Abstract
Introduction:
Noise exposure, the main cause of hearing loss in countries with lot of industries, may result both in temporary or permanent hearing loss. The goal of this study was to investigate the effects of parenteral papaverine and piracetam administration following an acoustic trauma on hearing function with histopathologic correlation.
Materials and Methods:
Eighteen Wistar albino rats exposed to noise for 8 h in a free environment were included. We divided the study population into three groups, and performed daily intraperitoneal injections of papaverine, piracetam, and saline, respectively, throughout the study. We investigated the histopathologic effects of cellular apoptosis on inner hair cells (IHCs) and outer hair cells (OHCs) and compared the distortion product otoacoustic emissions (DPOAEs) thresholds among the groups.
Results and Discussion:
On the 3rd and 7th days, DPOAE thresholds at 8 kHz were significantly higher both in papaverine and piracetam groups compared with the control group (P = 0.004 for 3rd day, P = 0.016 and P = 0.028 for 7th day, respectively). On the 14th day, piracetam group had significantly higher mean thresholds at 8 kHz (P = 0.029); however, papaverine group had similar mean thresholds compared to the control group (P = 0.200). On the 3rd and 7th days following acoustic trauma, both IHC and OHC loss were significantly lower in both papaverine and piracetam groups. On the 7th day, the mean amount of apoptotic IHCs and OHCs identified using Caspase-3 method were significantly lower in both groups, but the mean amount identified using terminal deoxynucleotidyl transferase dUTP nick end labeling method were similar in both groups compared to the control group.
Conclusion:
We demonstrated the effects of papaverine and piracetam on the recovery of cochlear damage due to acoustic trauma on experimental animals using histopathologic and electrophysiologic examinations.
Keywords: Acoustic trauma, cochlea, otoacoustic emissions, papaverine, piracetam
Introduction
Noise exposure, main cause of hearing loss in industrial countries, may result both in temporary or permanent hearing loss. Noise induced hearing loss (NIHL) is the most frequent occupational disease in the USA. Sixteen percent of adults worldwide suffered from hearing loss are related to occupational noise exposure.[1] Previous studies focus on the usage of antioxidant agents and/or agents increasing the blood support of inner ear, which prevents cochlear damage, supports the hypothesis that cochlear damage due to noise exposure is caused by hypoxia and oxidative stress.[2] In accordance with this purpose, many agents such as N-acetyl cysteine,[3] corticosteroids,[4] and vasodilators[5] have been used for treatment.
Piracetam, an agent for the treatment of many conditions such as sudden idiopathic hearing loss and cochlear damage due to radiation for head and neck cancers, is commonly used for its neuroprotective and antioxidant effects.[6] Papaverine has also been used for the treatment of many diseases such as sudden hearing loss and meniere for its booster effects on microcirculation and reorganizing effects. In this experimental animal study, we used piracetam and papaverine, which have neuroprotective effects, antioxidant effects, and booster effects on the blood supply of the inner ear, preventing or reducing cochlear damage.
The aim of this study was to demonstrate the effects of parenteral papaverine and piracetam administration following an acoustic trauma to the cochlear hair cells using electrophysiologic and histopathologic methods. Prior archival human temporal bone studies demonstrated the cochlear changes in many diseases can be seen in the literature,[7] yet to the best of our knowledge, this is the first study investigated the effects of papaverine on cochlear hair cells.
Material and Methods
Subjects and study design
Animal experiment local ethical committee approved this trial performed at the animal experiment laboratory (No. 2013-15-111, September 4, 2013). We followed the principles of the Declaration of Helsinki. Eighteen adult and male Wistar albino rats with a weight of 220–250 g were included in the study. Firstly, all rats underwent endoscopic ear examination under general anesthesia and had debris and plugs in their external ears cleaned to provide a normal tympanic membrane view. We performed distortion product otoacoustic emission (DPOAE; ILO V6, Otodynamics Ltd., Herts, UK) testing before noise exposure and confirmed the normal hearing function of all rats included in the study. We placed the rats into a silent cabin in a cage and exposed them to white noise at a frequency of 110 dB for 8 h in a free environment with Interacoustics AC 40 clinical audiometry device and binaural speakers. The cage of the rats was 5–10 cm away from the speaker. In many prior studies, a white noise at 110 dB or higher, applied for durations varying from 10 min to 24 h, was successfully used to achieve an acoustic trauma. Like previous literature, we generated a model of acoustic trauma using a white noise at 110 dB sound pressure level (SPL) for 8 h because of its lower cost, repeatability, and ease of application. After the noise exposure, eighteen subjects were randomly divided into three groups (n = 6, for each group) and performed daily intraperitoneal injections of papaverine, piracetam and saline (as control group) to group 1, group 2, and group 3, respectively, throughout the study. All rats underwent DPOAE testing immediately after the trauma, on the 3rd, 7th and 14th days. We performed electrophysiologic testing and the parenteral injections under general anesthesia achieved by administering 5 mg/kg intramuscular xylazine hydrogen chloride (HCL) (Rhompun ampoule; Bayer, Istanbul, Turkey) and 40 mg/kg of intramuscular ketamine HCl (Ketalar ampoule; Pfizer, Istanbul, Turkey).
Histopathologic assessment
On the 3rd day following noise exposure, we sacrificed one rat from each group after DPOAE testing as did we another from each group after DPOAE testing on the 7th day. On the 14th day, we sacrificed remaining four rats from each group after DPOAE testing and isolated the cochleae of all rats. Removed tissues were fixed in neutral formalin for 24 h. Then, they washed under a water flow, and were decalcificated in an ethylenediaminetetraacetic acid (EDTA) solution. Alcohol series and xylene were used to dehydrate the tissues for transparency. We mounted the serial sections on polylysine-coated slides (5 μm in thickness). We obtained the sections from the basal turn of cochlea. Some of the sections were spared for immunohistochemical staining and others for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. We used the Caspase-3 method for TUNEL and immunohistochemical staining that was described in the literature.[8] Two observers blinded to the experimental information evaluated the TUNEL and immunolabeling scores independently. The average number of apoptotic cells was determined by counting the TUNEL-positive cells that were in randomly chosen fields of each case. In each case, a total of hundred, both TUNEL positive or negative, cells were counted and the TUNEL-positive cells were shown in a percentage form.
The staining intensity of the slides with their immunohistochemical protocol was graded semi-quantitatively, and the HSCORE was calculated using the following formula: HSCORE=Pi (i + 1), where i is the intensity of staining with a value of 1, 2, or 3 (weak, moderate, or strong, respectively), and where Pi is the percentage of stained cells for each intensity, varying from 0 to 100%. Morphometric analysis was performed for all subjects using image-analyzing software (Leica Q Win V3 Plus Image, Leica, Germany).
We investigated the histopathologic effects of cellular apoptosis on inner hair cells (IHCs) and outer hair cells (OHCs) and compared the mean DPOAE thresholds among the groups.
Statistical analysis
Results are presented using descriptive statistics [mean ± standard deviation (SD), median ± SD, frequency, ratio, minimum and maximum). The normal distribution of data from each group was confirmed using Kolmogorov–Smirnov normality test. To compare the quantitative data from three groups, we used Kruskal–Wallis test. To detect the group causing the statistical difference and to compare the mean ratio of apoptotic IHCs and OHCs, we used Mann–Whitney U test. For all of our data analysis, we used NCSS (Number Cruncher Statistical System) 2007&PASS (Power Analysis and Sample Size) 2008 Statistical Software (Utah, USA). A P value <0.05 was considered statistically significant.
Results
Distortion product otoacoustic emission testing results
Mean DPOAE thresholds recorded at 750, 1500, 2000, and 4000 Hz before noise exposure, immediately after noise exposure, on the 3rd day, 7th day, and 14th day following noise exposure did not significantly differ among three groups (P > 0.05).
On the 3rd day following acoustic trauma, mean DPOAE thresholds at 6000 Hz were significantly higher in piracetam group compared with the control group (P = 0.010) but on the other days following noise exposure, they did not significantly differ among three groups (P > 0.05) [Table 1].
Table 1.
Comparison of mean DPOAE thresholds at 6 kHz among the groups on the throughout the study
| 6 kHz DPOAE | Control1 Min–Max (median) Mean ± SD | Papaverine2 Min–Max (median) Mean ± SD | Piracetam3Min–Max (median) Mean ± SD | P a | P b |
|---|---|---|---|---|---|
| Before noise trauma (n = 6) | 20.3–26.3 (23) | 18.1–29.1 (22.4) | 19.3–29.8 (26.3) | 0.796 | |
| 23.21 ± 2.70 | 23.24 ± 3.86 | 25.01 ± 4.57 | |||
| Immediately after the trauma (n = 6) | 10.9–15.3 (12.4) | 9.6–15 (14.7) | 11–16.7 (14.9) | 0.522 | |
| 12.84 ± 1.63 | 13.59 ± 2.15 | 14.19 ± 2.62 | |||
| Day 3 (n = 6) | 14.8–17.2 (15.4) | 14.6–23.2 (17.9) | 16.3–24.4 (20.8) | 0.027* | 1,2 P = 0.073 |
| 15.75 ± 0.86 | 18.54 ± 3.15 | 20.52 ± 3.58 |
1,3
P = 0.010* 2,3 P = 0.337 |
||
| Day 7 (n = 5) | 16.9–19.3 (18.6) | 15.3–27.4 (19.2) | 17.2–26.5 (24.3) | 0.185 | |
| 18.14 ± 1.11 | 20.8 ± 4.79 | 22.70 ± 4.09 | |||
| Day 14 (n = 4) | 17.5–19.9 (18.6) | 17.5–28.5 (23.2) | 22.2–27.8 (27.2) | 0.077 | |
| 18.64 ± 1.03 | 23.10 ± 4.80 | 26.09 ± 2.63 |
Kruskal–Wallis test. bMann–Whitney U test. *P < 0.05.
On the 3rd and 7th days following acoustic trauma, mean DPOAE thresholds at 8000 Hz were significantly higher both in papaverine and piracetam groups compared with the control group (P = 0.004 for 3rd day, P = 0.016 and P = 0.028 for 7th day, respectively). Additionally no significant difference was found between piracetam and papaverine groups [Table 2].
Table 2.
Comparison of mean DPOAE thresholds at 8 kHz among the groups on the throughout the study
| 8 kHz DPOAE | Control1 Min–Max (median) Mean ± SD | Papaverine2 Min–Max (median) Mean ± SD | Piracetam3 Min–Max (median) Mean ± SD | P a | P b |
|---|---|---|---|---|---|
| Before noise trauma (n = 6) | 17.4–20.3 (18.6) | 16.7–25.2 (19.2) | 17.1–24.1 (21.7) | 0.244 | |
| 18.65 ± 1.10 | 20.19 ± 2.99 | 20.99 ± 2.49 | |||
| Immediately after the trauma (n = 6) | 4.8–9.8 (7.9) | 7.8–11.7 (9) | 7.9–11.1 (9.7) | 0.182 | |
| 7.89 ± 1.81 | 9.27 ± 1.42 | 9.61 ± 1.36 | |||
| Day 3 (n = 6) | 9–12.9 (11) | 12.9–19.8 (15.2) | 14.3–19.7 (16.9) | 0.003* | 1,2 P = 0.004** |
| 11.19 ± 1.48 | 15.84 ± 2.42 | 16.94 ± 2.63 |
1,3
P = 0.004** 2,3 P = 0.748 |
||
| Day 7 (n = 5) | 10.6–15.9 (13.2) | 13.8–23.5 (17.5) | 15.5–22.4 (20.8) | 0.023* | 1,2 P = 0.016* |
| 13.32 ± 1.91 | 18.13 ± 3.66 | 19.57 ± 2.75 |
1,3
P = 0.028* 2,3 P = 0.465 |
||
| Day 14 (n = 4) | 14.3–15.4 (15.3) | 15.1–24.7 (20.1) | 20.5–23.7 (21.4) | 0.059 | 1,2 P = 0.200 |
| 15.06 ± 0.51 | 20.02 ± 4.04 | 21.73 ± 1.36 |
1,3
P = 0.029* 2,3 P = 0.486 |
Kruskal–Wallis test. bMann–Whitney U test. *P < 0.05. **P < 0.01.
On the 14th day, piracetam group had significantly higher mean thresholds at 8000 Hz (P = 0.029), but papaverine group had similar mean thresholds compared to the control group (P = 0.200) [Table 2].
Histopathologic examination results
Both histopathologic examinations using Caspase-3 and TUNEL methods on the 3rd, 7th and 14th days revealed that each group had higher mean amount of apoptotic OHCs compared with the mean amount of IHCs (P = 0.001).
Comparison of mean amount of apoptotic OHCs and IHCs identified using Caspase-3 method on the 3rd and 7th days is shown in Table 3. On the 14th day following noise exposure, the mean amount of apoptotic OHCs and IHCs identified using Caspase-3 method were significantly lower in papaverine group compared both with piracetam and control groups (P = 0.001 for OHCs and P = 0.004 and P = 0.005 for IHCs, respectively). However, no significant difference was found between piracetam and control groups (P = 0.403 for OHCs and P = 0.818 for IHCs) [Table 3, Figure 1].
Table 3.
Comparison of mean amount of apoptotic OHCs and IHCs identified using Caspase-3 method on the 3rd, 7th and 14th days
| Control1 Min–Max (median) Mean ± SD | Papaverine2 Min–Max (median) Mean ± SD | Piracetam3 Min–Max (median) Mean ± SD | P a | P b | |
|---|---|---|---|---|---|
| OHC | |||||
| Day 3 | 165–189 (175.5) | 141–159 (148.5) | 90–132 (109.5) | 0.001** | 1,2 0.007** |
| 177.00 ± 7.32 | 148.90 ± 4.93 | 109.90 ± 13.75 |
1,3
0.001** 2,3 0.001** |
||
| Day 7 | 129–144 (135) | 114–138 (122) | 101–123 (115.5) | 0.001** | 1,2 0.001** |
| 136.00 ± 4.67 | 123.00 ± 6.67 | 114.90 ± 6.56 |
1,3
0.001** 2,3 0.015* |
||
| Day 14 | 93–120 (106.5) | 72–91 (82.5) | 104–120 (111) | 0.001** | 1,2 0.001** |
| 106.80 ± 7.57 | 81.90 ± 6.54 | 104.50 ± 4.60 |
1,30.403 2,3 0.001** |
||
| IHC | |||||
| Day 3 | 34–39 (36.5) | 25–31 (28) | 17–26 (20.5) | 0.001** | 1,2 0.001** |
| 36.50 ± 1.58 | 28.10 ± 1.91 | 20.70 ± 3.27 |
1,3
0.001** 2,3 0.001** |
||
| Day 7 | 23–28 (25.5) | 20–27 (23.5) | 19–24 (21.5) | 0.001** | 1,2 0.036* |
| 25.60 ± 1.71 | 23.50 ± 2.17 | 21.50 ± 1.58 |
1–3
0.001** 2,3 0.039* |
||
| Day 14 | 18–24 (21) | 15–24 (17.5) | 16–30 (22.5) | 0.005** | 1,2 0.004** |
| 21.00 ± 2.05 | 17.80 ± 1.93 | 20.70 ± 1.94 |
1,30.818 2,3 0.005** |
||
Kruskal–Wallis test. bMann–Whitney U test. *P < 0.05. **P < 0.01.
Figure 1.
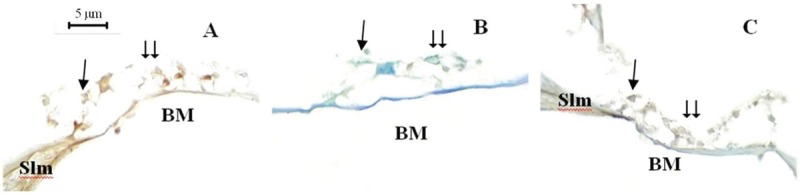
Fourteenth day study cochlea sections, Caspase-3 staining method, ×1000. Single arrow = inner hair cells, double arrow = outer hair cells, Slm = spiral limbus, BM = basilar membrane. Control (A), papaverine (B), and piracetam (C)
Comparison of mean amount of apoptotic OHCs and IHCs identified using TUNEL method on the 3rd and 7th days are shown in Table 4. On the 14th day following noise exposure, the mean amount of apoptotic OHCs and IHCs identified using TUNEL method were significantly lower in papaverine group compared both with piracetam and control groups (P = 0.001 for OHCs and P = 0.015 for IHCs). However, no significant difference was found between piracetam and control groups (P = 0.454 for OHCs and P = 0.863 for IHCs) [Table 4, Figure 2].
Table 4.
Comparison of mean amount of apoptotic OHCs and IHCs identified using TUNEL method on the 3rd, 7th and 14th days
| Control1 Min–Max (median) Mean ± SD | Papaverine2 Min–Max (median) Mean ± SD | Piracetam3Min–Max (median) Mean ± SD | P a | P b | |
|---|---|---|---|---|---|
| OHC | |||||
| Day 3 | 8–13 (10.5) | 7–10 (8.5) | 4–7 (5.5) | 0.001** | 1,2 0.007** |
| 10.60 ± 1.51 | 8.60 ± 1.17 | 5.60 ± 0.97 |
1,3
0.001** 2,3 0.001** |
||
| Day 7 | 8–11 (10) | 4–10 (7.5) | 4–12 (7.5) | 0.004** | 1,2 0.001** |
| 9.70 ± 0.95 | 7.20 ± 1.62 | 7.80 ± 2.20 |
1,3
0.019* 2,30.588 |
||
| Day 14 | 7–11 (9) | 4–7 (5.5) | 6–10 (9) | 0.001** | 1,2 0.001* |
| 9.00 ± 1.15 | 5.60 ± 0.97 | 8.50 ± 1.27 |
1,30.454 2,3 0.001* |
||
| IHC | |||||
| Day 3 | 1–3 (1.5) | 1–2 (1) | 0–1 (1) | 0.011* | 1,2 0.049* |
| 1.70 ± 0.82 | 1.10 ± 0.32 | 0.90 ± 0.32 |
1,3
0.010** 2,30.168 |
||
| Day 7 | 1–3 (2) | 1–2 (1) | 1–2 (1) | 0.187 | |
| 1.80 ± 0.79 | 1.30 ± 0.48 | 1.30 ± 0.48 | |||
| Day 14 | 1–3 (1) | 0–1 (1) | 1–3 (1) | 0.023* | 1,2 0.015* |
| 1.60 ± 0.84 | 0.80 ± 0.42 | 1.50 ± 0.71 |
1,30.863 2,3 0.015* |
||
Kruskal–Wallis test. bMann–Whitney U test. *P < 0.05. **P < 0.01.
Figure 2.
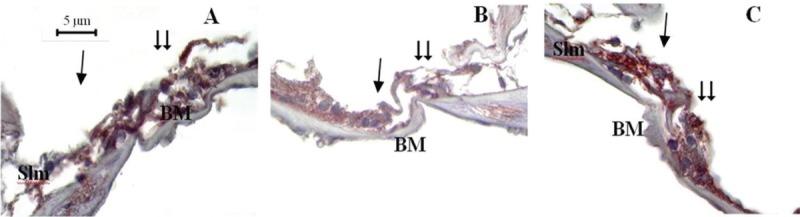
Fourteenth day study cochlea sections, TUNEL staining method, ×1000. Single arrow = inner hair cells, double arrow = outer hair cells, Slm = spiral limbus, BM = basilar membrane. Control (A), papaverine (B), and piracetam (C)
Discussion
In this study, we demonstrated that papaverine and piracetam might be effective on the inner ear to recover the negative effects due to noise exposure using histopathologic and electrophysiologic examinations.
Noise exposure may cause permanent or temporary hearing loss in animal and human being due its direct mechanical and/or secondary metabolic effects.[9] The mechanism of the NIHL is still unclear and agents considered useful for prevention and treatment of the NIHL have not become a routine medication protocol yet.[10] Therefore, prophylaxis and treatment of the NIHL remain a matter of debate, as does its pathophysiology. Agents for prophylaxis and treatment of NIHL might reduce medical costs and have significantly positive effects on the quality of life.
It is commonly known that ischemia/reperfusion damage, hypoxia, accumulation of free oxygen radicals in hypoxic regions, apoptosis, and necrosis due to excessive secretion of excitatory neurotransmitters have a role in the mechanism of the cochlear damage following noise exposure.[11] Accordingly, many agents in a wide spectrum have been used for treatment of NIHL.[12,13]
Papaverine plays an important role on preserving the homeostasis of inner ear and cochlear microcirculation, reducing the oxygen stress in perilymph. Supporting cochlear micro vascularization, it increases oxygenation of perilymph, endolymph and cochlear cells, which has a crucial role for cochlear functions.[14] Prior studies reported that papaverine is as effective as the other agents are that commonly used for treatment of sudden hearing loss.[15] Another study revealed that topical papaverine might be effective on prevention of vasospasm of internal auditory artery.[16]
Piracetam has neuroprotective and antioxidant effects, and it arranges the fluidity of the membranes in reorganizing cells. Additionally, it has an anti-thrombotic effect inhibiting thrombocyte aggregation. It reduces the levels of fibrinogens and Von Willebrand factor. Thus, it reduces capillary vasospasm and viscosity of blood, and reorganizes blood supply increasing cerebral perfusion and oxygen extraction in circulation.[17] Psillas et al. reported that piracetam and corticosteroid application in the first hour following noise exposure is effective on recovery of the hearing levels of the patients.[18]
In this study, lower amount of apoptosis in papaverine group was remarkable compared to the piracetam and control groups particularly on the 14th day. Although piracetam group had lower amount of apoptosis compared to the papaverine group on the first 7 days, this might not be a powerful significance because of limited number of the animals. However, the early recovery effect of piracetam has an implication that piracetam might have a similarity to caspase inhibitors and metilprednisolone, which are more effective at early period to prevent apoptosis following nose exposure.[19]
Like previous publications, our DPOAE findings suggest that noise exposure has destructive effects on hearing more at higher frequencies.[20] Also, we demonstrated that noise exposure has higher destructive effects on OHCs compared with IHCs in all groups of our study. In consistence with histopathologic examination, we found that piracetam group had higher mean DPOAE thresholds at 6000 Hz on the 3rd day compared with the papaverine and control groups, while mean thresholds taken before noise exposure, immediately after noise exposure, on the 7th and 14th days following noise exposure did not significantly differ among three groups. Histopathologic correlation of our findings was remarkable and important for the clinical implication of the study. On the 14th day following acoustic trauma, papaverine group had lower amount of apoptotic hair cells, while piracetam group had higher mean DPOAE thresholds in our study. To explain this inconsistency between DPOAE testing and histopathologic examination logically, we can hypothesize that piracetam may have multiple effects; such as short term hair cell protection and long term neuroprotective properties. Furthermore, in our study, DPOAE thresholds are generally statistically higher at 3rd and 7th days and, although there is no statistically significant difference between the groups in terms of DPOAE thresholds at 14th days, treated animals have better DPOAE thresholds. It may be explained that there is self-healing or recovery mechanism for hair cells by the time after 7 days.
The limitations of this study are as follows: limited number of animals, short following period and lack of a group received piracetam, and papaverine together. In addition, DPOAEs are a useful method to assess the activity of the OHCs but ABRs would provide data about IHCs and afferent neuronal transmission. It would be better if we assessed the hair cell loss for the different location of cochlea.
Conclusions
We demonstrated the affirmative effects of papaverine and piracetam on recovery of cochlear damage due to acoustic trauma on experimental animals using histopathologic and electrophysiologic examinations. Although we evaluated the effects of papaverine and piracetam following noise exposure independently, this study revealed that piracetam was more effective at earlier period while papaverine was more effective at later period following noise exposure. Thus, we are of opinion that together usage of piracetam and papaverine might be more effective because of combination of earlier and later effects and potentialization each other.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nelson DI, Nelson RY, Concha-Barrientos M, Fingerhut M. The global burden of occupational noise-induced hearing loss. Am J Ind Med. 2005;48:446–58. doi: 10.1002/ajim.20223. [DOI] [PubMed] [Google Scholar]
- 2.Kopke RD, Coleman JK, Liu J, Campbell KC, Riffenburgh RH. Candidate’s thesis: enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss. Laryngoscope. 2002;112:1515–32. doi: 10.1097/00005537-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kopke RD, Weisskopf PA, Boone JL, Jackson RL, Wester DC, Hoffer ME, et al. Reduction of noise-induced hearing loss using L-NAC and salicylate in the chinchilla. Hear Res. 2000;149:138–46. doi: 10.1016/s0378-5955(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 4.Ozdogan F, Ensari S, Cakir O, Ozcan KM, Koseoglu S, Ozdas T, et al. Investigation of the cochlear effects of intratympanic steroids administered following acoustic trauma. Laryngoscope. 2012;122:877–82. doi: 10.1002/lary.23185. [DOI] [PubMed] [Google Scholar]
- 5.Kansu L, Ozkarakas H, Efendi H, Okar I. Protective effects of pentoxifylline and nimodipine on acoustic trauma in Guinea pig cochlea. Otol Neurotol. 2011;32:919–25. doi: 10.1097/MAO.0b013e3182267e06. [DOI] [PubMed] [Google Scholar]
- 6.Karakurt SE, Ozkul MD, Cukurova I, Demirhan E, Yigitbasi OG. [Combined treatment supported by piracetam and/or acyclovir in idiopathic sudden sensorineural hearing loss: experience with 81 cases] Kulak Burun Bogaz Ihtis Derg. 2009;19:22–7. [PubMed] [Google Scholar]
- 7.Kaya S, Tsuprun V, Hizli O, Paparella MM, Cureoglu S. Quantitative Assessment of Cochlear Histopathologic Findings in Patients With Suppurative Labyrinthitis. JAMA Otolaryngol Head Neck Surg. 2016;142:364–9. doi: 10.1001/jamaoto.2015.3803. [DOI] [PubMed] [Google Scholar]
- 8.Sagit M, Korkmaz F, Gurgen SG, Kaya M, Akcadag A, Ozcan I. The protective role of thymoquinone in the prevention of gentamicin ototoxicity. Am J Otolaryngol. 2014;35:603–9. doi: 10.1016/j.amjoto.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Clark WW. Recent studies of temporary threshold shift (TTS) and permanent threshold shift (PTS) in animals. J Acoust Soc Am. 1991;90:155–63. doi: 10.1121/1.401309. [DOI] [PubMed] [Google Scholar]
- 10.Abaamrane L, Raffin F, Gal M, Avan P, Sendowski I. Long-term administration of magnesium after acoustic trauma caused by gunshot noise in guinea pigs. Hear Res. 2009;247:137–45. doi: 10.1016/j.heares.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Chen GD. Effect of hypoxia on noise-induced auditory impairment. Hear Res. 2002;172:186–95. doi: 10.1016/s0378-5955(02)00582-8. [DOI] [PubMed] [Google Scholar]
- 12.Probst R, Tschopp K, Ludin E, Kellerhals B, Podvinec M, Pfaltz CR. A randomized, double-blind, placebo-controlled study of dextran/pentoxifylline medication in acute acoustic trauma and sudden hearing loss. Acta Otolaryngol. 1992;112:435–43. doi: 10.3109/00016489209137424. [DOI] [PubMed] [Google Scholar]
- 13.Alagic Z, Goiny M, Canlon B. Protection against acoustic trauma by direct application of D-methionine to the inner ear. Acta Otolaryngol. 2011;131:802–8. doi: 10.3109/00016489.2011.564652. [DOI] [PubMed] [Google Scholar]
- 14.Axelsson A. The cochlear blood vessels in guinea pigs of different ages. Acta Otolaryngol. 1971;72:172–81. doi: 10.3109/00016487109122470. [DOI] [PubMed] [Google Scholar]
- 15.Bittar RS, Oiticica J, Zerati FE, Bento RF. Sudden hearing loss: a ten-year outpatient experience. Int Tinnitus J. 2009;15:196–202. [PubMed] [Google Scholar]
- 16.Morawski K, Telischi FF, Merchant F, Namyslowski G, Lisowska G, Lonsbury-Martin BL. Preventing internal auditory artery vasospasm using topical papaverine: an animal study. Otol Neurotol. 2003;24:918–26. doi: 10.1097/00129492-200311000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winblad B. Piracetam: a review of pharmacological properties and clinical uses. CNS Drug Rev. 2005;11:169–82. doi: 10.1111/j.1527-3458.2005.tb00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Psillas G, Pavlidis P, Karvelis I, Kekes G, Vital V, Constantinidis J. Potential efficacy of early treatment of acute acoustic trauma with steroids and piracetam after gunshot noise. Eur Arch Otorhinolaryngol. 2008;265:1465–9. doi: 10.1007/s00405-008-0689-6. [DOI] [PubMed] [Google Scholar]
- 19.Sendowski I, Abaamrane L, Raffin F, Cros A, Clarencon D. Therapeutic efficacy of intra-cochlear administration of methylprednisolone after acoustic trauma caused by gunshot noise in guinea pigs. Hear Res. 2006;221:119–27. doi: 10.1016/j.heares.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Bonfils P, Avan P. Distortion-product otoacoustic emissions. Values for clinical use. Arch Otolaryngol Head Neck Surg. 1992;118:1069–76. doi: 10.1001/archotol.1992.01880100061014. [DOI] [PubMed] [Google Scholar]


