Abstract
Background & objectives:
Psoriasis is a recurrent hyper-proliferative skin disease which is often associated with free radical generation, abnormal lipid metabolism and increased inflammatory secretion that induce cardiovascular risk in these patients. The present study was intended to evaluate serum lipids, lipoprotein and oxidants-antioxidants status and to establish their relationship with atherogenic risk markers [oxidized low-density lipoprotein (oxLDL) and high-sensitivity C-reactive protein (hsCRP)] in patients with psoriasis.
Methods:
The study was conducted on 150 psoriasis patients and 150 age- and sex-matched healthy controls. Overnight fasting blood samples were obtained for lipids, lipoproteins, lipid oxidation and peroxidation products [oxLDL, malondialdehyde (MDA)], antioxidant enzymes [reduced glutathione (GSH) and total antioxidant status] levels and hsCRP estimations.
Results:
The mean levels of atherogenic lipids [total cholesterol (P<0.001), triacylglycerol (P<0.01)], lipid peroxidation products (P<0.001) and oxLDL and hsCRP (P<0.001) levels in patients with psoriasis were found to be significantly higher than those of healthy controls. On the other hand, ferric-reducing ability of plasma (FRAP, P<0.001) and antioxidant enzyme activities (reduced GSH, P<0.01) were significantly lower when compared to healthy controls. The plasma oxLDL was positively correlated to LDL cholesterol (P<0.001) and MDA (P<0.001) and negatively associated with antioxidant status in these patients. Serum MDA, FRAP and oxLDL were correlated with risk of atherosclerosis in the patients with psoriasis; however, no significant association was found between reduced GSH and hsCRP.
Interpretation & conclusions:
The study results suggest that LDL oxidation and reactive oxygen species in addition to inflammatory markers may play a pivotal role in inducing atherosclerosis in patients of psoriasis.
Keywords: Antioxidants, ferric-reducing ability of plasma, glutathione, high-sensitivity C-reactive protein, malondialdehyde, oxidative stress, psoriasis
Psoriasis is an autoimmune inflammatory disease of the skin. Various exogenous and endogenous factors along with other biochemical and genetic parameters are known to affect the severity of disease, but the exact mechanism of the disease is not known1. T-lymphocytes along with other immune and phagocytic cells contain reduced nicotinamide adenine dinucleotide phosphate (NADPH) which produces reactive oxygen species (ROS) in immunocompromised condition. The sedentary lifestyle, along with emotional and behavioural (alcoholism and smoking habit) factors, can further stimulate these inflammatory responses, thereby further inducing generation of free radicals2,3. The endogenous antioxidant defence system of the body fails to replenish the damage, and the unfavourable skin metabolism further worsens the situation for patients of psoriasis4. Peroxynitrite and hydroxyl radicals produced by lipid peroxidation [malondialdehyde (MDA)] damage cell membranes, lipoproteins and a large number of lipid molecules. Uptake of oxidation product of low-density lipoprotein (oxLDL) by macrophages in the vascular wall can lead to the development of atherosclerosis5.
Psoriasis besides being a skin disorder is also a systemic inflammatory disease leading to atherosclerosis6,7. Different cross-sectional and case-control studies across the country have reported higher prevalence of cardiac risk in patients of psoriasis8,9. Khunger et al9 reported increased risk (22%) of metabolic syndrome (MS) in patients with psoriasis as compared to healthy controls. Since the age of onset of psoriasis can be as early as 15 (early adolescence), it becomes even more important to emphasize on the mechanism of the disease which leads to increased cardiac risk in these patients.
Recent biomedical data have revealed that psoriasis and other inflammatory diseases such as rheumatoid arthritis and systemic lupus erythematosus are often associated with increased incidence of atherosclerosis due to shared pathogenic mechanisms of oxidative stress, dyslipidaemia and inflammation6,10,11. However, contradictory results have also been reported12. Small LDL particles can accumulate in the tunica intima to initiate atherosclerosis. These LDL particles undergo oxidative modification producing oxLDL that may enter macrophages to get transformed into foam cells, leading to the development of atherosclerotic plaques. Products of oxLDL may aggravate vascular wall cells to produce cytokines and inflammatory mediators, thereby promoting low-grade inflammation and progression of atherosclerotic plaques. One such known inflammatory mediator that is atherogenic is high-sensitivity C-reactive protein (hsCRP). The present study was undertaken to measure the circulating oxLDL and hsCRP in relation to oxidants/antioxidants status of psoriasis patients. The effect of severity of disease on relationship between oxidative stress and atherogenic parameters was also evaluated.
Material & Methods
One hundred and fifty consecutive psoriasis patients (>18 yr of age) attending the outpatient department of Dermatology of University College of Medical Sciences (UCMS) and Guru Teg Bahadur (GTB) Hospital, Delhi, India, from 2013 to 2015 who satisfied the inclusion and exclusion criteria and equal number of age and sex matched healthy volunteers (from hospital staff who were free from any systemic disease) were included in the study. Measurable confounders [age, sex and body mass index (BMI)] were adjusted. Participants on medication for any systemic disease and retinoid therapy were excluded from the study. This study was approved by the Institutional Ethical Committee and written informed consent was obtained from all patients and controls.
Anthropometric and clinical data pertaining to disease and family history of cardiovascular disease or/and psoriasis in the first- and second-degree relatives were collected from patients, at the time of recruitment. History of medication and presence of any other comorbidities were investigated, particularly dyslipidaemia, type 2 diabetes and depression. Information concerning lifestyle factors, including physical activity, diet, smoking and alcohol habits of patients was collected.
Weight, height, waist circumference13 and blood pressure were measured during physical examination. BMI was calculated using the standard formula: BMI = weight (kg)/height (m)2.
Psoriasis area severity index (PASI) was used to assess severity of the disease. Patients with PASI score >20 were considered to have severe psoriasis14.
Assay for biochemical parameters: Fasting blood samples (5 ml) were collected from these patients for the laboratory investigations including lipid profiling and blood glucose estimation as described earlier15. According to the National Cholesterol Education Programme Adult Treatment Panel III (NCEP ATP III) Guidelines16, total cholesterol (TC) ≥200 mg/dl and/or LDL-cholesterol (LDL-C) ≥160 mg/dl and/or high-density lipoprotein (HDL)-cholesterol <50 mg/dl for women or <40 mg/dl for men and/or triglycerides (TG) ≥150 mg/dl were defined as dyslipidaemia.
The assessment of metabolic risk was based on the presence of three or more conventional risk factors which take into account central obesity, smoking index, hypertension, dyslipidaemia and diabetes (The American Heart Association Guidelines)17.
OxLDL levels in patients and healthy controls were measured using the method of Ahotupa et al18. MDA was taken as a marker of oxidative stress and its level in serum samples was estimated by Satoh method19. Blood reduced glutathione (GSH) levels were determined by a method developed by Beutler et al20 which was based on the development of a stable yellow colour, when 5-5 dithionine 2-nitrobenzoic acid was added to a sulphydryl compound. Ferric-reducing ability of plasma (FRAP) method was based on the reduction of ferric tripyridyltriazine complex to ferrous tripyridyltriazine [Fe (II)-TPTZ] by a reductant at low pH. The blue-coloured Fe(II)-TPTZ was monitored at 593nm21. Serum hsCRP levels were evaluated using an ELISA Kit (DRG International, Inc., USA).
Statistical analysis: The statistical analysis was done using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). The normality of continuous data was assessed by the Kolmogorov–Smirnov test. Comparison between cases and controls was done using Student's t test. Continuous variables were expressed as mean±standard deviation while categorical variables as percentage. Various patient groups were compared using one-way ANOVA test followed by Tukey's test for post hoc multiple comparisons. Pearson's correlation analysis was used to determine the correlation between hsCRP and oxidative parameters.
Results
A total of 150 psoriasis patients (95 males and 55 females) and equal number of age-, sex- and BMI-matched controls participated in the study. Enrolled participants were between 18 and 65 yr of age (patients; 39.56±11.86 yr vs controls; 37.50±12.55 yr). Most of the patients were from 31 to 40 yr of age group. Early exacerbation (<20 yr of age) was observed in 18 per cent of diseased population. Eighty six per cent (129) patients were suffering with most common variant of psoriasis, psoriasis vulgaris. Next most common variant was guttate psoriasis (n=14, 9.3%) followed by erythrodermic psoriasis (n=4, 2.6%) and pustular psoriasis (n=3, 2%) (Table I). The mean of PASI score was 14.96±9.33 for patients.
Table I.
Frequency distribution of clinical variables of the psoriasis patients (n=150)
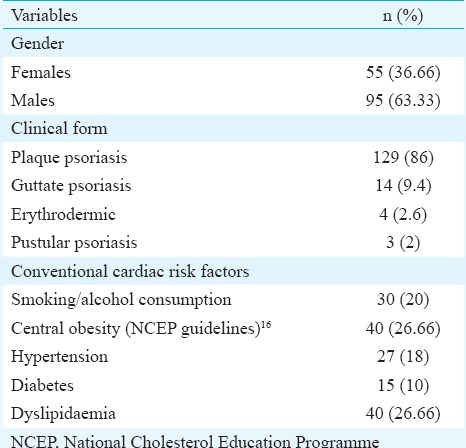
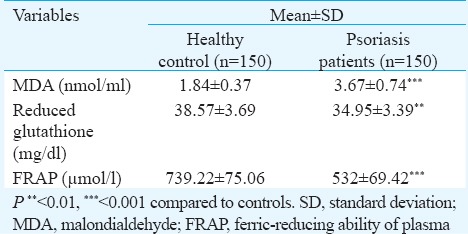
Assessing the anthropometric factors between the two groups, no significant difference was found between BMI of the two groups (patients; 25.35 ± 3.10 vs controls 24.57 ± 3.65 kg/m2). The diastolic blood pressure was significantly higher in psoriasis patients. Twenty per cent of patient population had more than three conventional cardiac risk factors making them more susceptible to MS. Examining the lipid profile of the two groups (Table II), levels of TC (P<0.001) and TG (P<0.01) were significantly higher in patients when compared with healthy controls. Though the difference in serum LDL of the two groups was not significant, plasma oxLDL (P<0.01) levels and oxLDL-to-LDL ratio (P<0.01) were significantly higher in psoriasis patients. Plasma oxLDL was also found to be associated with oxLDL/LDL ratio (r=0.685, P<0.001) and LDL levels (r=0.871, P<0.001) in psoriasis patients. A significant increase was observed in levels of hsCRP (P<0.001) of psoriasis patients when compared with healthy controls. Psoriasis patients had significantly higher serum MDA (P<0.001) levels and decreased reduced GSH (P<0.01) and FRAP (P<0.001) level as compared to healthy controls (Table III).
Table II.
Biochemical parameters of healthy controls and psoriasis patients
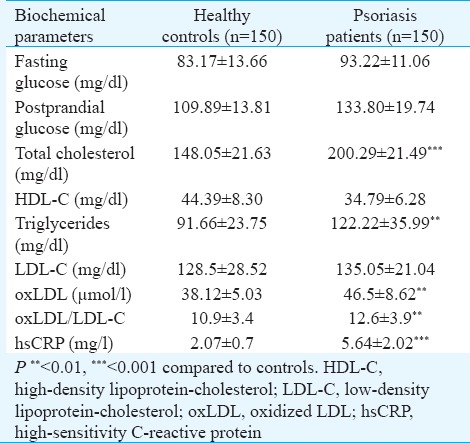
Table III.
Comparison of oxidative stress parameters of patients and controls
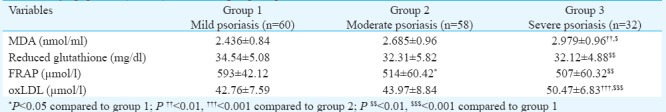
When the psoriasis patients were segregated on the basis of severity index (Table IV) - mild (PASI: 1-10), moderate (PASI: 11-20) and severe (PASI: 21and above) groups, oxLDL levels were significantly increased in severe cases when compared with mild (P<0.001) and moderate (P<0.001) psoriasis patients. There was severity-wise increase in serum MDA levels. FRAP activity was significantly reduced in patients with high severity index when compared with those with low PASI score. However, GSH levels were comparable in patients with moderate-to-severe psoriasis. Serum hsCRP levels were higher in severe cases when compared to patients with mild-to-moderate severity.
Table IV.
Levels of serum malondialdehyde (MDA), reduced glutathione, ferric-reducing ability of plasma (FRAP) and oxidized low-density lipoprotein (oxLDL) in different groups of psoriasis
There was significant (P<0.001) association between oxLDL and serum MDA in different patient groups. A negative correlation of reduced GSH (moderate: P<0.05; severe: P<0.001) and FRAP (P<0.001) with oxLDL was observed among the three different subgroups of psoriasis patients (Table V). Plasma oxLDL was positively correlated to serum hsCRP (r=0.305, P<0.01). An association of serum MDA and plasma FRAP levels was observed with oxLDL and hsCRP (P<0.001). However, reduced GSH was not associated to serum hsCRP (Table VI).
Table V.
Correlation analysis between oxidative stress markers and oxidized low-density lipoprotein in different groups of patients of psoriasis
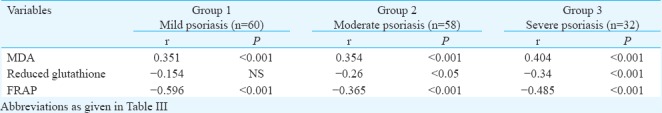
Table VI.
Correlation analysis between oxidative stress markers and high-sensitivity C-reactive protein in different groups of patients of psoriasis
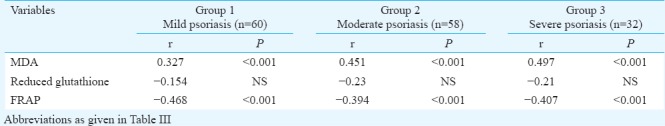
Discussion
Psoriasis is known to be affected by a variety of exogenous and endogenous factors, but the aetiology of the disease is yet not fully understood. Studies have reported that psoriasis closely shares its pathogenesis with other chronic inflammatory diseases and atherosclerosis6,10,11. Thus, it can be postulated that risk of atherosclerosis in psoriasis patients depends on the potential of intrinsic antioxidant system to overcome oxidant stress condition. ROS produced due to inflammatory process leads to the formation of lipid oxidation and peroxidation products such as oxLDL and MDA.
In the present study, dyslipidaemia was observed characterized by increased TC and TG levels in patients of psoriasis22. However, there was no difference in HDL levels of patients when compared with controls. Praveenkumar et al23 reported a significant decrease in HDL levels of psoriasis patients when compared to healthy controls. A previous study from our laboratory has revealed that psoriasis patients with abnormal apolipoprotein profile (decreased levels of apoA-containing lipoproteins and increased levels of apoB-containing lipoproteins)15 are susceptibility to subclinical atherosclerosis.
Our findings were also consistent with others24 as LDL levels in the study groups were not significantly different. The present study showed oxLDL/LDL ratio a better predictor for atherosclerotic risk as compared to LDL. As reported in the previous studies25, a rise in serum MDA levels was observed. However, some studies have shown no significant change in serum MDA levels but have reported increased level of MDA in tissue and RBCs in psoriasis patients26,27.
In our study, a significant rise was observed in plasma oxLDL and a significant decrease in levels of antioxidants activities as measured by reduced GSH and FRAP in different groups of psoriasis patients when compared with healthy controls. These findings were consistent with those of Kadam et al28.
As reported in other studies29,30, increase in LDL oxidation and peroxidation products in psoriasis cases was found to be associated with hsCRP levels. However, Balci et al31 did not find any difference in hsCRP levels of patients with mild-to-moderate psoriasis. Small sample size was a limitation of the present study.
In conclusion, our findings showed that an imbalance between antioxidants/oxidants in psoriasis might be associated with inflammatory responses in psoriasis, thereby increasing risk of premature atherosclerosis in these patients. Thus, the dietary plan, lifestyle change, and drugs used to treat psoriasis should induce antioxidant response against endogenous and exogenous oxidative stress. The monitoring of oxidative injury in these patients may help develop new effective treatment targeting both psoriasis and atherosclerosis.
Acknowledgment
The study was supported by funding received from University Grants Commission, New Delhi, India and senior research fellowship (SRF) to the first author (KA) from Indian Council of Medical Research, New Delhi, India.
Footnotes
Conflicts of Interest: None.
References
- 1.Gudjonsson JE, Elder JT. Psoriasis. In: Wolff K, Goldsmith L, Katz S, Gilchrest B, Paller A, Lefell D, editors. Fitzpatrick's dermatology in general medicine. 7th ed. Vol. 1. New York: McGraw-Hill; 2008. pp. 169–93. [Google Scholar]
- 2.Fuchs J, Zollner TM, Kaufmann R, Podda M. Redox-modulated pathways in inflammatory skin diseases. Free Radic Biol Med. 2001;30:337–53. doi: 10.1016/s0891-5849(00)00482-2. [DOI] [PubMed] [Google Scholar]
- 3.Trouba KJ, Hamadeh HK, Amin RP, Germolec DR. Oxidative stress and its role in skin disease. Antioxid Redox Signal. 2002;4:665–73. doi: 10.1089/15230860260220175. [DOI] [PubMed] [Google Scholar]
- 4.Baz K, Cimen MY, Kokturk A, Yazici AC, Eskandari G, Ikizoglu G, et al. Oxidant/antioxidant status in patients with psoriasis. Yonsei Med J. 2003;44:987–90. doi: 10.3349/ymj.2003.44.6.987. [DOI] [PubMed] [Google Scholar]
- 5.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–61. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flammer AJ, Ruschitzka F. Psoriasis and atherosclerosis: Two plaques, one syndrome? Eur Heart J. 2012;33:1989–91. doi: 10.1093/eurheartj/ehr425. [DOI] [PubMed] [Google Scholar]
- 7.Dogra S, Mahajan R. Psoriasis: Epidemiology, clinical features, co-morbidities, and clinical scoring. Indian Dermatol Online J. 2016;7:471–80. doi: 10.4103/2229-5178.193906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma A, Gopalakrishnan D, Kumar R, Vijayvergiya R, Dogra S. Metabolic syndrome in psoriatic arthritis patients: A cross-sectional study. Int J Rheum Dis. 2013;16:667–73. doi: 10.1111/1756-185X.12134. [DOI] [PubMed] [Google Scholar]
- 9.Khunger N, Gupta D, Ramesh V. Is psoriasis a new cutaneous marker for metabolic syndrome? A study in Indian patients. Indian J Dermatol. 2013;58:313–4. doi: 10.4103/0019-5154.113958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: Mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J. 2015;36:482–9c. doi: 10.1093/eurheartj/ehu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE -mechanisms and management. Nat Rev Rheumatol. 2012;8:214–23. doi: 10.1038/nrrheum.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowlatshahi EA, Kavousi M, Nijsten T, Ikram MA, Hofman A, Franco OH, et al. Psoriasis is not associated with atherosclerosis and incident cardiovascular events: The Rotterdam study. J Invest Dermatol. 2013;133:2347–54. doi: 10.1038/jid.2013.131. [DOI] [PubMed] [Google Scholar]
- 13.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome - A new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 14.Ali AR, Abdulhadi J, Sahal S, Nesreen M, Taha H, Majed L, et al. Psoriasis: Correlation between severity index (PASI) and quality of life index (DLQI) based on the type of treatment. J Dermatol Dermatol Surg. 2016;20:15–8. [Google Scholar]
- 15.Asha K, Sharma SB, Singal A, Aggarwal A. Association of carotid intima-media thickness with leptin and apoliprotein b/apoliprotein a-I ratio reveals imminent predictors of subclinical atherosclerosis in psoriasis patients. Acta Medica (Hradec Kralove) 2014;57:21–7. doi: 10.14712/18059694.2014.4. [DOI] [PubMed] [Google Scholar]
- 16.Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. for American Heart Association & National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 18.Ahotupa M, Ruutu M, Mäntylä E. Simple methods of quantifying oxidation products and antioxidant potential of low density lipoproteins. Clin Biochem. 1996;29:139–44. doi: 10.1016/0009-9120(95)02043-8. [DOI] [PubMed] [Google Scholar]
- 19.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 20.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 21.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 22.Rocha-Pereira P, Santos-Silva A, Rebelo I, Figueiredo A, Quintanilha A, Teixeira F, et al. The inflammatory response in mild and in severe psoriasis. Br J Dermatol. 2004;150:917–28. doi: 10.1111/j.1365-2133.2004.05984.x. [DOI] [PubMed] [Google Scholar]
- 23.Praveenkumar U, Ganguly S, Ray L, Nanda SK, Kuruvila S. Prevalence of metabolic syndrome in psoriasis patients and its relation to disease duration: A hospital based case-control study. J Clin Diagn Res. 2016;10:WC01–5. doi: 10.7860/JCDR/2016/17791.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uyanik BS, Ari Z, Onur E, Gündüz K, Tanülkü S, Durkan K, et al. Serum lipids and apolipoproteins in patients with psoriasis. Clin Chem Lab Med. 2002;40:65–8. doi: 10.1515/CCLM.2002.013. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh A, Mukhopadhyay S, Kar M. Role of free reactive iron in psoriasis. Indian J Dermatol Venereol Leprol. 2008;74:277–8. doi: 10.4103/0378-6323.41390. [DOI] [PubMed] [Google Scholar]
- 26.Yildirim M, Inaloz HS, Baysal V, Delibas N. The role of oxidants and antioxidants in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:34–6. doi: 10.1046/j.1468-3083.2003.00641.x. [DOI] [PubMed] [Google Scholar]
- 27.Kökçam I, Naziroǧlu M. Antioxidants and lipid peroxidation status in the blood of patients with psoriasis. Clin Chim Acta. 1999;289:23–31. doi: 10.1016/s0009-8981(99)00150-3. [DOI] [PubMed] [Google Scholar]
- 28.Kadam DP, Suryakar AN, Ankush RD, Kadam CY, Deshpande KH. Role of oxidative stress in various stages of psoriasis. Indian J Clin Biochem. 2010;25:388–92. doi: 10.1007/s12291-010-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. Circulating levels of adiponectin, oxidized LDL and C-reactive protein in Portuguese patients with psoriasis vulgaris, according to body mass index, severity and duration of the disease. J Dermatol Sci. 2009;55:202–4. doi: 10.1016/j.jdermsci.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Sunitha S, Rajappa M, Thappa DM, Chandrashekar L, Munisamy M, Revathy G, et al. Comprehensive lipid tetrad index, atherogenic index and lipid peroxidation: Surrogate markers for increased cardiovascular risk in psoriasis. Indian J Dermatol Venereol Leprol. 2015;81:464–71. doi: 10.4103/0378-6323.163734. [DOI] [PubMed] [Google Scholar]
- 31.Balci DD, Yonden Z, Dogramaci CA, Duran N. Serum high sensitivity C reactive protein and homocysteine levels in patients with mild to moderate psoriasis. Turkderm Deri Hast Frengi Ars. 2009;43:53–7. [Google Scholar]


