Since 2012, the confluence of knowledge on treatment and prevention of HIV infection has injected new enthusiasm in responses to the epidemic to shift the discourse to ending AIDS by 2030. While ending AIDS will necessitate the discovery of a vaccine and a cure, changing the response to the epidemic to a point where it no longer poses a public health threat is achievable with currently available interventions. It requires detailed knowledge of the epidemic at a local level in all its diversity; identification of the combination of strategies most suited in each setting; and applying these at a community, group and individual level in ways that enhance equity and justice.
Globally in 2016, there were about 37 million people living with HIV; about one million AIDS-related deaths and 1.8 million new HIV infections1. About 70 per cent of those living with HIV, dying or newly infected with HIV were from sub-Saharan Africa and notably in eastern and southern Africa1. While approximately 20 million HIV-infected persons are now on antiretroviral (ARV) treatment, a similar number of individuals still need to be initiated on treatment to fully realize its individual survival benefits and its prevention benefits at a population level. Preventing HIV infection remains a challenge. The majority of new HIV infections are taking place in Eastern Europe, in key populations in different regions of the world and in adolescent girls and young women in eastern and southern Africa1.
There are an increasing number of countries who are achieving epidemic control, India is one of them. India with a population of nearly one billion is home to about six per cent of the people living with HIV making it the third highest burden country in the world2,3. Approximately 49 per cent of people living with HIV are accessing antiretroviral therapy. A total of 80,000 new HIV infections were observed in 2016, with the key populations including people who inject drugs (9.9%), transgender individuals (7.2%), men who have sex with men (4.3%) and sex-workers (2.2%)3. Concerns about the rapid spread of HIV anticipated in the early 2000s have not materialized due to strategic intervention put into place early in the emerging epidemic.
Overall, the epidemic in India appears to be on a good trajectory to meet the 2020 UNAIDS targets2,3, but there are States, geographical locations and key populations where focused attention is needed. There are multiple modes of transmission of HIV including sexual and through drug use4,5. In the early days, truck drivers and sex workers were initially the main populations infected with HIV, but a rapid spread to their spouses and the general population occurred. Currently, men having sex with men, injecting drug users, migrant workers and their wives represent the majority of people living with HIV in India5. States in the south of India or where there is a substantial amount of migrant labour appear to have higher rates of infection as do States that border countries where drug trafficking is common5. Interventions such as communication, education provision, increasing the availability of condoms, treatment of sexually transmitted infections (STIs), needle exchange programmes and opiate substitution therapy for drug users have been pivotal in containing the spread of HIV and early access to ARV treatment may have also played a role5,6. There is, however, no room for complacency to ensure current trends are not reversed and that India continues its current glide path to meet the UNAIDS 2020 targets.
In contrast, while South Africa had about the same number of infections as India in about 20027 the trajectory in South Africa has been very different. Despite being home to <1 per cent of the global population, South Africa accounts for one in five people living with HIV or who have newly acquired HIV2. A unique feature of the generalized, hyperendemic, predominantly sexually transmitted epidemic in South Africa, and indeed in the southern and eastern African region, is the early age of HIV infection in young women 15-24 yr of age8 who acquire HIV infection 5-7 yr earlier than men and have up to six times more infection compared to their male peers9,10.
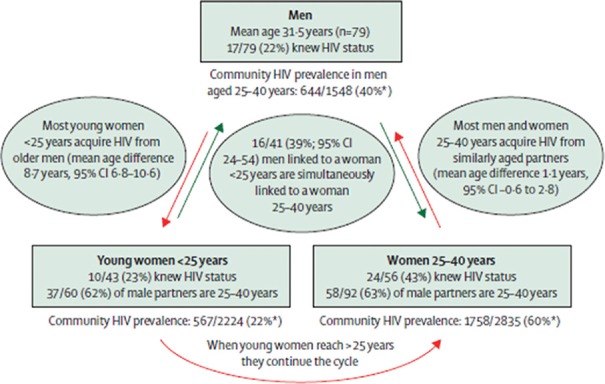
In terms of advancing knowledge of the age-sex difference in HIV infection, a recent study using phylogenetics has provided a more nuanced understanding of cycles of transmission in eastern and southern Africa11. This cross-sectional, population-based survey conducted in one of the highest HIV burden districts in South Africa included sequencing of about 1500 viruses isolated from recently infected men and women has demonstrated that there are three important groups who are critical for the spread of HIV (Figure); these include young women under 25 yr who are primarily acquiring HIV from men who are on average about eight years older than them; these men are primarily acquiring infection from women aged 25-35 yr who are their peers. Significantly about 40 per cent of the men 25-40 yr old are having sex simultaneously with young women <25 yr and women >25 yr11. These data highlight that effective prevention of HIV infection in adolescent girls and young women will require working with all three groups that are part of this cycle of transmission rather than any one group.
Figure.
Depicting the cycle of transmission of HIV in a South African cohort. *Weighted HIV prevalence. Source: Ref 10, reproduced with permission.
In terms of advancing understanding of the high rates of new HIV infection in young women, several studies utilizing specimens from the CAPRISA 004 study12 participants have enhanced our understanding of the role of genital health. The study by Masson et al13 highlights that women with genital inflammation, as measured by a panel of cytokines, are about three times more likely to acquire HIV compared to women without genital inflammation. Importantly, about 20 per cent of the genital inflammation is attributable to traditional STIs while the cause remains unknown for 80 per cent of the cases13. Another study looking at viral isolates sequenced from women with genital inflammation compared to those with no inflammation demonstrate that the presence of genital inflammation enables acquisition of very low virulent strains of HIV14. A third study of the vaginal microbiome has demonstrated a dominance of anaerobic pathogens and notably Prevotella bivia in those with genital inflammation and who subsequently acquire HIV infection15. Recent data from Klatt et al16 demonstrate the decreased potency of topical pre-exposure prophylaxis (PrEP) (tenofovir gel) can be attributed to rapid metabolism of tenofovir by Gardnerella vaginalis one of the organisms that is common in a non-Lactobacillus crispatus dominant genital tract17. Collectively, these studies highlight the importance of good vaginal health in reducing HIV acquisition in young women and the need to integrate HIV prevention efforts with sexual reproductive health services.
A retrospective analysis of data from the highly efficacious partners PrEP study showed no association between bacterial vaginosis and oral PrEP efficacy18. Similarly, a study on the dapiravine intravaginal ring showed no association with vaginal microbiome composition and vaginal dapiravine levels19. There are several new antiretroviral-based products currently in development that are less user dependent and the findings from these studies are eagerly awaited.
Notwithstanding, the vulnerability of adolescent girls and young women, prevention options for those who are unable to negotiate mutually faithful monogamous relations; or consistent use of male or female condoms; medical male circumcision or knowledge of HIV status and if positive linkage to care and be virally suppressed, prevention options are limited. Daily, oral tablets combining tenofovir and emtricitabine (PrEP) is the only available option in terms of women initiated prevention technologies.
Preventing HIV infection in adolescent girls and young women is a complex, multi-faceted problem that includes biological, behavioural, social and structural facets at an individual, dyadic and community/societal level with no quick or easy solutions. The PEPFAR (President's Emergency Plan for AIDS Relief) initiated DREAMS (Determined, Resilient, Empowered, AIDS-free, Mentored, and Safe) project20 targeted at preventing HIV infection in young women is an important initiative that takes these complexities into account and provides a useful opportunity to integrate some of the new knowledge to enhance prevention efforts in eastern and southern Africa. Of note in the cycles of transmission, the study is that only one in five women <25 yr of age and one in five men 25-40 yr were aware of their HIV status, while two out of five women >25 yr of age were aware of their HIV status11. A key starting point to break this cycle of transmission is to enhance knowledge of HIV status and based on the outcome of the test, linkage to antiretroviral treatment and viral suppression if infected or appropriate prevention services including oral PrEP for those who are uninfected especially in adolescent girls and young women <25 yr and men aged between 25 and 40 yr. We need to enhance our understanding of barriers to access to testing, treatment and prevention services in these priority populations. For adolescent girls and younger women, we need to recognise that the root cause of their vulnerability lies in complex gender-power dynamics wherein adolescent girls and young women are most vulnerable but have the least amount of power21,22.
We have achieved much globally and locally in the treatment of AIDS and reducing infant acquisition through science, prevention of mother-to-child transmission (PMTCT) programmes, advocacy, social mobilization, political will and commitment. We need to bring the same energy and commitment to prevention of HIV infection in men, adolescent girls and young women, if not more, as the next phase in our response is going to be even more challenging and we cannot afford to reverse the gains already made to date.
References
- 1.UNAIDS. Fact sheet - Latest statistics on the status of the AIDS epidemic 2017, global statistics. Geneva: UNAIDS; 2017. [Google Scholar]
- 2.UNAIDS. Global AIDS Update 2016. Geneva: UNAIDS; 2016. [Google Scholar]
- 3.UNAIDS. HIV overview India. [accessed on November 15, 2017]. Available from: http://www.unaids.org/en/regionscountries/countries/india .
- 4.Lucas GM, Solomon SS, Srikrishnan AK, Agrawal A, Iqbal S, Laeyendecker O, et al. High HIV burden among people who inject drugs in 15 Indian cities. AIDS. 2015;29:619–28. doi: 10.1097/QAD.0000000000000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paranjape RS, Challacombe SJ. HIV/AIDS in India: An overview of the Indian epidemic. Oral Dis. 2016;22(Suppl 1):10–4. doi: 10.1111/odi.12457. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishnan L, Ramanathan S, Chakrapani V, Goswami P, Deshpande S, Yadav D, et al. Comparison of sexual risk, HIV/STI prevalence and intervention exposure among men who have sex with men and women (MSMW) and men who have sex with men only (MSMO) in India: Implications for HIV prevention. AIDS Behav. 2015;19:2255–69. doi: 10.1007/s10461-015-1058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNAIDS. AIDS Epidemic Update, December 2003. Geneva: UNAIDS; 2003. [Google Scholar]
- 8.UNAIDS. Geneva: UNAIDS; 2016. HIV prevention among adolescent girls and young women: Putting HIV Prevention among adolescent girls and young women on the fast-track and engaging men and boys. [Google Scholar]
- 9.Dellar RC, Dlamini S, Karim QA. Adolescent girls and young women: Key populations for HIV epidemic control. J Int AIDS Soc. 2015;18:19408. doi: 10.7448/IAS.18.2.19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdool Karim Q, Abdool Karim SS, Singh B, Short R, Ngxongo S. Seroprevalence of HIV infection in rural South Africa. AIDS. 1992;6:1535–9. doi: 10.1097/00002030-199212000-00018. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira T, Kharsany AB, Gräf T, Cawood C, Khanyile D, Grobler A, et al. Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: A community-wide phylogenetic study. Lancet HIV. 2017;4:e41–50. doi: 10.1016/S2352-3018(16)30186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masson L, Passmore JA, Liebenberg LJ, Werner L, Baxter C, Arnold KB, et al. Genital inflammation and the risk of HIV acquisition in women. Clin Infect Dis. 2015;61:260–9. doi: 10.1093/cid/civ298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selhorst P, Masson L, Ismail SD, Samsunder N, Garrett N, Mansoor LE, et al. Cervicovaginal inflammation facilitates acquisition of less infectious HIV variants. Clin Infect Dis. 2017;64:79–82. doi: 10.1093/cid/ciw663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passmore JA, Williams B. 21st International AIDS Conference (AIDS 2016) Durban, South Africa. Durban: International AIDS Society; 2016; 2016. Jul 18-22, Role of vaginal microbiota in genital inflammation and enhancing HIV acquisition in women. Abstract No. TUSS0604. [Google Scholar]
- 16.Klatt NR, Cheu R, Birse K, Zevin AS, Perner M, Noël-Romas L, et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science. 2017;356:938–45. doi: 10.1126/science.aai9383. [DOI] [PubMed] [Google Scholar]
- 17.Hillier SL, Meyn LA, Bunge K, Austin M, Moncla BJ, Dezzutti CS, et al. Conference on Retroviruses and Opportunistic Infections (CROI 2017) Seattle, USA. Seattle: CROI; 2017; 2017. Feb 13-16, Impact of vaginal microbiota on genital tissue and plasma concentrations of tenofovir. Abstract no. 86 LB. [Google Scholar]
- 18.Heffron R, McClelland RS, Balkus J, Celum CL, Cohen C, Mugo N, et al. Conference on Retroviruses and Opportunistic Infections (CROI 2017) Seattle, USA. Seattle: CROI; 2017; 2017. Feb 13-16, Daily oral PrEP is effective among women with abnormal vaginal microbiota. Abstract no. 85. [Google Scholar]
- 19.Hillier S, Meyn L, Bunge K, Austin M, Moncla B, Dezzutti C, et al. IAS 2017: Conference on HIV Pathogenesis Treatment and Prevention. Paris, France. Paris: International AIDS Society; 2017; 2017. Jul 23-26, Impact of microbiota on female genital tissue and plasma concentrations of dapivirine. [Google Scholar]
- 20.PEPFAR. DREAMS Innovation Challenge. [accessed on November 5, 2017]. Available from: https://www.pepfar.gov/documents/organization/247602.pdf .
- 21.Teitelman AM, Jemmott JB, Bellamy SL, Icard LD, O’Leary A, Heeren GA, et al. Partner violence, power, and gender differences in South African adolescents’ HIV/sexually transmitted infections risk behaviors. Health Psychol. 2016;35:751–60. doi: 10.1037/hea0000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdool Karim Q, Dellar R. Inclusion of adolescent girls in HIV prevention research -An imperative for an AIDS-free generation. J Int AIDS Soc. 2014;17:19075. doi: 10.7448/IAS.17.1.19075. [DOI] [PMC free article] [PubMed] [Google Scholar]