Abstract
Background
The aim of this study was to investigate the effects of various ratios of hemodilution on the survival of McFarlane’s skin flaps.
Material/Methods
An experimental study was performed on 42 adult male Wistar rats (weighing 260 to 305 g) allocated to a control group without any volume loss and to 6 study groups with hemodilution ratios of 5%, 10%, 15%, 20%, 25%, and 30%. In all subjects, random-pattern McFarlane’s skin flaps were uniformly elevated and re-sutured to the donor sites. The amount of necrosis was evaluated on the 7th day postoperatively and compared among the groups.
Results
The amounts of flap necrosis in the groups with 5%, 10%, 15%, and 20% hemodilution ratios were significantly lower than that of the control group (p<0.001). In the 25% and 30% hemodilution groups, although there was less necrosis than in the control group, the differences were not statistically significant. Hematocrit levels, which initially decreased in conjunction with the hemodilution ratios, returned to normal levels on the 7th day after the operation.
Conclusions
Our results indicated that 20% or less of the total blood volume loss that may be compensated by the normovolemic hemodilution with dextran can improve flap survival.
MeSH Keywords: Blood Transfusion, Hemodilution, Plasma Substitutes, Surgical Flaps, Survival
Background
The delivery of oxygen to tissues is the most critical determinant on survival of flaps, and regulation of microcirculation must be accomplished based on local requirements [1]. Recent publications have focused on improvements of the therapeutic modalities for facilitation of perfusion and oxygenation of ischemic flap tissue [2–4].
Theoretically, increasing the blood flow using vasodilatation can be useful to improve oxygenation. In this aim, antagonizing the sympathetic adrenergic system, which is effective for regulation of cutaneous blood flow, can be considered. On the other hand, the depressive effect of the systemic vasodilators on arterial blood pressure may consequently lead to a diminution of flap perfusion [5]. To achieve a beneficial effect, vasodilatation should be confined to the target tissue. However, further evidence is necessary to prove that blockade of sympathetic perfusion improves flap perfusion.
Decreasing the vascular resistance through diminishing blood viscosity by exchanging blood with crystalloid or colloid solutions is another way to facilitate the blood flow and microcirculation [6]. This objective can be accomplished by hemodilution, which has been utilized in the treatment of various ischemic diseases [7,8]. Hemodilution may also have beneficial effects on skin flap survival, and normovolemic hemodilution after the replacement of surgical blood losses with crystalloid and/or colloid solutions has been reported to support the flap circulation [9,10].
In clinical practice, decrease in hematocrit levels below 30% is accepted as an indication for transfusion of red blood cells because the compromise of oxygen carriers from excessive hemodilution may result in hypoxic damage in tissues vulnerable to ischemia after trauma and surgery [7]. Hemodilution can be compensated for by the increased peripheral blood flow that supports the tolerance of lower hematocrit levels, diminishing the risk of infection with blood-borne diseases [11,12]. Failure of the free flaps has been mostly associated with venous and global ischemia, while in random-pattern flaps, factors influencing distal ischemia are more important [13]. Elucidation of the biochemical basis of cellular injury and oxidative stress provided better insights into the physiology and survival of flaps. Research is needed to elucidate the impacts of the dynamic parameters of circulation, such as hypovolemia or hemodilution, on flap survival. In previous studies, the outcomes of different plasma expanders used for normovolemic hemodilution were compared. Erni et al. [7] reported better results in ischemic tissue oxygen tension with 6% dextran compared with the 8% human serum albumin. In a clinical study with 40 adult patients who underwent moderate hemodilution to a target hemoglobin level of 9 gm/dL, 4 replacement fluids – Ringer’s lactate, 5% albumin, 6% dextran 70, or 6% hetastarch – were used in different groups. In that study, although acute normovolemic hemodilution was well-tolerated irrespective of the replacement fluid used, hetastarch or dextran as the replacement fluid was associated with a more stable mean arterial pressure [14], but the data are limited, especially regarding the effects of different hemodilution ratios on survival of skin flaps.
The aim of the present study was to assess the impacts of different ratios of normovolemic hemodilution on the survival of random-pattern skin flaps in an experimental rat model.
Material and Methods
Study design
This experimental trial was performed in the Experimental Animal Laboratory of our institution after approval by the local Animal Subject Committee. All experimental procedures were carried out according to the National Institutes of Health (NIH) guidelines and the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments for the care and use of laboratory animals. A total of 42 adult, male, albino Wistar rats (weighing 260 g to 305 g) allocated in 7 groups were used. Six rats served as the control group (Group I) without any blood volume loss, while the remaining 36 subjects were randomly distributed to 6 groups (n = 6 for each group) with various ratios of hemodilution.
All animals were maintained in plastic rodent cages under controlled conditions of light (12-h day/night cycle), temperature (21±2°C), and humidity (50–60%). Access to food (commercially available rodent pellets) and water was allowed ad libitum. Body weights of all animals were recorded in the preoperative period, and all surgical procedures were carried out by the same surgeon (CYD).
Surgical procedure
Anesthesia was induced by the intraperitoneal injection of ketamine HCl (100 mg/kg body weight) and Xylazine (5 mg/kg body weight). Following the fixation of the animal on a surgical table, the femoral vein of the rat was identified, dissected, and catheterized with a thin cannula. Before the intervention, the average blood volume of every rat was estimated as 54–70 ml/kg (mean: 60 ml/kg). In the 6 experimental normovolemic hemodilution groups, 5%, 10%, 15%, 20%, 25%, or 30% of the calculated blood volume was withdrawn through the cannula in the femoral veins. The control group, without any volume loss, is referred to as Group I, while the experimental groups with the loss of 5%, 10%, 15%, 20%, 25%, or 30% of volume are referred to as Groups II–VII, respectively. Dextran-70 isotonic saline (Macrodex®) was administered at a maximum daily dose of 20 ml/kg to replace the loss of blood volume. For instance, a rat weighing 300 g that lost 5% of its total blood volume received 0.9 ml of dextran-70 isotonic saline, whereas in another rat with the same body weight, 30% of blood volume was exchanged with 5.4 ml of dextran-70 isotonic saline through the femoral vein. The site of incision for access to the femoral vein was sutured.
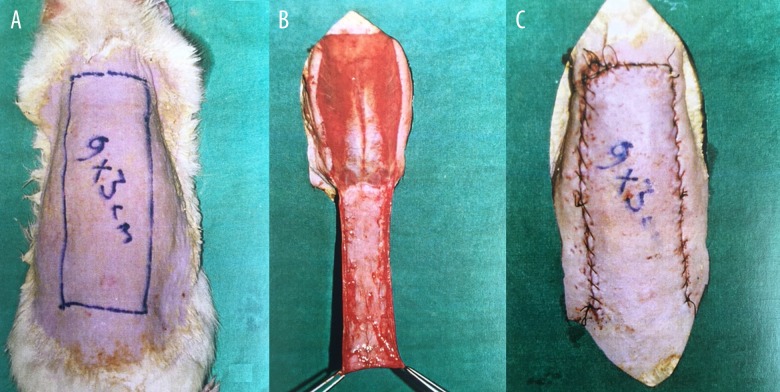
Before the surgical procedure, blood samples were collected from the tail veins and centrifuged for 30 min at 3000 rpm. After shaving and epilating the dorsal skin, this surgical field was cleansed with povidone iodine. As described by McFarlane et al., a caudal-based random skin flap (with a width of 3 cm and length of 9 cm) was elevated from the dorsal skin [15] (Figure 1).
Figure 1.
(A–C) Preparation of caudal-based McFarlane dorsal skin flap in rat model.
Outcome parameters
Estimation of hematocrit
Blood samples were collected from the tail veins of the rats for estimation of the hematocrit on the 2nd hour, and on the 1st, 3rd, and 7th days after the surgical procedure. Heparin-washed microtubes were utilized for the measurement of hematocrit levels (Hct, Readcrit Centrifuge, Clay Adams Division of Becton, Dickinson, Parsippany, NJ, USA).
Assessment of necrotic areas in skin flaps
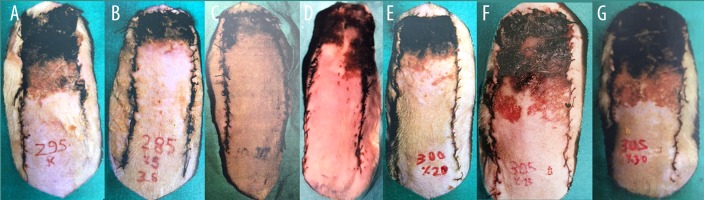
All experimental animals were evaluated for the necrotic areas of skin flaps on the 7th day postoperatively (Table 1). The necrotic areas in Groups I–VII are shown in Figure 2. Acetate templates designed in the same dimensions with the flap were used for marking the necrosis. The ratio of necrosis in skin flaps was measured by the paper template method suggested by Sasaki [16]. The surface of necrotic areas was counted on millimeter graph papers and was calculated in mm2. After measurements were performed and biopsies were obtained from the transitional zone of necrosis area, the animals were sacrificed using an overdose of KCl solution.
Table 1.
Descriptive statistics and comparison results for the sizes of necrotic areas (mm2) in experimental groups with various haemodilution ratio groups.
| Haemodilution ratio (%) | Size of necrotic area (mm2) | p | |
|---|---|---|---|
| I | 0 | 1283.33±29.17a# | 0.01 |
| II | 5 | 627.33±110.97b | |
| III | 10 | 613.33±37.03b | |
| IV | 15 | 651.676±29.80b | |
| V | 20 | 613.33±37.39b | |
| VI | 25 | 1190.00±101.06a | |
| VII | 30 | 1208.33±48.39a |
Different lower cases represent statistically significant differences.
Figure 2.
The amount of flap necrosis in (A). Group I, (B). Group II, (C). Group III, (D). Group IV, (E). Group V, (F). Group VI, and (G). Group VII.
Histopathological examination
Biopsies were obtained from the transitional zones of necrotic areas on the dorsal skin flap, and these specimens were preserved in 10% neutral buffered formalin. Sections with a thickness of 7 mm were taken in parallel to the skin surface and stained with hematoxylin and eosin. Epidermal necrosis was evaluated under light microscopy, which was evident in all biopsy samples.
Statistical analysis
Analysis of data was performed using Statistical Package for Social Sciences program version 17.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics for the studied variables (characteristics) are presented as mean and standard deviation. The Kruskal-Wallis test was performed to compare groups. The Friedman test was also performed to compare periods. Spearman correlation coefficients were calculated to determine relationships between hematocrit levels and necrotic areas in each group and period. The statistical significance level was set at 5%.
Results
Comparison of the flap necrosis areas in the control group and 6 experimental groups with different ratios of hemodilution is presented in Table 1. The necrotic surfaces in Groups I–VII were 1283.33±29.17, 627.33±110.97, 613.33±37.03, 651.676±29.8, 613.33±37.39, 1190±101.06, and 1208.33±48.39 mm2, respectively. There were no statistically significant differences between the control group and Groups VI (p=0.248) and VII (p=0.163) regarding the necrotic area. The sizes of necrotic areas were similar in Groups II, III, IV, and V without any significant differences and, notably, they were significantly lower compared with the control group. The necrotic areas in Groups II–V were also significantly lower than those of the Groups VI and VII (p=0.003).
The hematocrite levels of all 7 groups are summarized in Table 2. An obvious diminution was evident in the hematocrit value at the 2nd hour and on 1st day, and this decrease was associated with an increase in the ratio of hemodilution. Compared with Group I, hematocrit values on the 3rd day were remarkably lower in Groups II to VII (p<0.001). Regarding hematocrit values on the 3rd day and 7th day, there was a notable difference in Groups VI and VII compared with the Groups II, III, IV, and V. In all groups, the lowest hematocrit levels were obtained on the 3rd day of study, which was improved on the 7th day.
Table 2.
Descriptive statistics and comparison result for Hematocrit levels of study groups measured at various time intervals.
| Group | Hematocrit level (%) | p | ||||
|---|---|---|---|---|---|---|
| Preoperative | Postoperative 2nd hour | Postoperative 1st day | Postoperative 3rd day | Postoperative 7th day | ||
| I | 54.33±0.21aA | 48.33±0.21aB | 42.00±0.52aD | 41.00±0.77aE | 43.00±0.26bC | 0.01 |
| II | 52.33±0.56bA | 45.00±0.26bB | 36.00±0.93cD | 33.67±0.92bE | 41.00±0.45dC | 0.01 |
| III | 53.00±0.68bA | 41.33±0.80cC | 38.33±0.42bD | 32.33±0.76cE | 44.33±0.42aB | 0.01 |
| IV | 55.00±0.26aA | 34.33±0.61fC | 32.33±0.21dD | 34.00±0.37bC | 41.33±0.42dB | 0.01 |
| V | 48.67±1.58cA | 39.33±1.82dC | 32.33±0.61dD | 33.67±0.42bC | 42.33±0.42cB | 0.01 |
| VI | 55.00±0.45aA | 38.00±0.52eC | 30.00±0.26eE | 31.67±0.42dD | 39.00±0.26eB | 0.01 |
| VII | 54.67±0.21aA | 34.67±0.42fC | 27.67±0.21fE | 29.00±0.26eD | 39.50±0.43eB | 0.01 |
| p | 0.01 | 0.01 | 0.01 | 0.01 | 0,01 | |
a, b, c; ↓: Different lower cases in the same column represent statistically significant differences among the groups;
A, B, C; →: Different upper cases in the same row represent statistically significant differences among the periods
We also investigated the correlation between the hematocrit levels and the sizes of necrotic areas in every group, and we did not find any statistically significant correlation at any time interval in any groups (Table 3).
Table 3.
Spearman’s correlation coefficients between Necrotic area and Hematocrit levels in each group and period.
| Groups | Preoperative | Postoperative 2nd hour | Postoperative 1st day | Postoperative 3rd day | Postoperative 7th day | |
|---|---|---|---|---|---|---|
| Necrotic area | I | 0.314 | −0.257 | 0.314 | −0.443 | −0.486 |
| II | −0.086 | −0.143 | 0.714 | −0.371 | −0.486 | |
| III | −0.143 | 0.086 | 0.771 | 0.429 | −0.257 | |
| IV | −0.314 | −0.314 | −0.371 | 0.143 | −0.486 | |
| V | −0.371 | −0.029 | −0.543 | −0.029 | −0.086 | |
| VI | −0.486 | −0.600 | 0.371 | 0.771 | −0.200 | |
| VII | −0.314 | −0.714 | 0.429 | −0.371 | 0.486 |
Discussion
The aim of this study was to evaluate the impacts of different ratios of normovolemic hemodilution on the survival of random-pattern skin flaps in an experimental rat model, and we determined that use of normovolemic hemodilution for compensation of loss of less than 20% of total blood volume exhibits favorable effects on the survival of skin flaps. However, in conditions of the blood loss exceeding 20% of total volume, beneficial effects of hemodilution were not detected. Moreover, normovolemic hemodilution was also well-tolerated in all groups, and on the 7th day of the experiment, hematocrit levels were much higher compared with the 1st and 3rd days after the hemodilution.
There have been few published reports on the association of normovolemic hemodilution with flap survival. Schram et al. [10] reported transiently improved oxygen tension and venous base excess during hemodilution, and they reported the maximal benefit to be expected at a hemoglobin concentration at or slightly less than 9 g/dl. Amoroso et al. [13] reported that both normovolemic and hypervolemic hemodilutions were effective in providing better flap survival rates. It is very important to determine the amount of blood volume withdrawn in normovolemic hemodilution because tissue oxygenation is important for all viable cells. In another experimental study, Kanayama et al. also determined that, after withdrawal of the 35% of blood volume, in the normovolemic hemodilution group flap survival was better compared with the control (sham) and blood transfusion groups. To the best of our knowledge, the present report is one of the first studies comparing different hemodilution ratios.
Flap viability may be affected by many conditions; general risk factors for the failure of flaps include extensive trauma, smoking, age, and peripheral vascular disease. Due to the lack of lymphatic drainage, compromise of innervations, and reduced ability to reabsorb excessive interstitial fluid, there is an increased risk of edema development for flaps, and the choice of appropriate fluid to be administered is very important to maintain intravascular volume and optimize blood flow and oxygen transport. However, there is controversy on the ideal perioperative fluid management: the replacement of intravascular volume with physiologic crystalloid or colloid solutions during microsurgery [17].
The delivery of oxygen to the tissues is associated with local perfusion, arterial oxygen concentration, local oxygen consumption, and the amount of oxygen carried in arterial blood [18]. Blood viscosity determines the perfusion of tissues during ischemia, and it is associated with hematocrit levels, the shape of the erythrocytes, and tissue fibrinogen concentration [18].
Diminution of blood viscosity using normovolemic hemodilution enhances the blood flow, which will serve for compensation of the decrease in the oxygen transport capacity of blood [18,19]. Thus, controlled hemodilution may be useful for facilitation of healing in ischemic tissues after surgery [20,21]. It was clearly shown that hemodilution reduces microcirculatory occlusion, thus increasing microcirculatory blood flow.
Our results indicated that normovolemic hemodilution ratios ranging from 5% to 20% were associated with better survival of flaps. The use of dextran-70 was accompanied by a 25–50% decrease in blood viscosity. We did not evaluate the blood viscosity or oxygen tension at the tissue level, which is a limitation of this study. However, a decrease in hematocrit levels is linked with a decrease in blood viscosity. On the capillary level, reduction of blood viscosity causes an acceleration of erythrocyte velocity. Previous reports also demonstrated that anemia did not necessarily lead to a delay in wound healing, and normovolemic hemodilution has been successfully used in treatment of ischemia [9,13,18]. Decrease in blood viscosity and total peripheral resistance resulted in increased stroke volume and cardiac output, which consequently increases the circulating blood volume [22]. It can be postulated that hemodilution enhances the microcirculation by influencing the vasomotor state at various hematocrit levels [18]. Based on this hypothesis, we aimed to investigate the flap survival rates at different hemodilution ratios, and we found better rates at 5%, 10%, 15%, and 20% hemodilution ratios. In contrast, better flap survival was not detected at hemodilution ratios of 25% and 30%.
The compromise of blood flow causes an increase in blood viscosity attributed to the aggregation of erythrocytes. This effect becomes more prominent if hematocrit levels are increased, whereas blood viscosity will be lower in the case of decreased hematocrit levels, such as in the case of hemodilution. Therefore, the impact of hemodilution on tissue perfusion will be more obvious with reduction of blood flow [23]. Our findings are consistent with this data, since hemodilution ratios of 5%, 10%, 15%, and 20% were associated with less necrotic areas in skin flaps. On the other hand, hemodilution ratios of 25% and 30% resulted in adverse outcomes regarding flap necrosis in our study.
The deformation of erythrocyte shape and concentration of plasma fibrinogen are other determinants of blood viscosity. These circumstances occur in cases of ischemia and acidosis, and result in an elevated rate of erythrocyte aggregation [23]. Hemodilution can restore local blood flow and tissue oxygenation, which may subsequently reverse the pathological changes in erythrocyte shape and acid-base balance [23]. In the relevant literature, results similar to our data were reported, showing that normovolemic hemodilution was associated with improved oxygen concentrations in both subcutaneous tissue and random-pattern skin flaps [23].
The hematocrit values at the 2nd hour after the procedure seemed to decline in proportion to the amount of blood volume drawn and in parallel with higher ratios of hemodilution. On the 7th day, the hematocrit values started to return to previous levels and differences between groups became less apparent. Our data show that controlled hemodilution up to a certain ratio improves flap survival and, after a cut-off point, hemodilution did not exhibit any further beneficial effects, suggesting therapeutic implications of improved skin flap survival by controlled hemodilution and involving ischemia. On the other hand, unnecessary blood transfusions should be avoided unless hematocrit values fall below a critical threshold. Otherwise, not only flap circulation may be adversely affected, but also risks such as dissemination of blood transfusion-related diseases, anaphylaxis, or immunosuppression may also occur [24]. In this perspective, replacement of blood volume loss can be compensated for using hemodilution by plasma volume-expander agents.
McFarlane flaps are frequently used for assessment of random-pattern flaps. The vascular supply of skin in rats is directly provided by cutaneous vessels, while musculocutaneous perforator vessels fulfill this function in humans, who have fixed skin structure [25,26]. No correlation has been previously reported between rat sizes and flap necrosis [26]. We preferred caudal-based dorsal flaps (Figure 1A–1C) in this study since they were less likely to undergo flap necrosis [27].
Another recent study reported a strong correlation between necrosis rate and hemodilution [28]. However, in the present study, we did not find any correlation between the necrotic area and the hematocrit levels at any time period.
The main strength of our study is the simultaneous investigation of the relationship between various hemodilution ratios and skin flap necrosis. There are some limitations that should be mentioned. First, due to the experimental design, the influence of neovascularization from the skin flap bed could not be excluded. Secondly, blood viscosity or oxygen tension at the tissue level or microcirculation were not evaluated directly or indirectly. Instead, we analyzed the overall outcome as the necrotic area. Finally, the impact of confounding environmental, genetic, and metabolic factors may have influenced our results.
Conclusions
In conclusion, the loss of less than 20% of total blood volume by normovolemic hemodilution with dextran may improve survival of skin flaps. If the amount of loss exceeds 20% of total blood volume, normovolemic hemodilution with dextran does not have any beneficial effects on flap survival. In light of the promising results determined in this experimental study, we suggest that normovolemic hemodilution, with the loss of less than 20% of total blood volume, may also improve flap survival in human patients. Larger prospective studies are warranted to define the role of normovolemic hemodilution in flap survival in humans.
Footnotes
Conflict of interests
None.
Source of support: Departmental sources
References
- 1.Memarzadeh K, Sheikh R, Blohmé J, et al. Perfusion and oxygenation of random advancement skin flaps depend more on the length and thickness of the flap than on the width to length ratio. Eplasty. 2016;19:16. [PMC free article] [PubMed] [Google Scholar]
- 2.Tao XY, Wang L, Gao WY, Yang LH. The effect of inducible nitric oxide synthase on multiterritory perforator flap survival in rats. J Reconstr Microsurg. 2016;32:643–49. doi: 10.1055/s-0036-1584808. [DOI] [PubMed] [Google Scholar]
- 3.Fichter AM, Ritschl LM, Rau A, et al. Free flap rescue using an extracorporeal perfusion device. J Craniomaxillofac Surg. 2016;44:1889–95. doi: 10.1016/j.jcms.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Slater NJ, Zegers HJ, Küsters B, et al. Ex-vivo oxygenated perfusion of free flaps during ischemia time: A feasibility study in a porcine model and preliminary results. J Surg Res. 2016;205:292–95. doi: 10.1016/j.jss.2016.06.096. [DOI] [PubMed] [Google Scholar]
- 5.Demir B, Engin MS, Keleş MK, et al. Comparison of the effects of different vasoactive and antiplatelet drugs on perforator flap viability: An experimental study. Hand Surg Rehabil. 2016;35:55–59. doi: 10.1016/j.hansur.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Weiskopf R. Efficacy of acute normovolemic hemodilution assessed as a function of fraction of blood volume lost. Anesthesiology. 2001;94:439–46. doi: 10.1097/00000542-200103000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Erni D, Wettstein R, Schramm S, et al. Normovolemic hemodilution with Hb vesicle solution attenuates hypoxia in ischemic hamster flap tissue. Am J Physiol Heart Circ Physiol. 2003;284:1702–9. doi: 10.1152/ajpheart.00821.2002. [DOI] [PubMed] [Google Scholar]
- 8.Hyodo A, Heros RC, Tu YK, et al. Acute effects of isovolemic hemodilution with crystalloids in a canine model of focal cerebral ischemia. Stroke. 1989;20:534–40. doi: 10.1161/01.str.20.4.534. [DOI] [PubMed] [Google Scholar]
- 9.Plock JA, Contaldo C, Sakai H, et al. Is hemoglobin in hemoglobin vesicles infused for isovolemic hemodilution necessary to improve oxygenation in critically ischemic hamster skin? Am J Physiol Heart Circ Physiol. 2005;289:2624–31. doi: 10.1152/ajpheart.00308.2005. [DOI] [PubMed] [Google Scholar]
- 10.Schramm S, Wettstein R, Wessendorf R, et al. Acute normovolemic hemodilution improves oxygenation in ischemic flap tissue. Anesthesiology. 2002;96:1478–84. doi: 10.1097/00000542-200206000-00030. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara G, Kanoore Edul VS, Martins E, et al. Intestinal and sublingual microcirculation are more severely compromised in hemodilution than in hemorrhage. J Appl Physiol. 2016;120:1132–40. doi: 10.1152/japplphysiol.00007.2016. [DOI] [PubMed] [Google Scholar]
- 12.Kanayama K, Mineda K, Mashiko T, et al. Blood congestion can be rescued by hemodilution in a random-pattern skin flap. Plast Reconstr Surg. 2017;139:365–74. doi: 10.1097/PRS.0000000000002935. [DOI] [PubMed] [Google Scholar]
- 13.Amoroso M, Ozkan O, Ozkan O, et al. The effect of normovolemic and hypervolemic hemodilution on a microsurgical model: Experimental study in rats. Plast Reconstr Surg. 2015;136:512–19. doi: 10.1097/PRS.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 14.Jones SB, Whitten CW, Monk TG. Influence of crystalloid and colloid replacement solutions on hemodynamic variables during acute normovolemic hemodilution. J Clin Anesth. 2004;16:11–17. doi: 10.1016/j.jclinane.2003.03.003. Erratum in: J Clin Anesth 2008; 20: 324. [DOI] [PubMed] [Google Scholar]
- 15.McFarlane RM, Deyoung G, Henry RA. The design of a pedicle flap in the rat to study necrosis and its prevention. Plast Reconstr Surg. 1965;35:177–82. doi: 10.1097/00006534-196502000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki GH, Pang CY. Hemodynamics and viability of acute neurovascular island skin flap in rats. Plast Reconstr Surg. 1980;65:152–58. doi: 10.1097/00006534-198002000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Namdar T, Bartscher T, Stollwerck PL, et al. Complete free flap loss due to extensive hemodilution. Microsurgery. 2010;30:214–17. doi: 10.1002/micr.20736. [DOI] [PubMed] [Google Scholar]
- 18.Barile L, Fominskiy E, Di Tomasso N, et al. Acute normovolemic hemodilution reduces allogeneic red blood cell transfusion in cardiac surgery: A systematic review and meta-analysis of randomized trials. Anesth Analg. 2017;124:743–52. doi: 10.1213/ANE.0000000000001609. [DOI] [PubMed] [Google Scholar]
- 19.Nisi G, Barberi L, Ceccaccio L, et al. Effect of repeated subcutaneous injections of carbon dioxide (CO2) on inflammation linked to hypoxia in adipose tissue graft. Eur Rev Med Pharmacol Sci. 2015;19:4501–6. [PubMed] [Google Scholar]
- 20.Xie P, Jia S, Tye R, et al. Systemic administration of hemoglobin improves ischemic wound healing. J Surg Res. 2015;194:696–705. doi: 10.1016/j.jss.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 21.Kimmel HM, Grant A, Ditata J. The presence of oxygen in wound healing. Wounds. 2016;28:264–70. [PubMed] [Google Scholar]
- 22.Lipowsky HH, Firrell JC. Microvascular hemodynamics during systemic hemodilution and hemoconcentration. Am J Physiol. 1986;250:908–22. doi: 10.1152/ajpheart.1986.250.6.H908. [DOI] [PubMed] [Google Scholar]
- 23.Hansen ES, Gellett S, Kirkegård L, et al. Tissue oxygen tension in random pattern skin flaps during normovolemic hemodilution. J Surg Res. 1989;47:24–29. doi: 10.1016/0022-4804(89)90043-7. [DOI] [PubMed] [Google Scholar]
- 24.Garraud O, Tariket S, Sut C, et al. Transfusion as an inflammation hit: Knowns and unknowns. Front Immunol. 2016;7:534. doi: 10.3389/fimmu.2016.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ersel M, Uyanikgil Y, Akarca FK, et al. Effects of Silk Sericin on Incision Wound Healing in a Dorsal Skin Flap Wound Healing Rat Model. Med Sci Monit. 2016;22:1064–78. doi: 10.12659/MSM.897981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conoyer JM, Toomey JM. Dorsal skin flaps in rats as an experimental model. Surg Forum. 1979;30:510–11. [PubMed] [Google Scholar]
- 27.Sen H, Oruc M, Isik VM, et al. The effect of omeprazole usage on the viability of random pattern skin flaps in rats. Ann Plast Surg. 2017;78:e5–e9. doi: 10.1097/SAP.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 28.Amoroso M, Özkan Ö, Başsorgun Cİ, et al. The effect of Normovolemic and hypervolemic hemodilution on a perforator flap with twisted pedicle model: Experimental study in rats. Plast Reconstr Surg. 2016;137:339–46. doi: 10.1097/01.prs.0000475782.06704.8a. [DOI] [PubMed] [Google Scholar]