Abstract
Background.
Transient ischaemic attacks (TIA) and minor strokes (TIAMS) have the same pathophysiological mechanism as stroke and carry a high risk of recurrent ischaemic events. Diagnosis of TIAMS can be challenging and often occurs in general practice. Absolute cardiovascular risk (ACVR) is recommended as the basis for vascular risk management. Consideration of cardiovascular risk in TIAMS diagnosis has been recommended but its utility is not established.
Objectives.
Firstly, to document the ACVR of patients with incident TIAMS and with TIAMS-mimics. Secondly, to evaluate the utility of ACVR calculation in informing the initial diagnosis of TIAMS.
Methods.
The International comparison of Systems of care and patient outcomes in minor Stroke and TIA (InSiST) study is an inception cohort study of patients of 17 Australian general practices presenting as possible TIAMS. An expert panel determines whether participants have had TIAMS or TIAMS-mimics. ACVR was calculated at baseline for each participating patient. In this cross-sectional baseline analysis, ACVR of TIAMS and TIAMS-mimics were compared univariately and, also, when adjusted for age and sex. The diagnostic utility of ACVR was evaluated via receiver operating characteristic (ROC) curves.
Results.
Of 179 participants, 87 were adjudicated as TIAMS. The presence of motor and speech symptoms and body mass index were associated with a diagnosis of TIAMS. ACVR was associated with TIAMS diagnosis on univariate analysis, but not when age- and sex-adjusted. ACVR did not significantly improve area under ROC curves beyond that of age and sex.
Conclusion.
In patients presenting with transient or minor neurological symptoms, calculation of ACVR did not improve diagnostic accuracy for TIAMS beyond that of age and sex.
Keywords: Cardiovascular diseases, diagnosis, family practice, risk assessment, stroke, transient ischaemic attack.
Introduction
Transient ischaemic attack (TIA) and ischaemic stroke constitute a continuum of acute cerebrovascular syndrome (1). TIA is a potent risk factor for stroke. Of patients with ischaemic stroke, 23% have had a prior TIA, usually within hours to days of the stroke (2). TIAs and minor strokes (TIAMS) can be considered a single entity, having a similar prognosis in terms of recurrent stroke (3) and are managed similarly. The early identification and treatment of TIAMS results in a substantial reduction in risk of recurrent stroke, mortality and disability (4).
Diagnosis of a TIA is based on clinical history and is often challenging as symptoms and signs have usually disappeared by the time of assessment. There is also considerable symptom overlap with TIA-mimics: migraine, partial seizure, vestibular disorders, intracranial lesions, metabolic causes, transient global amnesia, syncope or psychogenic illness are often initially misdiagnosed as a TIA (5). Studies involving written case histories suggest GPs have variable diagnostic ability concerning TIA (6). Approximately 50% of referrals to TIA clinics are eventually diagnosed as having a TIA (7,8). Given the urgency of TIA management, improved diagnostic precision would be of value in general practice where TIA patients usually first present [and in Emergency Departments (EDs), the next most common site of presentation].
To distinguish TIAs from mimics, multiple diagnostic support tools have been evaluated in secondary care settings (9,10), but only a few have been evaluated in general practice (11). TIA diagnostic tools appear to perform less well in general practice than in secondary care (11).
As well as the history of clinical symptoms, GPs are advised to elicit risk factors for vascular disease as an element in TIA diagnosis (12,13). Risk factors included in these recommendations include hypertension, diabetes mellitus, previous cardiac or vascular disease, dyslipidaemia, smoking and obesity (12,13). These risk factors are similar to those incorporated in the Framingham risk score (14) and its derivative, the absolute cardiovascular risk (ACVR) score (15). The Stroke Foundation of Australia currently recommends primary vascular disease prevention based on ACVR calculation (15). Some risk factors are more strongly associated with certain types of cardiovascular disease, e.g. blood pressure (BP) is more strongly associated with stroke (14), and a specific Framingham stroke risk can be calculated (16). But specific stroke risk is as well predicted in primary care by the Framingham general cardiovascular risk as by the Framingham stroke-specific profile (17).
ACVR score is currently part of preventative health guidelines for Australian GPs and has shown promise in improvement of cardiovascular risk prevention. Little is known about its importance in diagnosis, though calculation of ACVR has been advocated as part of GPs’ diagnostic differentiation of TIAs and TIA-mimics (5).
We aimed to establish the vascular risk profile of patients with incident possible TIAMS and to compare the ACVR scores of TIAMS and TIAMS-mimics. We also aimed to determine the potential for ACVR score calculation to improve TIAMS diagnostic performance.
Methods
The International comparison of Systems of care and patient outcomes in minor Stroke and TIA (InSiST) study is an inception cohort study in which patients with possible TIAMS are followed for 12 months post-event. Participants are recruited from within the patient-base of 17 general practices in the Hunter and Manning Valley regions of New South Wales, Australia. All practices are within the referral footprint of a single hospital-based secondary care Acute Neurovascular Clinic which provides a service for urgent diagnosis and management of TIAMS.
In InSiST, a clinical definition of TIA is used: a rapidly developed clinical symptom of focal disturbance of cerebral function lasting <24 hours with no apparent non-vascular cause. Minor strokes are defined as lasting >24 hours and with a National Institutes of Health Stroke Scale score of ≤4. Multiple overlapping means of ascertainment are used. These are within participating general practices, EDs, the regional Acute Neurovascular Clinic, GP after-hours services, Stroke Units and hospital separations.
This is a cross-sectional analysis of baseline data from the first 13 months of the InSiST study (August 2012 to August 2013).
Participants completed an extensive baseline interview, either by phone or face-to-face. Interviews were conducted by an experienced stroke nurse or a recent medical graduate provided with specific training for the task. The interviewers elicited a detailed patient narrative of the index event and its context as well as a structured interview eliciting any salient features of the index event (positive or negative) not captured by the narrative. Further data was obtained from general practice and hospital clinical records. Index events were classified as TIAMS or TIAMS-mimic by an adjudication panel of three clinician neurologists and GPs (including at least one stroke physician and one GP). As well as the study interview findings, the data available to the panel included general practice, ED and Acute Neurovascular Clinic clinical records, including imaging reports, and direct viewing of imaging studies ordered during ED or Acute Neurovascular Clinic consultations. The final adjudication for each event was the consensus of the panel, arrived at by consideration and discussion of all available information.
A baseline ACVR score was calculated for each participant, utilizing the Australian ACVR charts (15). In these charts, patient age, sex, smoking status, diabetes and ratio of total cholesterol to high density lipoprotein cholesterol are used to calculate ACVR. A Framingham-based general ACVR rather than stroke-specific risk was employed as the Framingham stroke-specific risk incorporates left ventricular hypertrophy (16) (for which we did not have complete data) and does not perform better in stroke prediction than the general ACVR (17). Furthermore, Australian GPs are familiar with the general ACVR tool and, thus, its demonstrated validity in TIAMS diagnosis would be of immediate clinical utility.
Participants were categorized as ‘low’ (<10% risk of cardiovascular event in next 5 years), ‘moderate’ (10–15% risk), ‘high’ (>15% risk), or ‘automatically-high’ (>15% risk) risk. The ‘automatically-high’ risk category is defined by current or previous cardiovascular disease, stroke or TIA, or carotid endarterectomy/stent. The ‘automatically-high’ risk category also includes those with diabetes and age >60, moderate/severe chronic kidney disease (estimated glomerular filtration rate <45 and/or persistent proteinuria), serum total cholesterol >7.5, systolic BP ≥180, diastolic BP ≥110 and Aboriginal or Torres Strait Islander adults aged >74, as per the National Vascular Disease Prevention Alliance (NVDPA) Guidelines (15). The NVDPA indicators of ‘automatically-high’ ACVR not included in our formulation were familial hypercholesterolaemia and diabetic microalbuminuria, as this data was not collected.
Statistical analysis
Categorical data are presented as number and percent of individuals with the outcome of interest. Continuous data are presented as means and SDs. All P values for TIAMS/non-TIAMS comparisons were calculated using logistic regression. Adjusted P values were adjusted for age and sex. Factors were then placed into receiver operating characteristic (ROC) curves and the area under the ROC (AUROC) curve was compared between factors of interest to assess improvements in diagnostic accuracy. All analyses were programmed in Stata v13 or SAS v 9.3.
Sample size calculation: for our primary outcome of comparison of ACVR in TIAMS and mimics, with an alpha of 0.05, power of 0.80, ratio of TIAMS to mimics of 1 (7,8,18), high or automatically high ACVR in mimics of 51% (19) and clinically significant difference of 0.2, we needed a sample size of 184.
Results
A total of 179 participants presenting as possible TIAMS (of 365 ascertained cases: response rate 49%) were recruited into the cohort under analysis (87 TIAMS and 92 TIAMS-mimics). Of the 179 participants, 72 (40%) presented to an ED, 68 (38%) were managed exclusively in general practice, 53 (30%) were referred to the regional Acute Neurovascular Clinic and 7 (4%) to a non-Acute Neurovascular Clinic specialist.
Migraine was the most common TIAMS-mimic (see Table 1). Of migrainous neurological events, 74% were in female participants and the mean age was 55 (SD = 13.6) years, compared with 69% female and 70 (SD = 11.8) years for other TIAMS-mimics.
Table 1.
TIAMS-mimic types by number and percentage
| Mimic type | N (%) |
|---|---|
| Migraine | 27 (29.4) |
| Vestibular dysfunction | 16 (17.4) |
| Seizure | 7 (7.6) |
| Syncope/presyncope | 7 (7.6) |
| Transient global amnesia | 4 (4.4) |
| Visual disturbances | 2 (2.2) |
| Isolated cranial nerve lesions | 2 (2.2) |
| Non-organic causes | 4 (4.4) |
| Other | 5 (5.4) |
| Uncertain | 18 (19.6) |
Categorized by ACVR, 96 of the 179 participants (53.6%) were high or automatically high risk, 25 (14.0%) were moderate risk, and 47 (26.3%) were low risk. ACVR was unable to be calculated for 11 participants (6.1%) with missing data.
Comparisons of TIAMS/non-TIAMS risk factors and clinical presentation variables are presented in Tables 2 and 3. On univariate analysis age, male sex, higher systolic BP, higher vascular risk, history of hypertension, atrial fibrillation, history of vascular disease and smoking history were significantly associated with TIAMS diagnosis. History of hypertension, atrial fibrillation and history of cardiovascular disease remained significantly associated with TIAMS diagnosis when adjusted for age and sex. Body mass index (BMI) became a significant association when adjusted for age and sex. Presentation to an ED rather than general practice, shorter delay in presentation, speech symptoms and motor symptoms were also associated with TIAMS diagnosis when adjusted for age and sex.
Table 2.
Demographic and other baseline characteristics of patients by outcome: risk factors
| Variable | Category | Diagnosis | P | Adj Pa | |
|---|---|---|---|---|---|
| TIAMS-mimic (n = 92) | TIAMS (n = 87) | ||||
| Age | Mean (SD) | 66 (14) | 73 (11) | 0.0009 | 0.0017 |
| Systolic BP | Mean (SD) | 142 (20) | 149 (24) | 0.0472 | 0.1025 |
| Cholesterol | Mean (SD) | 4.89 (1.33) | 4.60 (1.24) | 0.1479 | 0.5344 |
| Weekly alcohol consumption SDEb | Mean (SD) | 5.0 (7.7) | 7.5 (12.9) | 0.1265 | 0.2498 |
| Exercise (hours/week) | Mean (SD) | 5.4 (5.9) | 5.0 (5.4) | 0.6719 | 0.5045 |
| Sex | Male | 27 (39%) | 42 (61%) | 0.0099 | 0.0225 |
| Vascular risk | High | 38 (40%) | 58 (60%) | 0.0057 | 0.4010 |
| Mod | 14 (56%) | 11 (44%) | |||
| Low | 32(68%) | 15 (32%) | |||
| Hypertension | Yes | 53 (43%) | 70 (57%) | 0.0012 | 0.0076 |
| Hyperlipidaemia | Yes | 42 (47%) | 48 (53%) | 0.2036 | 0.3099 |
| Atrial fibrillation | Yes | 12 (32%) | 25 (68%) | 0.0123 | 0.1130 |
| History of cardiovascular disease | Yes | 25 (36%) | 44 (64%) | 0.0015 | 0.1391 |
| Diabetes | Yes | 16 (50%) | 16 (50%) | 0.8615 | 0.6214 |
| Ever smoked | Yes | 37 (47%) | 42 (53%) | 0.2440 | 0.2157 |
| Smoking at time of event | Yes | 6 (55%) | 5 (45%) | 0.5820 | 0.7697 |
| BMI category | 17 to ≤25 | 34 (47%) | 39 (53%) | 0.1033 | 0.0047 |
| 25 to ≤30 | 32 (56%) | 25 (44%) | |||
| 30 to ≤35 | 19 (68%) | 9 (32%) | |||
| >35 | 7 (35%) | 13 (65%) | |||
aThe P value is adjusted for age and sex.
bSDE is standard drink equivalents.
Table 3.
Characteristics of the patient’s presentation by outcome: clinical presentation variables
| Variable | Category | Diagnosis | P | Adj Pa | |
|---|---|---|---|---|---|
| TIAMS-mimic (n = 92) | TIAMS (n = 87) | ||||
| Hours from symptom onset to first doctor | Mean (SD) | 362 (1103) | 99 (217) | 0.0351 | 0.0469 |
| Hours from symptom onset to Acute Neurovascular Clinic (acute referral) | Mean (SD) | 604 (1395) | 205 (157) | 0.4683 | 0.3575 |
| Presentation pattern | ED | 21 (36%) | 38 (64%) | 0.0128 | 0.0033 |
| GP | 62 (58%) | 44 (42%) | |||
| GP -> ED | 9 (64%) | 5 (36%) | |||
| Symptom duration | ≤60 minutes | 42 (55%) | 35 (45%) | 0.4642 | 0.3865 |
| >60 minutes | 50 (49%) | 52 (51%) | |||
| Motor impairment | Yes | 12 (27%) | 33 (73%) | 0.0002 | 0.0012 |
| Sensory impairment | Yes | 28 (57%) | 21 (43%) | 0.3461 | 0.4191 |
| Visual impairment | Yes | 29 (53%) | 26 (47%) | 0.8125 | 0.9405 |
| Speech impairment | Yes | 17 (27%) | 46 (73%) | <0.0001 | <0.0001 |
aThe P value is adjusted for age and sex.
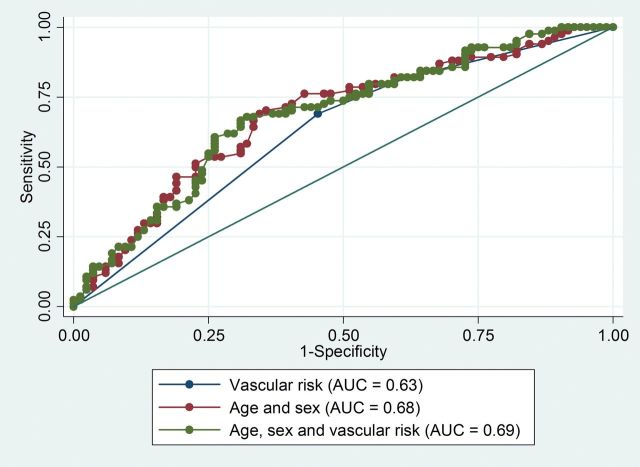
The prediction of TIAMS/TIAMS-mimic by baseline factors, adjusted for age and sex and adjusted for ACVR, is presented (via AUROC) in Table 4 and Figure 1. Most variables, including ACVR, did not improve diagnostic accuracy beyond that of age and sex. BMI, motor symptoms and speech symptoms, however, did improve the AUROC.
Table 4.
AUROC curve associated with various baseline variables and symptoms
| Variable | Area under the curve (95% confidence interval) | ||
|---|---|---|---|
| Unadjusted | Adjusted for age and sex | Adjusted for ACVR | |
| Age | 0.64 (0.56–0.72) | 0.67 (0.59–0.75) | |
| Sex | 0.59 (0.52–0.67) | 0.66 (0.58–0.74) | |
| Age and sex | 0.68 (0.60–0.76) | 0.69 (0.60–0.77) | |
| Vascular risk | 0.63 (0.55–0.70) | 0.69 (0.60–0.77) | |
| Systolic BP | 0.59 (0.50–0.68) | 0.68 (0.60–0.77) | 0.64 (0.55–0.73) |
| Hypertension | 0.61 (0.55–0.68) | 0.71 (0.63–0.78) | 0.67 (0.60–0.75) |
| Atrial fibrillation | 0.58 (0.52–0.64) | 0.69 (0.61–0.77) | 0.65 (0.57–0.73) |
| History of cardiovascular disease | 0.61 (0.54–0.69) | 0.68 (0.60–0.76) | 0.65 (0.57–0.72) |
| BMI category | 0.62 (0.54–0.70) | 0.75 (0.67–0.82) | 0.72 (0.64–0.79) |
| Hours from symptom onset to first doctor | 0.62 (0.54–0.71) | 0.70 (0.62–0.78) | 0.71 (0.63–0.79) |
| Motor impairment | 0.63 (0.56–0.69) | 0.74 (0.66–0.81) | 0.69 (0.61–0.76) |
| Sensory impairment | 0.53 (0.46–0.60) | 0.70 (0.62–0.78) | 0.62 (0.57–0.72) |
| Visual impairment | 0.52 (0.45–0.59) | 0.68 (0.60–0.76) | 0.63 (0.55–0.71) |
| Speech impairment | 0.66 (0.59–0.73) | 0.73 (0.66–0.81) | 0.71 (0.63–0.79) |
Figure 1.
Area under the curve of age and sex and age, sex and ACVR for diagnosis of transient ischaemic attack/minor stroke.
Discussion
Summary of main findings
Of participants presenting with a possible TIAMS, 49% were adjudicated as having had a TIAMS. ACVR was high or automatically high in 57% of study participants. ACVR was strongly associated with TIAMS diagnosis on univariate analysis, but not when age- and sex-adjusted.
A number of other risk factors, demographic and presentation factors were significantly associated with diagnosis of TIAMS rather than TIAMS-mimic, but only BMI, motor symptoms and speech symptoms showed any indication of usefulness in improving diagnostic accuracy.
Calculation of ACVR risk did not improve diagnostic accuracy beyond that of age and sex.
Interpretation of findings and comparison with previous literature
The finding that 49% of patients presenting with suspected TIAMS were adjudicated as TIAMS is similar to the 51–54% of attendees at Acute Neurovascular Clinics in Scotland, Australia and Germany (7,8,18). There is a higher proportion of migraines in our study and comparatively more ‘uncertain’ diagnoses compared to earlier Acute Neurovascular Clinic studies (7,10). This likely reflects our ascertainment of all patients within participating practices with a possible TIAMS rather than the ‘filtered’ population of referral secondary care clinics.
The strong association of age and sex with TIAMS diagnosis in our study may be partly explained by the large proportion of migraines (with bias to female sex and younger age) among our TIAMS-mimics.
We found variable evidence of individual elements of vascular risk calculation being associated with TIAMS on univariate analysis (age, sex, BP and a past history of vascular disease were significantly associated, but lipid levels, diabetes and smoking were not). History of hypertension and atrial fibrillation (not included in our ACVR calculation) were also associated with TIAMS analysis on univariate analysis. Our results are similar to an Irish Acute Neurovascular Clinic study: in that study, TIAMS patients were older, more likely male and to have had a previous TIA, hypertension, coronary artery disease or atrial fibrillation than were TIAMS-mimics (9). In comparison, in a Scottish Acute Neurovascular Clinic cohort (7), TIA patients differed from non-TIA counterparts by being older, having a higher BP and a higher burden of traditional cardiovascular risk factors (smoking, hypertension, hyperlipidaemia and diabetes).
We found ACVR to be univariately associated with TIAMS/TIAMS-mimic adjudication, but this association did not persist after adjustment for age and sex. Furthermore, addition of ACVR to age and sex did not appreciably increase the area under the curve (AUC) of ROC curves for TIAMS/TIAMS-mimic diagnosis. However, addition of motor symptoms or speech symptoms to age and sex increased the AUROC (from 0.68 to 0.74 and 0.73, respectively), similarly for the addition of BMI to age and sex (AUROC of 0.75). By comparison, in an Irish Acute Neurovascular Clinic population, use of ABCD2 score (3) (designed as a prognostic rather than diagnostic decision tool, and incorporating age, BP, speech symptoms, motor symptoms, duration of symptoms and history of diabetes) as an aid to TIAMS diagnosis resulted in an AUROC of 0.70 (9).
The finding of association of speech and motor symptoms with TIAMS diagnosis is consistent with previous studies (9,10).
The significant association of TIAMS diagnosis with BMI in our study was not linear, with a U-shaped relationship, so that BMIs <25 and >35 were strongly associated with TIAMS diagnosis. This is consistent with the U-shaped relationship of BMI with all-cause mortality (20) and with the shift to the right of this U-shaped curve with age (given the age of our study population). In previous studies, the upward limb of the stroke-specific mortality U-curve takes off at a BMI of 32.5 (20) which is consistent with our findings of the relationship of BMI and TIAMS/TIAMS-mimic.
Strengths and limitations
A strength of this study is the ascertainment of possible TIAMS from primary as well as secondary care. Previous studies have been of referred patients to secondary care settings. In our study, 38% of patients were managed exclusively in general practice and only 34% had attended an Acute Neurovascular Clinic or otherwise been seen by a stroke specialist. As a result of multiple overlapping means of ascertainment, we are confident that ascertainment has been good. The moderate response rate of 49% is a limitation of the study but reflects the difficulties of patient recruitment to research in primary care and in this context our response rate is relatively good.
Implications for clinical practice
GPs are encouraged to take cardiovascular risk factors into account when diagnosing TIAMS/mimics (5,12,13). Our study suggests that GPs should consider two component factors of ACVR, age and sex, in their diagnostic process for possible TIAMS in addition to clinical features of presentation (of which, we found speech/motor symptoms to be the most discriminatory). GPs diagnosed hemispheric TIAs more readily if the patient was older than 65 (6) in a Polish scenario-based study, indicating that some may already be using this approach. Patients being underweight or, especially, having a BMI >35 may also point to a diagnosis of TIAMS. However, we found no utility in formal calculation of ACVR beyond that provided by age and sex alone.
Conclusion
Calculation of ACVR does not appear to improve diagnostic accuracy for TIAMS in the situation of first contact with a patient with a possible TIAMS.
Declaration
Funding: The InSiST study is funded by the National Health and Medical Research Council , Project APP1027794. DL is supported by the National Institute for Health Research (NIHR), Oxford Biomedical Research Centre based at Oxford University Hospitals NHS Trust and University of Oxford.
Ethical approval: The InSiST study is approved by the Hunter New England Health and University of Newcastle Human Research Ethics Committees (12/04/18/4.02; H-2012-0154).
Conflict of interest: none.
Acknowledgement
The authors acknowledge the practice nurses at the participating practices for their diligent work in TIAMS identification and patient recruitment. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
References
- 1. Siket MS, Edlow JA. Transient ischemic attack: reviewing the evolution of the definition, diagnosis, risk stratification, and management for the emergency physician. Emerg Med Clin North Am 2012; 30: 745–70. [DOI] [PubMed] [Google Scholar]
- 2. Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology 2005; 64: 817–20. [DOI] [PubMed] [Google Scholar]
- 3. Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007; 369: 283–92. [DOI] [PubMed] [Google Scholar]
- 4. Rothwell PM, Giles MF, Chandratheva A, et al. ; Early use of Existing Preventive Strategies for Stroke (EXPRESS) study. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet 2007; 370: 1432–42. PubMed PMID: 17928046. English. [DOI] [PubMed] [Google Scholar]
- 5. Leung ES, Hamilton-Bruce MA, Koblar SA. Transient ischaemic attacks - assessment and management. Aust Fam Physician 2010; 39: 820–4. [PubMed] [Google Scholar]
- 6. Tomasik T, Windak A, Margas G, de Melker RA, Jacobs HM. Transient ischaemic attacks: desired diagnosis and management by Polish primary care physicians. Fam Pract 2003; 20: 464–8. [DOI] [PubMed] [Google Scholar]
- 7. Cameron AC, Dawson J, Quinn TJ, et al. Long-term outcome following attendance at a transient ischemic attack clinic. Int J Stroke 2011; 6: 306–11. [DOI] [PubMed] [Google Scholar]
- 8. Murray S, Bashir K, Lees KR, et al. Epidemiological aspects of referral to TIA clinics in Glasgow. Scott Med J 2007; 52: 4–8. [DOI] [PubMed] [Google Scholar]
- 9. Sheehan OC, Merwick A, Kelly LA, et al. Diagnostic usefulness of the ABCD2 score to distinguish transient ischemic attack and minor ischemic stroke from noncerebrovascular events: the North Dublin TIA Study. Stroke 2009; 40: 3449–54. [DOI] [PubMed] [Google Scholar]
- 10. Quinn TJ, Cameron AC, Dawson J, Lees KR, Walters MR. ABCD2 scores and prediction of noncerebrovascular diagnoses in an outpatient population: a case-control study. Stroke 2009; 40: 749–53. [DOI] [PubMed] [Google Scholar]
- 11. Lasserson DS, Mant D, Hobbs FD, Rothwell PM. Validation of a TIA recognition tool in primary and secondary care: implications for generalizability. Int J Stroke 2013. doi:101111/ijs12201.2013 [DOI] [PubMed] [Google Scholar]
- 12. Solenski NJ. Transient ischemic attacks: Part I. Diagnosis and evaluation. Am Fam Physician 2004; 69: 1665–74. [PubMed] [Google Scholar]
- 13. Simmons BB, Cirignano B, Gadegbeku AB. Transient ischemic attack: Part I. Diagnosis and evaluation. Am Fam Physician 2012; 86: 521–6. [PubMed] [Google Scholar]
- 14. Kannel WB, McGee D, Gordon T. A general cardiovascular risk profile: the Framingham Study. Am J Cardiol 1976; 38: 46–51. [DOI] [PubMed] [Google Scholar]
- 15. Alliance NVDP. Guidelines for the Management of Absolute Cardiovascular Disease Risk. 2012. [Google Scholar]
- 16. Wolf PA, D’Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991; 22: 312–8. [DOI] [PubMed] [Google Scholar]
- 17. D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117: 743–53. [DOI] [PubMed] [Google Scholar]
- 18. Magin P, Lasserson D, Parsons M, et al. Referral and triage of patients with transient ischemic attacks to an acute access clinic: risk stratification in an Australian setting. Int J Stroke 2013; 8 (suppl A100): 81–9. [DOI] [PubMed] [Google Scholar]
- 19. Heeley EL, Peiris DP, Patel AA, et al. Cardiovascular risk perception and evidence–practice gaps in Australian general practice (the AusHEART study). Med J Aust 2010; 192: 254–9. [DOI] [PubMed] [Google Scholar]
- 20. Whitlock G, Lewington S, Sherliker P, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373: 1083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]