Sir,
Noroviruses (NoVs) are the leading cause of acute gastroenteritis outbreaks across all age groups and the second most common cause of sporadic childhood gastroenteritis worldwide. In countries implementing routine rotavirus immunization, NoVs are now identified as the primary cause of viral gastroenteritis among children1. With rotavirus vaccine introduction in the National Immunization Programme of India2, NoV surveillance and research acquire importance.
NoVs are classified into seven genogroups, GI-GVII, of which GI, GII and GIV infect humans1,3. GII NoVs, most common in human infections, are classified into 23 genotypes4,5. The genotype GII.4 is predominant globally1 and exhibits further diversity in the form of 14 GII.4 variants4,5.
NoVs have a linear positive-sense single-stranded RNA genome consisting of three open-reading frames (ORFs), of which ORF1 codes for non-structural proteins, while ORF2 and 3 encode the capsid proteins. The major capsid protein, VP1, carries the viral antigenic determinants and is involved in interaction with host antibodies and cellular-binding ligands - the histoblood group antigens (HBGAs)6. On in vitro expression using baculovirus system, the recombinant VP1 molecules undergo self-assembly to form virus-like particles (VLPs)7. These VLPs are morphologically and antigenically similar to native virions and exhibit HBGA binding. Laboratory diagnosis of NoV infections currently relies on reverse transcription-polymerase chain reaction (RT-PCR)-based detection of NoV RNA in faecal specimens3. Although detection of NoV antigen employing anti-NoV VLP polyclonal antibodies and/or monoclonal antibodies (MAbs) has been reported8,9, development of sensitive, broadly reactive assays for diagnostic use remains a challenge due to the NoV genetic and antigenic diversity.
In the present study, rabbit polyclonal antibodies were generated against VLPs representing a NoV GII.4 DenHaag_2006b strain from Pune, India, and the utility of these antibodies in NoV antigen capture immunoassays was assessed. This study was conducted during the period of April 2013 to September 2015 at the Enteric Viruses Group, ICMR-National Institute of Virology, Pune, India. The study was approved by the institutional animal ethics committee.
The GII.4-DenHaag_2006b VLPs produced in Sf9 cells using baculovirus expression system were purified by sucrose gradient centrifugation, analyzed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and characterized by immunoblotting and electron microscopy, as reported elsewhere10. One New Zealand white rabbit was immunized with five doses of adjuvanted GII.4 VLPs by the intramuscular route. The post-immunization serum samples, Post 1 to Post 5, obtained at two weeks after the respective dose were subjected to detection and titration of anti-NoV IgG using VLP-based immunoassays as described earlier10. Briefly, VLP-coated micro-wells were incubated with the serum specimens (dilution range: 1:100 to 1:102,400) and the bound IgG was probed by horse-radish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Sigma, USA). Pre-immunization rabbit serum served as negative control. Test serum with absorbance greater than the cut-off value, i.e. mean of three negative controls + three times standard deviation, was considered to be positive.
The post-immunization rabbit serum samples were also tested for their ability to block VLP-HBGA binding using the VLP-pig gastric mucin (PGM) binding blockade assay11. Briefly, VLPs (1 μg/ml) pre-treated with serum specimen (dilution range: 1:100 to 1:102,400) were added onto micro-wells coated with PGM Type III (Sigma, USA) dissolved in phosphate-buffered saline (PBS pH: 7.4). The bound VLPs were detected using commercially available anti-NoV GII.4 MAb (Abcam, UK) and rabbit anti-mouse IgG-HRP conjugate (Abcam). The 50 per cent blocking titre (BT50) determined for each specimen was defined as the reciprocal of the highest dilution with ≥50 per cent blocking (reduction in absorbance) as compared to the VLP control (VLPs untreated with serum).
After first VLP dose, the rabbit serum (Post 1) showed an anti-NoV IgG titre of 400 and a BT50 value of 100. Both antibody titres increased significantly in the Post 2 serum (IgG titre: 12,800, BT50: 400) and also in the Post 3, 4 and 5 serum samples (IgG titre: ≥102,400, BT50: 1600-3200) (Figure). Thus, high-titre anti-VLP antibodies capable of blocking VLP-HBGA interaction indicated that the VLPs were highly immunogenic.
Figure.
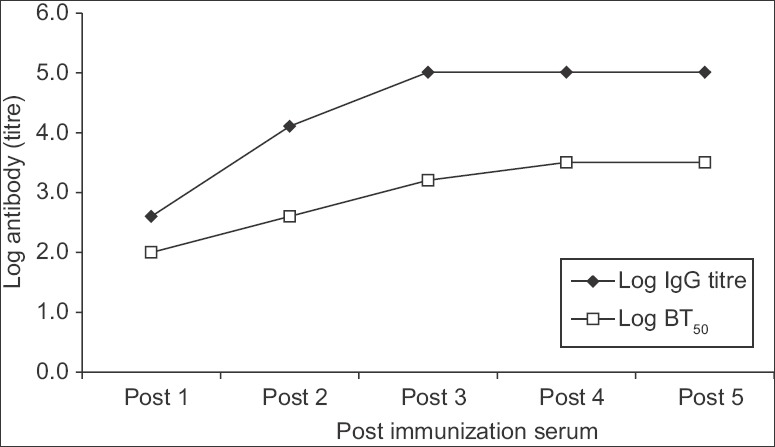
Anti-Norovirus serum antibody titres in rabbit immunized with Norovirus GII.4 virus-like particles. Log-transformed IgG titre and 50 per cent blocking titre (BT50) in the post-immunization rabbit serum samples, Post 1 to Post 5, obtained at two-week interval after the respective dose, are shown. Each serum dilution was tested in duplicate experiments. The IgG titre was defined as the reciprocal of the highest serum dilution indicating positive reaction while 50 per cent blocking titre was defined as the reciprocal of the highest dilution with ≥50 per cent blocking.
To use the anti-VLP rabbit polyclonal IgG in NoV antigen capture ELISA, IgG was purified from the hyperimmune serum by recombinant protein G agarose-based affinity column chromatography (Invitrogen, USA) and rabbit anti-NoV IgG-HRP conjugate was prepared using HRP Labelling Kit (Merck GeNei, India). The purified anti-NoV rabbit IgG was used as capture antibody in ELISA and its performance was compared to the commercial anti-GII.4 MAb (Abcam, UK). Micro-wells (Nunc-Immuno MaxiSorp) coated with 1 μg of rabbit IgG or MAb in 0.05 M carbonate-bicarbonate buffer (pH: 9.5) overnight at room temperature were blocked with five per cent skimmed milk in PBS (pH: 7.4) for 30 min at room temperature. After washing with PBS-0.2 per cent Tween-20, recombinant VLPs (100 ng/well) or 30 per cent suspensions of known NoV RT-PCR-positive and RT-PCR-negative faecal specimens12 were incubated (37°C, 1 h) in the wells. The bound NoV antigen was detected by incubation (37°C, 1 h) with the indigenously prepared rabbit anti-NoV IgG-HRP conjugate followed by addition of substrate (tetramethylbenzidine/H2O2, Merck GeNei). Absorbance was read at 450 nm. Faecal specimens with absorbance greater than the cut-off value, i.e. mean of three negative controls + three times standard deviation, were considered to be positive.
Both anti-VLP rabbit polyclonal IgG and the commercial MAb were found to detect the indigenously developed GII.4 DenHaag_2006b VLPs in the capture ELISA with lower absorbance values in MAb-coated wells. On assessment of NoV GII.4-DenHaag_2006b positive faecal specimens (n=8) collected from children with acute gastroenteritis, all specimens tested positive in rabbit IgG-coated wells. However, none were positive in MAb-coated wells. The inability of the commercial MAb to detect native NoV in faecal specimens may be due to generation of the MAb against a peptide immunogen representing a linear viral epitope. Alternatively, antigen capturing potential of the MAb may have been limited due to its specificity for a single viral epitope as against multiple epitopes capturing capacity of rabbit polyclonal anti-VLP IgG. Thus, only the indigenous anti-VLP rabbit IgG was found to be suitable for use as capture antibody in NoV antigen detection from faecal specimens by ELISA. The performance of this rabbit IgG as an HRP-conjugated detector antibody could not be compared with that of the MAb due to unavailability of sufficient quantity of MAb for HRP labelling.
The rabbit polyclonal IgG-based capture ELISA was then evaluated for its ability to detect other NoV GII.4 variants (Hunter_2004, Yerseke_2006a, Osaka_2007, Apeldoorn_2007, New Orleans_2009 and Sydney_2012) and GII genotypes (GII.2, GII.3, GII.6, GII.7, GII.9, GII.13, GII.14, GII.21, GII.P21/GII.3) in faecal specimens (n=70). None of these GII.4 variants or GII genotypes could be detected in the ELISA. However, limited reactivity of Apeldoorn_2007 variant was noted with one of the two tested specimens indicating absorbance equal to the set cut-off value.
The rabbit anti-NoV VLP polyclonal IgG-based ELISA, described in the present study, was found to be highly specific for NoV GII.4 DenHaag_2006b variant and was thus not useful for routine diagnostic purposes in its current format. To detect other GII.4 variants and NoV genotypes, further improvement involving incorporation of a cocktail of anti-NoV MAbs of different specificities or a broadly reactive anti-NoV MAb as capture antibody(s) in the ELISA is required. Evaluation of such ELISA by testing of a large number of NoV RT-PCR-positive faecal specimens comprising different genotypes would be useful for this purpose. Generation of cross-reactive anti-NoV VLP MAbs13,14 and their utility in diagnostic NoV antigen capture ELISAs have been described9. Development of indigenous MAb-based ELISA(s) needs to be pursued further using the anti-GII.4 rabbit IgG generated as a detector antibody in the present study. This anti-VLP polyclonal IgG persisting (≥15 months) in high titres in immunized rabbits would serve as an economical reagent for use in NoV diagnostics.
Acknowledgment
This study was supported by a financial grant from ICMR-National Institute of Virology, Pune, India.
Footnotes
Conflicts of Interest: None.
References
- 1.Ramani S, Atmar RL, Estes MK. Epidemiology of human noroviruses and updates on vaccine development. Curr Opin Gastroenterol. 2014;30:25–33. doi: 10.1097/MOG.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora R, Swaminathan S. Ready to measure impact? The introduction of rotavirus vaccine in India. Indian Pediatr. 2016;53:565–7. doi: 10.1007/s13312-016-0889-x. [DOI] [PubMed] [Google Scholar]
- 3.Vinjé J. Advances in laboratory methods for detection and typing of norovirus. J Clin Microbiol. 2015;53:373–81. doi: 10.1128/JCM.01535-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroneman A, Vennema H, Deforche K, v d Avoort H, Peñaranda S, Oberste MS, et al. An automated genotyping tool for enteroviruses and noroviruses. J Clin Virol. 2011;51:121–5. doi: 10.1016/j.jcv.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Kroneman A, Vega E, Vennema H, Vinjé J, White PA, Hansman G, et al. Proposal for a unified norovirus nomenclature and genotyping. Arch Virol. 2013;158:2059–68. doi: 10.1007/s00705-013-1708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green KY. Caliciviridae: The noroviruses. In: Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, et al., editors. Fields virology. 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 582–608. [Google Scholar]
- 7.Jiang X, Wang M, Graham DY, Estes MK. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992;66:6527–32. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang X, Wilton N, Zhong WM, Farkas T, Huang PW, Barrett E, et al. Diagnosis of human caliciviruses by use of enzyme immunoassays. J Infect Dis. 2000;181(Suppl 2):S349–59. doi: 10.1086/315577. [DOI] [PubMed] [Google Scholar]
- 9.Kou B, Crawford SE, Ajami NJ, Czakó R, Neill FH, Tanaka TN, et al. Characterization of cross-reactive norovirus-specific monoclonal antibodies. Clin Vaccine Immunol. 2015;22:160–7. doi: 10.1128/CVI.00519-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kulkarni R, Lole K, Chitambar SD. Seroprevalence of antibodies against GII.4 norovirus among children in Pune, India. J Med Virol. 2016;88:1636–40. doi: 10.1002/jmv.24495. [DOI] [PubMed] [Google Scholar]
- 11.Lindesmith LC, Debbink K, Swanstrom J, Vinjé J, Costantini V, Baric RS, et al. Monoclonal antibody-based antigenic mapping of norovirus GII.4-2002. J Virol. 2012;86:873–83. doi: 10.1128/JVI.06200-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkarni R, Patel A, Bhalla S, Chhabra P, Cherian S, Chitambar SD, et al. Characterization of GII.4 noroviruses circulating among children with acute gastroenteritis in Pune, India: 2005-2013. Infect Genet Evol. 2016;37:163–73. doi: 10.1016/j.meegid.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Kitamoto N, Tanaka T, Natori K, Takeda N, Nakata S, Jiang X, et al. Cross-reactivity among several recombinant calicivirus virus-like particles (VLPs) with monoclonal antibodies obtained from mice immunized orally with one type of VLP. J Clin Microbiol. 2002;40:2459–65. doi: 10.1128/JCM.40.7.2459-2465.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindesmith LC, Beltramello M, Donaldson EF, Corti D, Swanstrom J, Debbink K, et al. Immunogenetic mechanisms driving norovirus GII.4 antigenic variation. PLoS Pathog. 2012;8:e1002705. doi: 10.1371/journal.ppat.1002705. [DOI] [PMC free article] [PubMed] [Google Scholar]


