Abstract
Metal catalyzed reductive couplings of π-unsaturated reagents with carbonyl compounds via hydrogenation or transfer hydrogenation has emerged as an alternative to the use of stoichiometric organometallic reagents in carbonyl addition.
Introduction
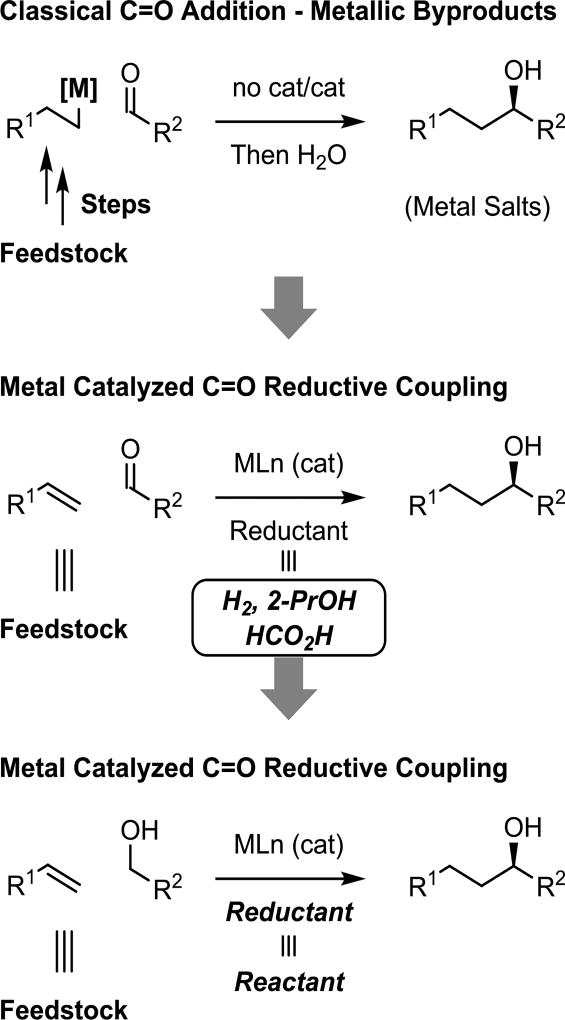
Carbonyl addition mediated by premetalated reagents has played an important role in synthetic chemistry for well over a century (Scheme 1, top).1,2 Despite their utility, the organometallic reagents commonly used in carbonyl addition pose safety issues and generate stoichiometric quantities of metallic byproducts, which complicates large-volume chemical manufacture.3 Metal-catalyzed reductive coupling of π-unsaturated reactants with carbonyl compounds represents an alternative to classical carbonyl addition.4–14 However, many frequently utilized terminal reductants (BEt3, ZnEt2) are just as problematic as the organometallic reagents they replace. In contrast, recently developed reductive couplings under the conditions of catalytic hydrogenation or transfer hydrogenation preclude the use of such hazardous reagents and the metallic byproducts they generate (Scheme 1, middle).15–18 An even more ideal approach to carbonyl reductive coupling is represented by hydrogen auto-transfer reactions that exploit the native reducing ability of reactant alcohols (Scheme 1, bottom).19–27 Here, alcohols serve dually as reductants and proelectrophiles (carbonyl precursors). Consequently, exogenous reductants are not necessary and byproduct-free carbonyl addition is achieved directly from the alcohol oxidation level. These processes convert lower alcohols to higher alcohols and, consequently, may be distinguished from related “borrowing hydrogen” reactions that deliver products of formal alcohol substitution.28–34
Scheme 1.
Carbonyl addition beyond stoichiometric metals.
Reductive coupling vs classical carbonyl addition
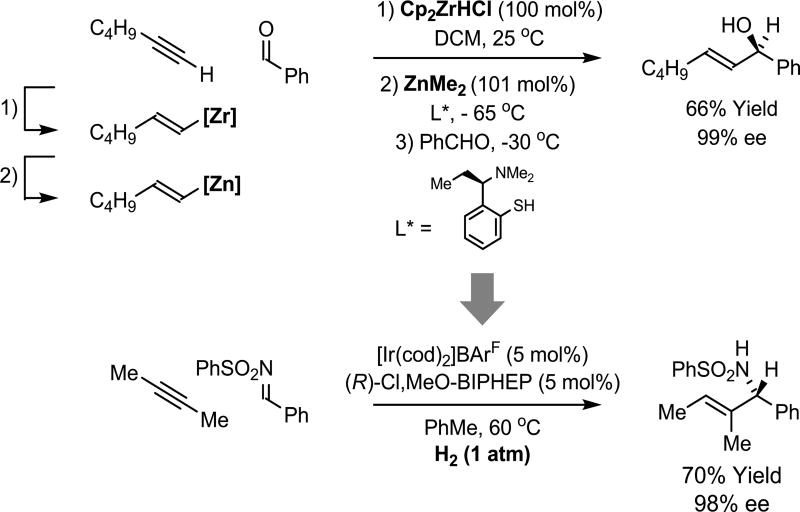
Under the conditions of hydrogen-mediated reductive coupling, reactions that traditionally have employed premetalated reagents may now be conducted catalytically in the absence of stoichiometric byproducts. To illustrate how these features streamline chemical synthesis, it is instructive to compare classical methods with the corresponding hydrogenative processes. For example, the Oppolzer-Wipf method for enantioselective carbonyl vinylation35,36 requires alkyne hydrozirconation mediated by Cp2ZrHCl, a mass-intensive reagent, with subsequent transmetalation to zinc mediated by ZnMe2, a pyrophoric liquid. Stoichiometric quantities of two organometallic reagents, Cp2ZrHCl and ZnMe2, are required to promote asymmetric carbonyl addition. In contrast, under hydrogenation conditions, alkyne-C=X (X = O, NR) asymmetric reductive coupling can be achieved in the absence of stoichiometric byproducts (Scheme 2).37
Scheme 2.
Beyond stoichiometric metals in asymmetric C=X (X = O, NR) vinylation.
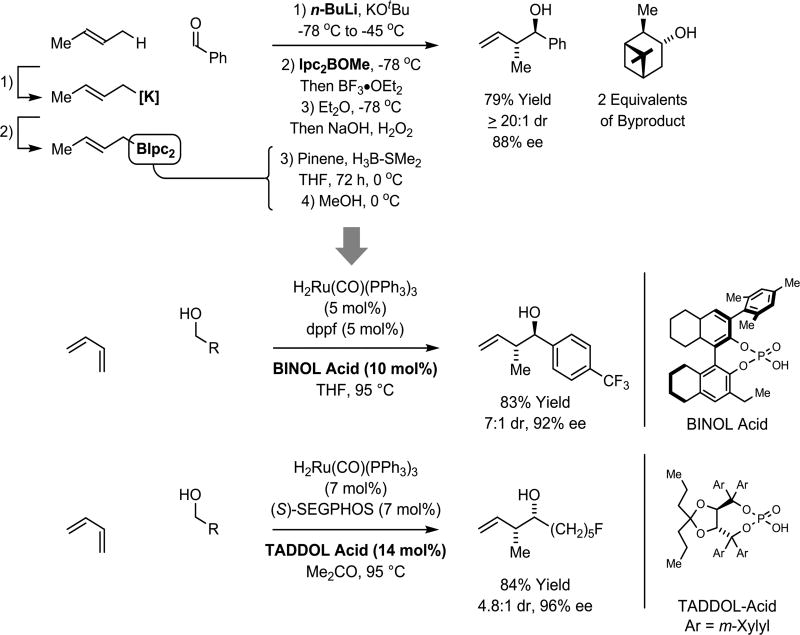
Similarly, as exemplified by Brown’s method,38,39 carbonyl crotylation is typically conducted using crotylmetal reagents. The requisite (E)- or (Z)-crotyldiisopinocampheylboranes are prepared through potassiation of butene followed by transmetalation to boron. Multiple manipulations and stoichiometric reagents (n-BuLi, KC4H7, Ipc2BOMe) are involved, and for each crotyl moiety that is transferred two equivalents of isopinocampheol are generated. Corresponding transfer hydrogenative processes for carbonyl crotylation are achieved through the direct coupling of primary alcohols with butadiene, an abundant petrochemical feedstock, in the absence of stoichiometric byproducts (Scheme 3).40,41
Scheme 3.
Beyond stoichiometric metals in asymmetric carbonyl crotylation.
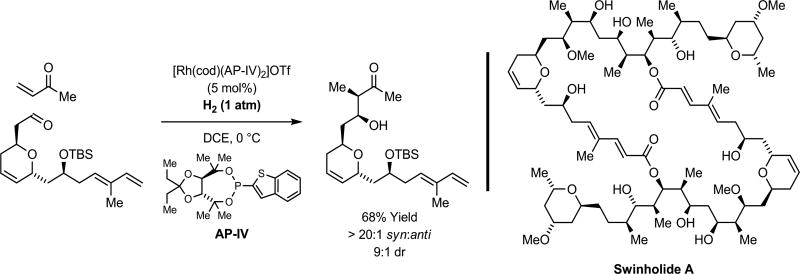
Enolates represent one of the most commonly utilized carbanions in chemical synthesis, and are frequently deployed in the context of asymmetric aldol addition for the construction of polyketide natural products.42–45 The control of relative and absolute stereochemistry in asymmetric aldol additions representing a longstanding challenge; one that is typically addressed through stereoselective enolization in combination with the use of chiral auxiliaries. Direct asymmetric reductive aldol addition is achieved upon hydrogenation of vinyl ketones in the presence of aldehydes using chiral rhodium complexes.46 This methodology was used to great effect in the total synthesis of the marine macrodiolide swinholide A.47 Notably, hydrogen-mediated aldol addition occurs in the presence of olefinic functional groups in a completely chemoselective fashion (Scheme 4).
Scheme 4.
Beyond stoichiometric metals in asymmetric aldol addition.
Conclusion
To conclude, by merging the characteristics of hydrogenation and carbonyl addition, we have developed a new family of catalytic asymmetric reductive couplings.7–13 These processes offer an alternative to the use of stoichiometric carbanions in a range of classical carbonyl additions, bypassing the issues of safety, selectivity and waste generation posed by premetalated reagents. Of potentially greater importance, the new patterns of reactivity underlying the present hydrogen-mediated C–C bond formations are empowering processes beyond classical transformations, unlocking hitherto unavailable volumes of chemical space. Among the many possibilities for further growth in this area, the development of catalytic systems for the catalytic C–C coupling of alcohols or amines with simple α-olefins represents a significant unmet challenge.11
Highlights.
H2-Mediated reductive coupling represents an alternative to classical carbonyl addition
Circumvents use of stoichiometric metals
Enhances efficiency by minimizing discrete redox and protecting group manipulations
Direct use of feedstock chemicals as partners for C-C coupling
Acknowledgments
The Robert A. Welch Foundation (F-0038) and the NIH-NIGMS (RO1-GM069445) are acknowledged for financial support.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Denmark SE, Fu J. Catalytic Enantioselective Addition of Allylic Organometallic Reagents to Aldehydes and Ketones. Chem Rev. 2003;103:2763–2794. doi: 10.1021/cr020050h. [DOI] [PubMed] [Google Scholar]
- 2.Yus M, González-Gómez JC, Foubelo F. Catalytic Enantioselective Allylation of Carbonyl Compounds and Imines. Chem Rev. 2011;111:7774–7854. doi: 10.1021/cr1004474. [DOI] [PubMed] [Google Scholar]
- 3.Dunn PJ. The Importance of Green Chemistry in Process Research and Development. Chem Soc Rev. 2012;41:1452–1461. doi: 10.1039/c1cs15041c. [DOI] [PubMed] [Google Scholar]
- 4.Weissermel K, Arpe HJ. Synthesis Involving Carbon Monoxide. Industrial Organic Chemistry 4th ed. Weinheim, Germany: WILEY-VCH; 2003. [Google Scholar]
- 5.van Leeuwen PWNM. Homogeneous Catalysis: Understanding the Art. Dordrecht: Springer Netherlands; 2004. [Google Scholar]
- 6.Kablaoui NM, Buchwald SL. Reductive Cyclization of Enones by a Titanium Catalyst. J Am Chem Soc. 1995;117:6785–6786. [Google Scholar]
- 7.Sam B, Breit B, Krische MJ. Paraformaldehyde and Methanol as C1 Feedstocks in Metal-Catalyzed C–C Couplings of π-Unsaturated Reactants: Beyond Hydroformylation. Angew Chem Int Ed. 2015;54:3267–3274. doi: 10.1002/anie.201407888. [DOI] [PubMed] [Google Scholar]
- 8.Dechert-Schmitt A-MR, Schmitt DC, Gao X, Itoh T, Krische MJ. Polyketide Construction via Hydrohydroxyalkylation and Related Alcohol C–H Functionalizations: Reinventing the Chemistry of Carbonyl Addition. Nat Prod Rep. 2014;31:504–513. doi: 10.1039/c3np70076c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bower JF, Krische MJ. Formation of C–C Bonds via Iridium-Catalyzed Hydrogenation and Transfer Hydrogenation. Top Organomet Chem. 2011;34:107–138. doi: 10.1007/978-3-642-15334-1_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan A, Krische MJ. Unlocking Hydrogenation for C–C Bond Formation: A Brief Overview of Enantioselective Methods. Org Process Res Dev. 2011;15:1236–1242. doi: 10.1021/op200195m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen KD, Park BY, Luong T, Sato H, Garza VJ, Krische MJ. Metal-Catalyzed Reductive Coupling of Olefin-Derived Nucleophiles: Reinventing Carbonyl Addition. Science. 2016;354:300. doi: 10.1126/science.aah5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin I, Krische MJ. Topics in Current Chemistry. Vol. 372. Springer International Publishing; 2015. Asymmetric Iridium-Catalyzed C–C Coupling of Chiral Diols via Site-Selective Redox-Triggered Carbonyl Addition; pp. 85–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez F, Oda S, Geary LM, Krische MJ. Ruthenium-Catalyzed Transfer Hydrogenation for C–C Bond Formation: Hydrohydroxyalkylation and Hydroaminoalkylation via Reactant Redox Pairs. Top Curr Chem. 2016;374:35. doi: 10.1007/s41061-016-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lumbroso A, Cooke ML, Breit B. Catalytic Asymmetric Synthesis of Allylic Alcohols and Derivatives and Their Applications in Organic Synthesis. Angew Chem Int Ed. 2013;52:1890–1932. doi: 10.1002/anie.201204579. [DOI] [PubMed] [Google Scholar]
- 15.Garza VJ, Krische MJ. Hydroxymethylation beyond Carbonylation: Enantioselective Iridium-Catalyzed Reductive Coupling of Formaldehyde with Allylic Acetates via Enantiotopic π-Facial Discrimination. J Am Chem Soc. 2016;138:3655–3658. doi: 10.1021/jacs.6b01078. ** Enantioselective reductive coupling of branched allylic acetates to formaldehyde enables formation of chiral building blocks that are inaccessible via classical carbonyl addition chemistry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena A, Perez F, Krische MJ. Ruthenium(0)-Catalyzed [4+2] Cycloaddition of Acetylenic Aldehydes with α-Ketols: Convergent Construction of Angucycline Ring Systems. Angew Chem Int Ed. 2016;55:1493–1497. doi: 10.1002/anie.201509646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oda S, Franke J, Krische MJ. Diene Hydroaminomethylation via Ruthenium-Catalyzed C–C Bond Forming Transfer Hydrogenation: Beyond Carbonylation. Chem Sci. 2016;7:136–141. doi: 10.1039/c5sc03854e. * This work merges transfer hydrogenation and imine addition, enabling access to a wide range of homoallylic amines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao H, Wang G, Krische MJ. Regioselective Hydrohydroxyalkylation of Styrene with Primary Alcohols or Aldehydes via Ruthenium-Catalyzed C–C Bond Forming Transfer Hydrogenation. Angew Chem Int Ed. 2016;56:1–5. doi: 10.1002/anie.201609056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park BY, Luong T, Sato H, Krische MJ. Osmium(0)-Catalyzed C–C Coupling of Ethylene and α-Olefins with Diols, Ketols, or Hydroxy Esters via Transfer Hydrogenation. J Org Chem. 2016;81:8585–8594. doi: 10.1021/acs.joc.6b01923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen KD, Herkommer D, Krische MJ. Enantioselective Formation of All-Carbon Quaternary Centers via C–H Functionalization of Methanol: Iridium-Catalyzed Diene Hydrohydroxymethylation. J Am Chem Soc. 2016;138:14210–14213. doi: 10.1021/jacs.6b09333. ** The first catalytic enantioselective C–C coupling of methanol. The reaction forms all-carbon quarternary stereocenters with exceptional levels of enantioselectivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang T, Woo SK, Krische MJ. C-Propargylation Overrides O-Propargylation in Reactions of Propargyl Chloride with Primary Alcohols: Rhodium-Catalyzed Transfer Hydrogenation. Angew Chem Int Ed. 2016;55:9207–9211. doi: 10.1002/anie.201603575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen KD, Herkommer D, Krische MJ. Ruthenium-BINAP Catalyzed Alcohol C–H Tert-Prenylation via 1,3-Enyne Transfer Hydrogenation: Beyond Stoichiometric Carbanions in Enantioselective Carbonyl Propargylation. J Am Chem Soc. 2016;138:5238–5241. doi: 10.1021/jacs.6b02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang T, Zhang W, Chen TY, Nguyen KD, Krische MJ. Ruthenium Catalyzed Diastereo- and Enantioselective Coupling of Propargyl Ethers with Alcohols: Siloxy-Crotylation via Hydride Shift Enabled Conversion of Alkynes to π-Allyls. J Am Chem Soc. 2015;137:13066–13071. doi: 10.1021/jacs.5b08019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G, Franke J, Ngo CQ, Krische MJ. Diastereo- and Enantioselective Iridium Catalyzed Coupling of Vinyl Aziridines with Alcohols: Site-Selective Modification of Unprotected Diols and Synthesis of Substituted Piperidines. J Am Chem Soc. 2015;137:7915–7920. doi: 10.1021/jacs.5b04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang T, Nguyen KD, Zhang W, Krische MJ. Enantioselective Ruthenium-Catalyzed Carbonyl Allylation via Alkyne-Alcohol C–C Bond-Forming Transfer Hydrogenation: Allene Hydrometalation vs Oxidative Coupling. J Am Chem Soc. 2015;137:3161–3164. doi: 10.1021/jacs.5b00747. * The first enantioselective carbonyl allylation using alkynes as allyl-metal precursors. A dual catalytic cycle involving alkyne-to-allene isomerization and alcohol dehydrogenation-allene hydrometalation-carbonyl addition is described. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sam B, Luong T, Krische MJ. Ruthenium-Catalyzed C–C Coupling of Fluorinated Alcohols with Allenes: Dehydrogenation at the Energetic Limit of β-Hydride Elimination. Angew Chem Int Ed. 2015;54:5465–5469. doi: 10.1002/anie.201500238. * This work applies transfer hydrogenative coupling to inexpensive and abundant fluorinated alcohols, delivering diverse organofluorine compounds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen TY, Tsutsumi R, Montgomery TP, Volchkov I, Krische MJ. Ruthenium-Catalyzed C–C Coupling of Amino Alcohols with Dienes via Transfer Hydrogenation: Redox-Triggered Imine Addition and Related Hydroaminoalkylations. J Am Chem Soc. 2015;137:1798–1801. doi: 10.1021/ja5130258. [DOI] [PubMed] [Google Scholar]
- 28.Guillena G, Ramón DJ, Yus M. Alcohols as Electrophiles in C–C Bond-Forming Reactions: The Hydrogen Autotransfer Process. Angew Chem Int Ed. 2007;46:2358–2364. doi: 10.1002/anie.200603794. [DOI] [PubMed] [Google Scholar]
- 29.Yang Q, Wang Q, Yu Z. Substitution of Alcohols by N-Nucleophiles via Transition Metal-Catalyzed Dehydrogenation. Chem Soc Rev. 2015;44:2305–2329. doi: 10.1039/c4cs00496e. [DOI] [PubMed] [Google Scholar]
- 30.Nandakumar A, Midya SP, Landge VG, Balaraman E. Transition-Metal-Catalyzed Hydrogen-Transfer Annulations: Access to Heterocyclic Scaffolds. Angew Chem Int Ed. 2015;54:11022–11034. doi: 10.1002/anie.201503247. [DOI] [PubMed] [Google Scholar]
- 31.Chelucci G. Metal-Catalyzed Dehydrogenative Synthesis of Pyrroles and Indoles from Alcohols. Coord Chem Rev. 2017;331:37–53. [Google Scholar]
- 32.Quintard A, Rodriguez J. A Step into an Eco-Compatible Future: Iron- and Cobalt-Catalyzed Borrowing Hydrogen Transformation. ChemSusChem. 2016;9:28–30. doi: 10.1002/cssc.201501460. [DOI] [PubMed] [Google Scholar]
- 33.Quintard A, Rodriguez J. Catalytic Enantioselective OFF ↔ ON Activation Processes Initiated by Hydrogen Transfer: Concepts and Challenges. Chem Commun. 2016;52:10456–10473. doi: 10.1039/c6cc03486a. [DOI] [PubMed] [Google Scholar]
- 34.Huang F, Liu Z, Yu Z. C-Alkylation of Ketones and Related Compounds by Alcohols: Transition-Metal-Catalyzed Dehydrogenation. Angew Chem Int Ed. 2016;55:862–875. doi: 10.1002/anie.201507521. [DOI] [PubMed] [Google Scholar]
- 35.Oppolzer W, Radinov RN. Catalytic Asymmetric Synthesis of Secondary (E)-Allyl Alcohols from Acetylenes and Aldehydes via (1-Alkenyl)zinc Intermediates. Helv Chim Acta. 1992;75:170–173. [Google Scholar]
- 36.Wipf P, Xu W. Preparation of Allylic Alcohols by Alkene Transfer from Zirconium to Zinc. Tetrahedron Lett. 1994;35:5197–5200. [Google Scholar]
- 37.Ngai M-Y, Barchuk A, Krische MJ. Enantioselective Iridium-Catalyzed Imine Vinylation: Optically Enriched Allylic Amines via Alkyne–Imine Reductive Coupling Mediated by Hydrogen. J Am Chem Soc. 2007;129:12644–12645. doi: 10.1021/ja075438e. [DOI] [PubMed] [Google Scholar]
- 38.Brown HC, Bhat KS. Enantiomeric Z- and E-Crotyldiisopinocampheylboranes. Synthesis in High Optical Purity of All Four Possible Stereoisomers of beta-Methylhomoallyl Alcohols. J Am Chem Soc. 1986;108:293–294. doi: 10.1021/ja00279a042. [DOI] [PubMed] [Google Scholar]
- 39.Brown HC, Bhat KS. Chiral Synthesis via Organoboranes. 7. Diastereoselective and Enantioselective Synthesis of Erythro- and Threo-β-Methylhomoallyl Alcohols via Enantiomeric (Z)- and (E)-Crotylboranes. J Am Chem Soc. 1986;108:5919–5923. doi: 10.1021/ja00279a042. [DOI] [PubMed] [Google Scholar]
- 40.Zbieg JR, Yamaguchi E, McInturff EL, Krische MJ. Enantioselective C–H Crotylation of Primary Alcohols via Hydrohydroxyalkylation of Butadiene. Science. 2012;336:324–327. doi: 10.1126/science.1219274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McInturff EL, Yamaguchi E, Krische MJ. Chiral-Anion-Dependent Inversion of Diastereo- and Enantioselectivity in Carbonyl Crotylation via Ruthenium-Catalyzed Butadiene Hydrohydroxyalkylation. J Am Chem Soc. 2012;134:20628–20631. doi: 10.1021/ja311208a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knochel P, Molander GA. Comprehensive Organic Synthesis Volume 2. 2. Amsterdam: Elsevier Ltd; 2014. [Google Scholar]
- 43.Arya P, Qin H. Advances in Asymmetric Enolate Methodology. Tetrahedron. 2000;56:917–947. [Google Scholar]
- 44.Romea P, Urpí F. Modern Methods in Stereoselective Aldol Reactions. Weinheim, Germany: Wiley-VCH; 2013. Stereoselective Acetate Aldol Reactions; pp. 1–81. [Google Scholar]
- 45.Chemler SR, Roush WR. Modern Carbonyl Chemistry. Weinheim, Germany: Wiley-VCH; 2000. Recent Applications of the Allylation Reaction to the Synthesis of Natural Products; pp. 403–490. [Google Scholar]
- 46.Bee C, Han SB, Hassan A, Iida H, Krische MJ. Diastereo- and Enantioselective Hydrogenative Aldol Coupling of Vinyl Ketones: Design of Effective Monodentate TADDOL-Like Phosphonite Ligands. J Am Chem Soc. 2008;130:2746–2747. doi: 10.1021/ja710862u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin I, Hong S, Krische MJ. Total Synthesis of Swinholide A: An Exposition in Hydrogen-Mediated C–C Bond Formation. J Am Chem Soc. 2016;138:14246–14249. doi: 10.1021/jacs.6b10645. ** A 15 step total sythesis of swinholide A is reported in which 10 C–C bonds are formed via enantioselective hydrogenative coupling reactions. [DOI] [PMC free article] [PubMed] [Google Scholar]