition, the group that received DIOA alone (DIOA->Veh) differed from the group that received bicuculline alone (Veh->Bic). No other group comparison was significant (p > 0.05).
In summary, DIOA treatment induced a state that emulated the effect of spinal transection, eliminating the EMR induced by bicuculline and inverting the drug’s effect on capsaicin-induced EMR.
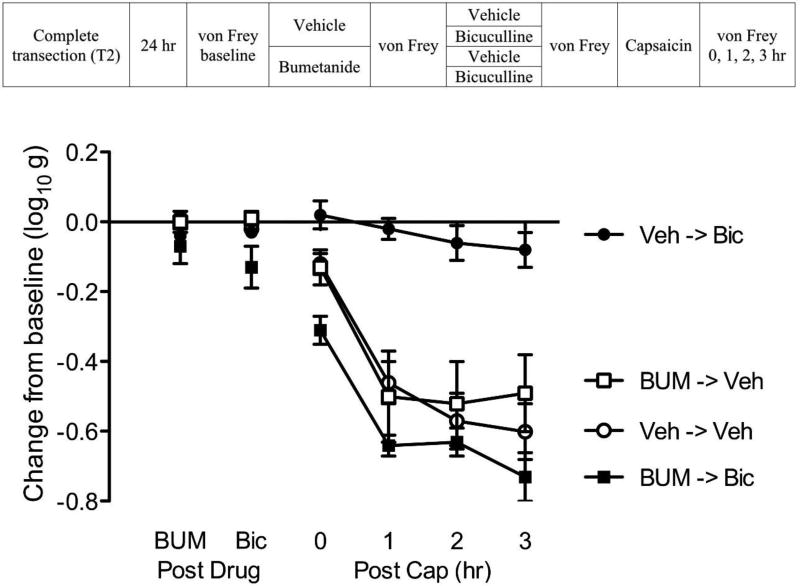
Bumetanide transforms GABA function in spinally-transected rats
Others have shown that blocking the inward flow of Cl− through the NKCC1 channel (with bumetanide) can compensate for reduced KCC2 membrane-bound expression and lower intracellular Cl− concentration, thereby reinstating GABA-dependent inhibitory tone (Cramer et al., 2008; Hasbargen et al., 2010). This suggests that administration of a NKCC1 antagonist may switch the effect of bicuculline in spinally transected rats, reinstating a behavioral pattern indicative of inhibition.
Spinally-transected and cannulized rats (n=6 per group) were microinjected with either vehicle or 1mM bumetanide (BUM; i.t.; see Fig. 12). Fifteen minutes after drug delivery, subjects in each group received either the vehicle (saline) or bicuculline i.t.. Fifteen minutes later, all subjects received an intradermal injection of capsaicin on one hindlimb (counter-balanced across subjects). Mechanical reactivity was assessed on each paw prior to drug delivery (baseline), after drug (BUM and BIC) injection, and again 0, 1, 2, 3 hr following capsaicin treatment.
Fig. 12. Bumetanide transforms GABA function in spinally-transected rats.
The experimental design is illustrated at the top of the figure. Subjects that received bumetanide (BUM) or its vehicle (Veh) are shown in black and white, respectively. Groups given bicuculline (Bic) or its vehicle (Veh) administration are depicted as squares and circles, respectively. The y-axis depicts the change from baseline after BUM and bicuculline treatment (Post Drug), and 0, 1, 2, 3 hr after the application of capsaicin (Post Cap). The error bars depict ± SEM.
Before bicuculline treatment (Post Drug, Fig. 12), BUM had no effect on mechanical reactivity (p > 0.05). Before capsaicin treatment, bicuculline induced EMR in BUM treated subjects (BUM->Bic). An ANOVA showed that the interaction of BUM with bicuculline treatment was statistically significant (p < 0.05). Post hoc comparisons confirmed that the group that received BUM and bicuculline (BUM->Bic) differed from the other groups (p < 0.05). No other group comparison was significant (p > 0.05).
Capsaicin treatment (Post Cap) induced a lasting EMR (Veh->Veh, BUM->Veh). This effect was blocked by pretreatment with bicuculline alone (Veh->Bic). Pretreatment with BUM blocked the effect of bicuculline (BUM->Bic). An ANOVA showed that the main effect of BUM and bicuculline, as well as their interaction, were statistically significant (all p < 0.05). Also, the main effect of time, and its interaction with BUM and bicuculline treatment, as well as the Time × BUM × Bicuculline three-way interaction, were significant (all p < 0.05). Post hoc comparisons of the group means confirmed that the group that received bicuculline alone (Veh->Bic) differed from the other groups (p < 0.05). No other group comparison was statistically significant (p > 0.05).
Blocking NKCC1 channel with bumetanide in transected rats emulated the pattern of results observed in intact rats, producing a state wherein bicuculline induced EMR and failed to attenuate capsaicin-induced EMR.
Discussion
Noxious stimulation and inflammation can enhance nociceptive processing within the spinal cord, a form of central sensitization that has been linked to the development of chronic pain (Woolf, 2011). The emergence of central sensitization is regulated by both descending fibers and GABAergic interneurons within the spinal cord that are designed to quell neural excitability (Gwak and Hulsebosch, 2011; Millan, 2002). We posited that SCI disrupts this homeostatic balance (Viemari et al., 2011; Wenner, 2014), recapitulating an earlier developmental state wherein GABA has an excitatory effect. Supporting this, we showed that pretreatment with the GABAA antagonist bicuculline blocks the EMR induced by noxious electrical stimulation, LPS-induced inflammation, or peripheral capsaicin treatment. The induction of capsaicin-induced EMR was also blocked by the GABAA antagonist gabazine. Bicuculline reversed capsaicin-induced EMR, implying that GABAergic neurons play a role in the maintenance of central sensitization after SCI.
The development of spinally-mediated central sensitization is associated with increased expression of c-fos and activation of ERK protein in dorsal horn neurons (Gao and Ji, 2009; Zhuang et al., 2005). Capsaicin treatment enhanced the expression of c-fos and pERK to ERK ratio after SCI and these effects were attenuated by bicuculline pretreatment. Taken together, the behavioral and cellular data imply that GABA drives, rather than inhibits, the development of central sensitization after SCI.
Our results stand in contrast to work examining the effect of bicuculline treatment on nociceptive reactivity in uninjured animals, where blocking GABA transmission has been shown to generally enhance nociceptive reactivity (Sorkin et al., 1998; Zhang et al., 2001). We replicated this observation demonstrating that in uninjured (sham operated) rats bicuculline per se induces EMR. Further, behavioral and cellular indices of capsaicin-induced central sensitization were not attenuated by bicuculline treatment. Finally, we showed that administration of a GABAA agonist (muscimol) inhibited the development of capsaicin-induced EMR in uninjured, but not spinally transected, rats.
Others have shown that SCI can impact GABA function by down-regulating KCC2 (Boulenguez et al., 2010). We confirmed that the ratio of membrane-bound to cytoplasmic KCC2 is reduced by SCI. We then attempted to emulate the effect of SCI using a drug (DIOA) that blocks KCC2 function. In uninjured rats, administration of DIOA eliminated bicuculline-induced EMR. More importantly, in DIOA treated intact rats, bicuculline attenuated capsaicin-induced central sensitization, an outcome that parallels the effect of SCI. We then asked whether a drug treatment (administration of the NKCC1 blocker bumetanide) that should restore low [Cl−]i, and GABA-dependent inhibition, eliminates the paradoxical effect of bicuculline after SCI. As predicted, in bumetanide treated transected rats, bicuculline induced EMR and failed to attenuate capsaicin-induced EMR—a pattern identical to that observed in intact rats.
Relation to other studies examining how KCC2 affects nociceptive processing
It is widely recognized that alterations in GABAergic function can impact nociceptive processing within the spinal cord (Gwak and Hulsebosch, 2011). In the absence of injury or pathology, GABA release appears to generally inhibit neural firing in nociceptive responsive neurons (Latremoliere and Woolf, 2009). Under these conditions, administration of a GABA antagonist typically enhances behavioral and cellular indices of nociceptive reactivity (Roberts et al., 1986; Sivilotti and Woolf, 1994). At the same time, it is becoming increasingly clear that how GABA affects nociceptive processing is altered by injury and pathology. After SCI, increased motor drive often leads to a lasting spasticity. This effect has been linked to a down-regulation of KCC2 and a loss of GABA-dependent neural inhibition (Boulenguez et al., 2010). Likewise, the enhanced mechanical reactivity observed after a spinal cord hemisection has been coupled to these effects (Drew et al., 2004; Lu et al., 2008). Pathology can also alter spinal function. For example, diabetes-induced neuropathy enhances nociceptive reactivity to a peripherally applied irritant (formalin; Jolivalt et al., 2008). It had been assumed that this reflected an increase in excitatory transmission. However, microdialysis revealed that the release of excitatory transmitters was reduced, and GABA was increased, in diabetic animals treated with formalin (Calcutt et al., 2000; Malmberg et al., 2006). Under these conditions, bicuculline attenuated formalin-induced pain and this effect too was related to a reduction in membrane-bound KCC2.
While it was clear from past work that alterations in KCC2 function can have a long-term effect, contributing to chronic pain and spasticity weeks after injury, little was known regarding the acute effect of injury. Further, to our knowledge, no study has examined how alterations in GABA function affect the development of spinally-mediated central sensitization. This is important because nociceptive input can induce a form of maladaptive plasticity that impairs adaptive motor behavior and undermines recovery after a contusion injury (Ferguson et al., 2012; Grau et al., 2014). Our hypothesis was that injury places spinal circuits in a vulnerable state because it transforms how GABA affects nociceptive circuits, replacing its usual inhibitory effect with an excitatory drive that contributes to the development of central sensitization. Supporting this, we showed that pretreatment with a GABAA antagonist blocks the development of central sensitization. We further showed that spinal injury brings about a reduction in membrane-bound KCC2 that is evident within 24 hrs. While others have reported a reduction 1–2 days after injury (Boulenguez et al., 2010), the effect reported here appears more robust. A stronger effect may have been observed because we used a remote (T2) cut to disrupt communication with the brain. Other studies have used a lower-level injury and explored changes at the site of injury (Bos et al., 2013; Boulenguez et al., 2010; Cramer et al., 2008).
GABAergic modulation of central sensitization: Potential mechanisms
The traditional model of central sensitization focuses on NMDAR-mediated plasticity within the spinal cord and assumes that this process is regulated by both descending circuits and GABAergic inhibition (Gjerstad et al., 2001; Gwak and Hulsebosch, 2011; Washburn et al., 2007; Woolf, 2004). This general framework is supported by 20+ years of research. The present paper, in conjunction with other recent findings, suggest that there is more than one path to central sensitization within the dorsal horn. Specifically, after spinal injury, neural over-excitation may be driven (rather than inhibited) by GABAergic neurons. Under these conditions, GABA plays an essential (necessary) role in the development of central sensitization and, for this reason, behavioral and cellular indices of central sensitization are blocked by bicuculline.
Within the spinal cord, GABA-dependent depolarization could impact the development of central sensitization in multiple ways. One possibility is that GABAergic neurons are wired to provide a source of negative feedback designed to limit over-excitation (Zeilhofer et al., 2012). Within this type of circuit, a switch in polarity would cause GABA release to drive, rather than inhibit, nociceptive circuits. An alternative is suggested by anatomical/electrophysiological work demonstrating that C and A fibers project to nociceptive neurons within the dorsal horn in two ways, directly and indirectly through inhibitory interneurons (Lu et al., 2008). Under normal conditions, the latter connections would (if anything) inhibit the development of central sensitization. However, when membrane-bound KCC2 is reduced, this GABAergic input could provide an excitatory drive, awakening silent circuits that would foster the development of central sensitization.
Brain-dependent processes regulate central sensitization and spinal KCC2
An intriguing feature of the present results is that capsaicin produced a comparable enhancement in mechanical reactivity in both transected and sham operated rats, but produced a much larger increase in pERK after spinal injury. The latter observation suggests that injury fosters the development of central sensitization within the spinal cord. Given this alone, we would have expected to observe a weaker EMR in uninjured animals. The fact this was not observed suggests that other (presumably, brain-dependent) processes contribute to the sensitization of nociceptive behavior in uninjured rats. Interestingly, other forms of noxious input (e.g., peripheral nerve injury) induce an EMR in spinally intact rats that is associated with the activation of microglia and the down-regulation of membrane-bound KCC2 within the dorsal horn (Coull et al., 2005; Miletic and Miletic, 2008). Combined with the present results, it could be suggested that a down-regulation in KCC2 is a prerequisite to the emergence of central sensitization within the spinal cord in both injured and uninjured animals.
Blocking GABA transmission has also been reported to have a paradoxical effect in anesthetized rats. For example, Garcia-Nicas et al. (2006) showed that capsaicin sensitizes neural activity within the dorsal horn in pentobarbital anesthetized rats and that this effect is blocked by pretreatment with a GABAA antagonist. A similar pattern was observed by Weng et al. (1998) who showed that bicuculline blocks central sensitization in halothane anaesthetized arthritic rats. Evidence suggests that pentobarbital anesthesia disrupts brain-dependent processes that normally counter the development of central sensitization (Washburn et al., 2007). This effect has been linked to serotonergic fibers that descend through the dorsolateral funiculus (DLF) (Crown and Grau, 2005). From this perspective, both anesthesia and spinal injury transform how bicuculline affects central sensitization for the same reason—they disrupt the regulation of spinal nociceptive circuits by descending 5-HT pathways. Supporting this, we have shown that i.t. application of a 5HT-1A agonist increases membrane-bound KCC2, and GABAergic inhibition, in spinally transected rats. Conversely, a state analogous to that produced by spinal transection can be induced in intact rats by lesioning the DLF or microinjecting a 5HT-1A antagonist i.t. prior to bicuculline treatment. Under these conditions, bicuculline blocks the development of central sensitization (Huang et al., 2015).
Implications
Alterations in GABAergic function have also been implicated in the recovery of locomotor behavior after SCI (Grillner and Wallen, 1985). Administration of a GABAA antagonist can foster hindlimb stepping on a treadmill (Edgerton et al., 1997). While this effect has been traditionally tied to a blockade of GABA-dependent neural inhibition, research examining the effect of SCI on spinal levels of KCC2 suggest an alternative interpretation—that administration of bicuculline fosters locomotor behavior because it reduces a GABA-dependent over-excitation that interferes with adaptive plasticity (Grau et al., 2014; Viemari et al., 2011). Here too, descending serotonergic fibers appear to play a key role (Gackiere and Vinay, 2014) and have been shown to impact KCC2 expression and the chloride equilibrium potential (Bos et al., 2013; Gackiere and Vinay, 2014). Our findings fit with an emerging view that suggests alterations in GABA function provide a form of ionic plasticity that regulates the capacity for neural modifications (Ferrini and De Koninck, 2013). Neural injury appears to push spinal systems towards a state akin to early development, wherein GABA has a depolarizing effect. On the one hand, this type of modification could foster recovery by promoting the adaptive re-wiring of spinal circuits. On the other hand, allowing GABA to have a depolarizing effect can awaken silent nociceptive circuits, promoting an over-excitation that fosters the development of chronic pain and spasticity. During the acute stage of injury, the transformation of GABAergic function could amplify the nociceptive impact of other tissue damage, to enhance secondary injury and promote pain. Treatments (e.g., bumetanide) that restore GABAergic inhibition by reducing intracellular Cl− concentrations could have a therapeutic effect and have been shown to reduce chronic pain after SCI (Cramer et al., 2008; Hasbargen et al., 2010).
Highlights.
Acute spinal cord injury induces a qualitative shift in how GABA affects nociceptive transmission.
GABAA receptor antagonism blocks the development of central sensitization after spinal cord injury.
Disrupting KCC2 function transforms how GABA impacts the development of nociceptive sensitization.
Acknowledgments
The authors wish to thank Misty Malamakal, Joel Turtle, Josh Reynolds, Melissa Brumley, Julia Forsberg and Jason Lu for comments on an earlier version of this article. This research was supported by National Institutes of Health Grant HD058412.
Abbreviations
- Bic
Bicuculline
- Cap
Capsaicin
- CNS
Central nervous system
- DIOA
dihydro-indenyl-oxy alkanoic acid
- EMR
Enhanced mechanical reactivity
- ERK
Extracellular signal-regulated kinases
- GABA
Gamma-aminobutyric acid
- GBZ
GABAzine
- 8-OH-DPAT
8-Hydroxy-2-dipropylaminotetralin hydrobromide
- i.t.
Intrathecal
- KCC2
K+-Cl− cotransporter 2
- LPS
Lipopolysaccharides
- LTP
Long-term potentiation
- Mus
Muscimol
- N-CAD
N-cadherin
- NKCC1
Na+-K+- Cl− cotransporter 1
- NMDAR
N-methyl-D-aspartate receptor
- pERK
Phosphorylated ERK
- SCI
Spinal cord injury
- Shk
Shock
- 5HT
Serontonin
- T2
Second thoracic
- TRPV1
Transient receptor potential vanilloid receptor subtype 1
- Unshk
Unshock
- Veh
Vehicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests
References
- Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Molecular and cellular neurosciences. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nature reviews. Neuroscience. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bos R, Sadlaoud K, Boulenguez P, Buttigieg D, Liabeuf S, Brocard C, Haase G, Bras H, Vinay L. Activation of 5-HT2A receptors upregulates the function of the neuronal K-Cl cotransporter KCC2. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:348–353. doi: 10.1073/pnas.1213680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nature medicine. 2010;16:302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- Calcutt NA, Stiller C, Gustafsson H, Malmberg AB. Elevated substance-P-like immunoreactivity levels in spinal dialysates during the formalin test in normal and diabetic rats. Brain research. 2000;856:20–27. doi: 10.1016/s0006-8993(99)02345-8. [DOI] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Cramer SW, Baggott C, Cain J, Tilghman J, Allcock B, Miranpuri G, Rajpal S, Sun D, Resnick D. The role of cation-dependent chloride transporters in neuropathic pain following spinal cord injury. Molecular pain. 2008;4:36. doi: 10.1186/1744-8069-4-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown ED, Ferguson AR, Joynes RL, Grau JW. Instrumental learning within the spinal cord. II. Evidence for central mediation. Physiology & behavior. 2002;77:259–267. doi: 10.1016/s0031-9384(02)00859-4. [DOI] [PubMed] [Google Scholar]
- Crown ED, Grau JW. Evidence that descending serotonergic systems protect spinal cord plasticity against the disruptive effect of uncontrollable stimulation. Experimental neurology. 2005;196:164–176. doi: 10.1016/j.expneurol.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Dougherty KJ, Hochman S. Spinal cord injury causes plasticity in a subpopulation of lamina I GABAergic interneurons. Journal of neurophysiology. 2008;100:212–223. doi: 10.1152/jn.01104.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew GM, Siddall PJ, Duggan AW. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain. 2004;109:379–388. doi: 10.1016/j.pain.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, de Leon RD, Tillakaratne N, Recktenwald MR, Hodgson JA, Roy RR. Use-dependent plasticity in spinal stepping and standing. Advances in neurology. 1997;72:233–247. [PubMed] [Google Scholar]
- Ferguson AR, Crown ED, Grau JW. Nociceptive plasticity inhibits adaptive learning in the spinal cord. Neuroscience. 2006;141:421–431. doi: 10.1016/j.neuroscience.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Huie JR, Crown ED, Grau JW. Central nociceptive sensitization vs. spinal cord training: opposing forms of plasticity that dictate function after complete spinal cord injury. Frontiers in physiology. 2012;3:396. doi: 10.3389/fphys.2012.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AR, Washburn SN, Crown ED, Grau JW. GABA(A) receptor activation is involved in noncontingent shock inhibition of instrumental conditioning in spinal rats. Behavioral neuroscience. 2003;117:799–812. doi: 10.1037/0735-7044.117.4.799. [DOI] [PubMed] [Google Scholar]
- Ferrini F, De Koninck Y. Microglia control neuronal network excitability via BDNF signalling. Neural plasticity. 2013;2013:429815. doi: 10.1155/2013/429815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gackiere F, Vinay L. Serotonergic modulation of post-synaptic inhibition and locomotor alternating pattern in the spinal cord. Frontiers in neural circuits. 2014;8:102. doi: 10.3389/fncir.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? The open pain journal. 2009;2:11–17. doi: 10.2174/1876386300902010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Nicas E, Laird JM, Cervero F. GABAA-Receptor blockade reverses the injury-induced sensitization of nociceptor-specific (NS) neurons in the spinal dorsal horn of the rat. Journal of neurophysiology. 2006;96:661–670. doi: 10.1152/jn.00377.2006. [DOI] [PubMed] [Google Scholar]
- Garraway SM, Woller SA, Huie JR, Hartman JJ, Hook MA, Miranda RC, Huang YJ, Ferguson AR, Grau JW. Peripheral noxious stimulation reduces withdrawal threshold to mechanical stimuli after spinal cord injury: role of tumor necrosis factor alpha and apoptosis. Pain. 2014;155:2344–2359. doi: 10.1016/j.pain.2014.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerstad J, Tjolsen A, Hole K. Induction of long-term potentiation of single wide dynamic range neurones in the dorsal horn is inhibited by descending pathways. Pain. 2001;91:263–268. doi: 10.1016/S0304-3959(00)00448-6. [DOI] [PubMed] [Google Scholar]
- Grau JW, Crown ED, Ferguson AR, Washburn SN, Hook MA, Miranda RC. Instrumental learning within the spinal cord: underlying mechanisms and implications for recovery after injury. Behavioral and cognitive neuroscience reviews. 2006;5:191–239. doi: 10.1177/1534582306289738. [DOI] [PubMed] [Google Scholar]
- Grau JW, Huie JR, Garraway SM, Hook MA, Crown ED, Baumbauer KM, Lee KH, Hoy KC, Ferguson AR. Impact of behavioral control on the processing of nociceptive stimulation. Frontiers in physiology. 2012;3:262. doi: 10.3389/fphys.2012.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau JW, Huie JR, Lee KH, Hoy KC, Huang YJ, Turtle JD, Strain MM, Baumbauer KM, Miranda RM, Hook MA, Ferguson AR, Garraway SM. Metaplasticity and behavior: how training and inflammation affect plastic potential within the spinal cord and recovery after injury. Frontiers in neural circuits. 2014;8:100. doi: 10.3389/fncir.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Wallen P. Central pattern generators for locomotion, with special reference to vertebrates. Annual review of neuroscience. 1985;8:233–261. doi: 10.1146/annurev.ne.08.030185.001313. [DOI] [PubMed] [Google Scholar]
- Gwak YS, Hulsebosch CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. 2011;60:799–808. doi: 10.1016/j.neuropharm.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbargen T, Ahmed MM, Miranpuri G, Li L, Kahle KT, Resnick D, Sun D. Role of NKCC1 and KCC2 in the development of chronic neuropathic pain following spinal cord injury. Annals of the New York Academy of Sciences. 2010;1198:168–172. doi: 10.1111/j.1749-6632.2010.05462.x. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain research reviews. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook MA, Huie JR, Grau JW. Peripheral inflammation undermines the plasticity of the isolated spinal cord. Behavioral neuroscience. 2008;122:233–249. doi: 10.1037/0735-7044.122.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YJ, Murphy LM, Lee KH, Grau JW. 2015 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2015. Loss of descending serotonergic fibers transforms how GABA regulates nociceptive systems within the spinal cord: Role of KCC2. [Google Scholar]
- Huie JR, Baumbauer KM, Lee KH, Bresnahan JC, Beattie MS, Ferguson AR, Grau JW. Glial tumor necrosis factor alpha (TNFalpha) generates metaplastic inhibition of spinal learning. PloS one. 2012;7:e39751. doi: 10.1371/journal.pone.0039751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummon AB, Lim SR, Difilippantonio MJ, Ried T. Isolation and solubilization of proteins after TRIzol extraction of RNA and DNA from patient material following prolonged storage. BioTechniques. 2007;42:467–470. 472. doi: 10.2144/000112401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JH, Yaksh TL. The effect of spinal GABA receptor agonists on tactile allodynia in a surgically-induced neuropathic pain model in the rat. Pain. 1997;70:15–22. doi: 10.1016/s0304-3959(96)03249-6. [DOI] [PubMed] [Google Scholar]
- Jergova S, Hentall ID, Gajavelli S, Varghese MS, Sagen J. Intraspinal transplantation of GABAergic neural progenitors attenuates neuropathic pain in rats: a pharmacologic and neurophysiological evaluation. Experimental neurology. 2012;234:39–49. doi: 10.1016/j.expneurol.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends in neurosciences. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Jolivalt CG, Lee CA, Ramos KM, Calcutt NA. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain. 2008;140:48–57. doi: 10.1016/j.pain.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan SA, Saade NE, Karam M, Khansa H, Jabbur SJ, Jurjus AR. Hyperalgesia and upregulation of cytokines and nerve growth factor by cutaneous leishmaniasis in mice. Pain. 2000;85:477–482. doi: 10.1016/S0304-3959(99)00297-3. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Hammond DL. Role of spinal gamma-aminobutyric acidA receptors in formalin-induced nociception in the rat. The Journal of pharmacology and experimental therapeutics. 1997;282:928–938. [PubMed] [Google Scholar]
- Khawaled R, Bruening-Wright A, Adelman JP, Maylie J. Bicuculline block of small-conductance calcium-activated potassium channels. Pflugers Archiv : European journal of physiology. 1999;438:314–321. doi: 10.1007/s004240050915. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. The journal of pain : official journal of the American Pain Society. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FY, Qu XX, Ding X, Cai J, Jiang H, Wan Y, Han JS, Xing GG. Decrease in the descending inhibitory 5-HT system in rats with spinal nerve ligation. Brain research. 2010;1330:45–60. doi: 10.1016/j.brainres.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zheng J, Xiong L, Zimmermann M, Yang J. Spinal cord injury-induced attenuation of GABAergic inhibition in spinal dorsal horn circuits is associated with down-regulation of the chloride transporter KCC2 in rat. The Journal of physiology. 2008;586:5701–5715. doi: 10.1113/jphysiol.2008.152348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, O'Connor WT, Glennon JC, Cesena R, Calcutt NA. Impaired formalin-evoked changes of spinal amino acid levels in diabetic rats. Brain research. 2006;1115:48–53. doi: 10.1016/j.brainres.2006.07.077. [DOI] [PubMed] [Google Scholar]
- Medina I, Friedel P, Rivera C, Kahle KT, Kourdougli N, Uvarov P, Pellegrino C. Current view on the functional regulation of the neuronal K(+)-Cl(−) cotransporter KCC2. Frontiers in cellular neuroscience. 2014;8:27. doi: 10.3389/fncel.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletic G, Miletic V. Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain. 2008;137:532–539. doi: 10.1016/j.pain.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Progress in neurobiology. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Reeve AJ, Patel S, Fox A, Walker K, Urban L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. European journal of pain. 2000;4:247–257. doi: 10.1053/eujp.2000.0177. [DOI] [PubMed] [Google Scholar]
- Rheims S, Holmgren CD, Chazal G, Mulder J, Harkany T, Zilberter T, Zilberter Y. GABA action in immature neocortical neurons directly depends on the availability of ketone bodies. Journal of neurochemistry. 2009;110:1330–1338. doi: 10.1111/j.1471-4159.2009.06230.x. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Beyer C, Komisaruk BR. Nociceptive responses to altered GABAergic activity at the spinal cord. Life sciences. 1986;39:1667–1674. doi: 10.1016/0024-3205(86)90164-5. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Learning and memory in pain pathways. Pain. 2000;88:113–118. doi: 10.1016/S0304-3959(00)00424-3. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiological reviews. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Sivilotti L, Woolf CJ. The contribution of GABAA and glycine receptors to central sensitization: disinhibition and touch-evoked allodynia in the spinal cord. Journal of neurophysiology. 1994;72:169–179. doi: 10.1152/jn.1994.72.1.169. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Puig S, Jones DL. Spinal bicuculline produces hypersensitivity of dorsal horn neurons: effects of excitatory amino acid antagonists. Pain. 1998;77:181–190. doi: 10.1016/S0304-3959(98)00094-3. [DOI] [PubMed] [Google Scholar]
- Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. The Journal of physiology. 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichaya EG, Baumbauer KM, Carcoba LM, Grau JW, Meagher MW. Spinal glia modulate both adaptive and pathological processes. Brain, behavior, and immunity. 2009;23:969–976. doi: 10.1016/j.bbi.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viemari JC, Bos R, Boulenguez P, Brocard C, Brocard F, Bras H, Coulon P, Liabeuf S, Pearlstein E, Sadlaoud K, Stil A, Tazerart S, Vinay L. Chapter 1--importance of chloride homeostasis in the operation of rhythmic motor networks. Progress in brain research. 2011;188:3–14. doi: 10.1016/B978-0-444-53825-3.00006-1. [DOI] [PubMed] [Google Scholar]
- Washburn SN, Patton BC, Ferguson AR, Hudson KL, Grau JW. Exposure to intermittent nociceptive stimulation under pentobarbital anesthesia disrupts spinal cord function in rats. Psychopharmacology. 2007;192:243–252. doi: 10.1007/s00213-007-0707-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- Weng HR, Laird JM, Cervero F, Schouenborg J. GABAA receptor blockade inhibits A beta fibre evoked wind-up in the arthritic rat. Neuroreport. 1998;9:1065–1069. doi: 10.1097/00001756-199804200-00019. [DOI] [PubMed] [Google Scholar]
- Wenner P. Homeostatic synaptic plasticity in developing spinal networks driven by excitatory GABAergic currents. Neuropharmacology. 2014;78:55–62. doi: 10.1016/j.neuropharm.2013.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD., Jr Dorsal root potentials and dorsal root reflexes: a double-edged sword. Experimental brain research. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Annals of internal medicine. 2004;140:441–451. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Young EE, Baumbauer KM, Elliot A, Joynes RL. Lipopolysaccharide induces a spinal learning deficit that is blocked by IL-1 receptor antagonism. Brain, behavior, and immunity. 2007;21:748–757. doi: 10.1016/j.bbi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Zeilhofer HU, Wildner H, Yevenes GE. Fast synaptic inhibition in spinal sensory processing and pain control. Physiological reviews. 2012;92:193–235. doi: 10.1152/physrev.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hefferan MP, Loomis CW. Topical bicuculline to the rat spinal cord induces highly localized allodynia that is mediated by spinal prostaglandins. Pain. 2001;92:351–361. doi: 10.1016/S0304-3959(01)00276-7. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]