Abstract
Homozygous or compound heterozygous for frameshift or nonsense mutations in the ATP–binding cassette transporter A3 (ABCA3) is associated with neonatal respiratory failure and death within the first year of life without lung transplantation. We report the case of a newborn baby girl who developed severe respiratory distress soon after birth. She was diagnosed with compound heterozygous frameshift mutation of the ABCA3 gene. Despite extensive treatment (intravenous corticosteroids pulse therapy, oral corticosteroids, azithromycin, and hydroxychloroquine), she developed chronic respiratory failure. As the parents refused cardio-pulmonary transplantation and couldn't resolve to an accompaniment of end of life, a tracheostomy was performed resulting in continuous mechanical ventilation. A neurodevelopmental delay and an overall muscular dystrophy were noted. At the age of 5 years, after 2 episodes of pneumothorax, the patient died from severe respiratory failure. To our knowledge, this was the first case of a child with compound heterozygous frameshift mutation who posed such an ethical dilemma with a patient surviving till the age of five years.
Keywords: ABCA3 deficiency, Compound heterozygous frameshift mutation, Neonatal respiratory failure, Tracheostomy, Mechanical ventilation, Ethical dilemma
Abbreviations: ABCA3, Adenosine triphosphate-binding cassette transporter subfamily A member 3
1. Introduction
ATP–binding cassette transporter A3 (ABCA3) is a member of a family of proteins that hydrolyses ATP to move substrates across biologic membrane [1]. This protein is expressed in alveolar cells type 2 and localized to the membrane of lamellar bodies. ABCA3 is essential for lamellar body biogenesis and transport of phospholipids into the lamellar body for assembly of the pulmonary surfactant. Lung disease resulting from ABCA3 mutations is usually expressed in an autosomal recessive manner. Mutations are distributed throughout the gene and include nonsense, frameshift, missense, splice site and insertions/deletions with an incidence of 3.6% in European descent individuals [2]. There are more than 200 distinct ABCA3 mutations, most of them leading to severe neonatal lung disease [3]. In absence of lung transplantation, homozygous or compound heterozygous for frameshift or nonsense mutations in ABCA3 result in neonatal respiratory failure and death before the end of the first year [4].
We describe the clinical course of a child with compound heterozygous frameshift mutation of the ABCA3 gene who survived till the age of five years.
2. Case report
A female infant, first child of non-consanguineous Caucasian parents, was born prematurely at 36 weeks of gestation by cesarean section for preterm premature rupture of membranes and failure to progress in labor. No meconium was noted at birth and there was no history of perinatal infection risk.
Within few hours from birth, she developed severe respiratory distress. White cell count and C reactive protein concentration were within normal range. Initial chest radiograph showed diffuse reticulonodular infiltrates consistent with respiratory distress syndrome. An echocardiography excluded pulmonary hypertension. The newborn was treated with an empiric course of antibiotics and exogenous surfactant was administered at 4 h of life. No notable improvement was noted so nasal continuous positive airway pressure was started.
At day 11 of life, the infant was intubated and ventilated for severe respiratory failure not responding to exogenous surfactant administration and systemic methylprednisolone.
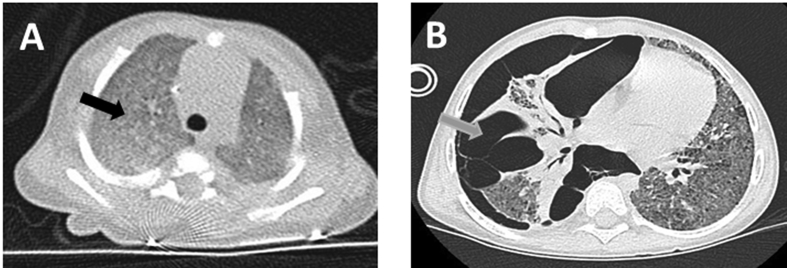
In view of high ventilatory pressure requirements and high oxygenation, lung high resolution computed tomography scan was performed at 22 days of life showing diffuse ground glass appearance (Fig. 1A). Genetic analysis returned positive for compound heterozygous frameshift mutation of the ABCA3 gene (c.3902del/c.5084_5097del or p. Pro1301Argfs*45/p.Leu1695Argfs*103). No mutation was found in the SP-C or SP-B genes. Parents refused genetic analysis and counseling.
Fig. 1.
A) Computed tomography scan of the chest at 22 days of life showing diffuse ground glass appearance (black arrow). B) Computed tomography scan of the chest showing pneumothorax at the right with multiple adhesions (grey arrow) along with shifting of the heart to the left and a diffuse ground glass appearance.
The infant was started on intravenous methylprednisolone pulse therapy at a dose of 300 mg/m2 (three day courses on a monthly base), associated with azithromycin at a dose of 20mg/Kg, three days per week, and hydroxychloroquine at a dose of 8 mg/Kg/day.
Multiple weaning trials were unsuccessful.
Our local medical ethics committees discussed several times the therapeutic management for this patient. Cardiopulmonary transplantation was proposed for technical facilities at these young ages, but refused by the parents because of the associated high mortality and morbidity rates. On the other hand, parents couldn't resolve to an accompaniment of end of life. So, tracheostomy was performed and patient was maintained on continuous (24h/24h) mechanical ventilation for four years resulting in an important neurodevelopmental delay and an overall muscular dystrophy. Because of her condition, she was living in a medicalized institution. In spite of continuous treatment with steroids, hydroxychloroquine, and azithromycin, her respiratory status continued to deteriorate. After a new multidisciplinary discussion at 4 years, the child was found ineligible for lung transplantation because of neurodevelopmental delay and diaphragmatic weakness resulting from her chronic illness. At the age of 5 years, after 2 episodes of severe respiratory distress both due to a pneumothorax (Fig. 1 B), the patient died from respiratory failure.
3. Discussion
We describe the clinical course of a child with compound heterozygote frameshift mutation of the ABCA3 gene who survived till the age of 5 years. This is the longest survival period observed for a patient with recessive frameshift mutation. However, during this period, our patient could not be weaned off invasive ventilation despite aggressive and continuous medical treatment. At the age of five years, the patient presented with a huge pneumothorax due to either the invasive ventilation or the natural outcome of surfactant deficiency with pulmonary fibrosis.
One case has been reported in the literature of a child with compound heterozygous mutation of the ABCA3 mutation. One of the mutations was a missense mutation and the other was a nonsense mutation. The child was weaned off mechanical ventilation at the age of 8 months after treatment with systemic and pulse therapy corticosteroids, azithromycin, and hydroxychloroquine. However, authors did not mention the respiratory evolution of this child past the age of 1 year except for the fact that he was still on supplemental oxygen (1–3 l/min). Interestingly the index case brother was found to have the same mutations but he was totally asymptomatic at the age of 4 years [5].
Mutations in ABCA3 appear to be the most common cause of genetic surfactant dysfunction in humans with compound heterozygous variants being most frequently reported in the literature [6,7].
The clinical spectrum and severity of disease caused by ABCA3 mutations is variable. Depending upon the degree of ABCA3 dysfunction, it can range from fulminant neonatal respiratory failure resulting in death during the first days or months of life to later onset of interstitial lung disease [8].
In a recent study that included the largest collective experience of subjects with ABCA3 deficiency, authors concluded to a consistent genotype-phenotype correlation for patients with homozygous or compound heterozygous for frameshift and/or nonsense mutations. All these subjects presented with respiratory distress at birth and died or underwent lung transplantation before one year of age. On the other hand, the prognosis for patients with other genotypes is more variable and difficult to predict depending on genetic and or environmental factors [4].
The role of modifier genes in altering the disease course caused by ABCA3 is still unknown [3]. However, ABCA3 can act as a modifier gene. For instance in a study that examined four children from the same family carrying the SFTPC 173T mutation and suffering from severe pulmonary diseases with asymptomatic parents, three of the four infants were found heterozygous for ABCA 3 mutations [9].
Treatment options for patients with ABCA3 mutations should be discussed on an individual patient basis. Given the almost uniformly poor prognosis for infants with homozygous or compound heterozygous for frameshift and/or nonsense mutations, discussion with families about lung transplantation or compassionate care should be conducted as soon as the diagnosis is made [4].
Lung transplantation provides an option to prolong survival in children with inherited disorders of surfactant metabolism. The five-year survival rate for children who underwent lung transplantation for inherited disorders of surfactant metabolism irrespective of the type of mutation in question is approximately 50%. This outcome reflects mainly the consequences of transplantation at a young age rather than the underlying disease itself [10]. Short and long-term complications and challenges should be well explained to families before proceeding to transplantation [11].
Tracheostomy and chronic ventilation along with anti-inflammatory agents (corticosteroids, azithromycin, hydroxychloroquine and/or azathioprine) have been suggested as alternatives to lung transplantation for infants with SFTPC mutations and severe respiratory failure with some children being totally weaned off oxygen over 2–6 years [12]. However for infants with recessive frameshift mutation of the ABCA 3 gene as is the case with our infant, this treatment has not proven to be beneficial.
This case created ethical difficulties. Parents refused early cardiopulmonary transplantation. On the other hand, they wanted to continue with aggressive management. Neurodevelopmental delay and muscular atrophy ensued. As a consequence, transplantation could no longer be considered and palliative care was the only remaining option.
The extent and nature of parents' involvement in decision making of whether to continue or to limit life-sustaining treatment in children with life limiting or life-threatening conditions remain contested. The two principle issues are parental rights and authority, and child's best interests. The level and nature of parental involvement in decision-making should be discussed with the parents in each case [13].
Further prospective studies are needed to guide clinical decision making and to evaluate benefits and risks of early lung transplantation and associated morbidity and mortality.
Funding source
No honorarium, grant, or other form of payment was given to anyone to produce the manuscript.
Financial disclosure
None.
Conflicts of interest
None.
Informed consent
Written informed consent for publication of this case report was obtained from both parents.
References
- 1.Dean M., Rzhetsky A., Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 2.Whitsett J.A., Wert S.E., Weaver T.E. Alveolar surfactant homeostasis and the pathogenesis of pulmonary disease. Ann. Rev. Med. 2010;61:105–119. doi: 10.1146/annurev.med.60.041807.123500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beers M.F., Mulugeta S. The biology of the ABCA 3 lipid transporter in lung health and disease. Cell Tissue Res. 2017;367:481–493. doi: 10.1007/s00441-016-2554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wambach J.A., Casey A., Fishman M.P., Wegner D.J., Wert S.E., Cole F.S., Hamvas A., Nogee L.M. Genotype–phenotype correlations for infants and children with ABCA3 deficiency. Am. J. Respir. Crit. Care Med. 2014;189(12):1538–1543. doi: 10.1164/rccm.201402-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hallik M., Annilo T., Ilmoja M.L. Different course of lung disease in two siblings with novel ABCA3 mutations. Eur. J. Pediatr. 2014;173:1553–1556. doi: 10.1007/s00431-013-2087-3. [DOI] [PubMed] [Google Scholar]
- 6.Shulenin S., Nogee L.M., Annilo T., Wert S.E., Whitsett J.A., Dean M. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N. Engl. J. Med. 2004;350:1296–1303. doi: 10.1056/NEJMoa032178. [DOI] [PubMed] [Google Scholar]
- 7.Somaschini M., Nogee L.M., Sassi I., Danhaive O., Presi S., Boldrini R., Montrasio C., Ferrari M., Wert S.E., Carrera P. Unexplained neonatal respiratory distress due to congenital surfactant deficiency. J. Pediatr. 2007;150:649–653. doi: 10.1016/j.jpeds.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Doan M.L., Guillerman R.P., Dishop M.K., Nogee L.M., Langston C., Mallory G.B., Sockrider M.M., Fan L.L. Clinical, radiological and pathological features of ABCA3 mutations in children. Thorax. 2008;63:366–373. doi: 10.1136/thx.2007.083766. [DOI] [PubMed] [Google Scholar]
- 9.Bullard J.E., Nogee L.M. Heterozygosity for ABCA3 mutations modifies the severity of lung disease associated with a surfactant protein C gene (SFTPC) mutation. Pediatr. Res. 2007;62:176–179. doi: 10.1203/PDR.0b013e3180a72588. [DOI] [PubMed] [Google Scholar]
- 10.Kirkby S., Hayes D., Jr. Pediatric lung transplantation: indications and outcomes. J. Thorac Dis. 2014;6:1024–1031. doi: 10.3978/j.issn.2072-1439.2014.04.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eldridge W.B., Zhang Q., Faro A., Sweet S.C., Eghtesady P., Hamvas A. Outcomes of lung transplantation for infants and children with genetic disorders of surfactant metabolism. J. Pediatr. 2017;184:157–164. doi: 10.1016/j.jpeds.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liptzin D.R., Patel T., Deterding R.R. Chronic ventilation in infants with surfactant protein C mutations: an alternative to lung transplantation. Am. J. Respir. Crit. Care Med. 2015;191:1338–1340. doi: 10.1164/rccm.201411-1955LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillam L., Sullivan J. Ethics at the end of life: who should make decisions about treatment limitation for young children with life-threatening or life-limiting conditions? J. Paediatr. Child Health. 2011;47:594–598. doi: 10.1111/j.1440-1754.2011.02177.x. [DOI] [PubMed] [Google Scholar]