Abstract
Background.
Most frequent attendance in primary care is temporary, but persistent frequent attendance is expensive and may be suitable for psychological intervention. To plan appropriate intervention and service delivery, there is a need for research involving standardized psychiatric interviews with assessment of physical health and health status.
Objective.
To compare the mental and physical health characteristics and health status of persistent frequent attenders (FAs) in primary care, currently and over the preceding 2 years, with normal attenders (NAs) matched by age, gender and general practice.
Methods.
Case–control study of 71 FAs (30 or more GP or practice nurse consultations in 2 years) and 71 NAs, drawn from five primary care practices, employing standardized psychiatric interview, quality of life, health anxiety and primary care electronic record review over the preceding 2 years.
Results.
Compared to NAs, FAs were more likely to report a lower quality of life ( P < 0.001), be unmarried ( P = 0.03) and have no educational qualifications ( P = 0.009) but did not differ in employment status. FAs experienced greater health anxiety ( P < 0.001), morbid obesity ( P = 0.02), pain ( P < 0.001) and long-term pathological and ill-defined physical conditions ( P < 0.001). FAs had more depression including dysthymia, anxiety and somatoform disorders (all P < 0.001).
Conclusions.
Persistent frequent attendance in primary care was associated with poor quality of life and high clinical complexity characterized by diverse and often persistent physical and mental multimorbidity. A brokerage model with GPs working in close liaison with skilled psychological therapists is required to manage such persistent complexity.
Keywords: Frequent attendance, health anxiety, medically unexplained symptoms, primary care, quality of life.
Introduction
Frequent attenders (FAs; top decile of face-to-face attendance) account for 38% of all primary care attendance, generating a high proportion of total cost and workload, and increased prescriptions and secondary care attendance compared to other attenders in primary care ( 1–3 ). Recent prospective research in Europe shows that only one in seven frequently attending patients continue to attend primary care so frequently over the next 2 years ( 4 , 5 ). Therefore, psychological interventions might be best focussed on persistent FAs in primary care. In the UK, unlike long-term conditions that are typically managed by a multi-disciplinary team of GPs, nurses and other professionals, the management of persistent FAs in primary care has largely been the responsibility of GPs with little assistance from others, in particular, mental health professionals ( 6 ).
Initiatives such as Improving Access to Psychological Therapies (IAPT) in England are being extended to offer psychological treatment to manage patients with both long-term physical and mental health problems to address these issues in primary care ( 5 ). Although there are a number of record-based and questionnaire studies on FAs in primary care ( 4 , 5 , 7–9 ), there are no case–control studies employing a full standardized psychiatric interview with a standardized assessment of long-term conditions and health status. Such data might guide service planning, for example, of relevant therapist expertise or commissioning of care more tailored to patient need. In this regard, identification of FAs in ways that can be realistically achieved in routine primary care without being burdensome for practices may also be helpful. Using a method of case identification that could be easily replicated in routine practice, this study aimed to compare the psychiatric, physical health and health status characteristics of FAs and normal attenders (NAs) in primary care.
Methods
Design
This matched case–control study compared the clinical and socio-demographic characteristics of FAs with NA controls ( 10 ). We piloted an approach in one practice (practice A) before extending the approach to four other practices.
Practice selection
Five practices across Nottinghamshire were purposively selected to obtain a wide variation in existing organization of care and socio-demographic contexts ( Table 1 ).
Table 1.
Practice profiles recruiting matched cases of persistent FAs and NAs
| Practice A | Practice B | Practice C | Practice D | Practice E | |
|---|---|---|---|---|---|
| Registered patients | 26977 | 11552 | 12915 | 14067 | 15325 |
| No. of sites | 4 | 1 | 2 | 1 | 1 |
| GPs | 14 | 7 | 14 | 8 | 6 |
| Deprivation decile a | 4 | 6 | 9 | 5 | 2 |
a Based on Index of Multiple Deprivation (11): 10 represents least deprived.
Practice A was selected because it prioritized access to care over continuity of GP care. It covered four sites in close proximity to a general hospital and served a deprived population.
Practice B was selected because it emphasized continuity of GP care, seeking to ensure patients saw the same GP on each occasion. It served a more affluent population and was purpose built on the site of a hospital.
Practice C had some university links, served an affluent population on two sites but was not close to a general hospital. It followed a policy of trying to meet patient preferences to see the GP of the patient’s choice but suggested alternatives if appointments would be delayed.
Practice D prioritized same-day access to a GP rather than continuity of care with the same doctor if that meant waiting longer. Direct access to mental health professionals and welfare advice with financial problems was available at the practice. It served a deprived population and was not close to a general hospital.
Practice E was similar to practice C organizationally but served an inner city ethnically diverse population in an area of high social deprivation.
Inclusion criteria
Regular attenders
For data protection reasons, practice staff, rather than the research team, selected FAs from their practice lists. Initially, we tried to recruit the top 10% of FAs by age and gender as previously suggested ( 12 ) but the pilot practice found this too burdensome to operate. Therefore, we established the consultation rate that was within the top 10% of all face-to-face contacts with GPs in a pilot phase in practice A and then applied that rate in all five practices in the study ( 10 ). That rate was 15 face-to-face contacts with GPs per year so we set a cut-off of 30 or more face-to-face contacts with GP or practice nurse within the last 2 years.
Normal attenders
Up to 22 face-to-face contacts with GP or practice nurse over 2 years. This upper limit was based on a previous study of eight Nottinghamshire practices showing a median annual attendance of eight, interquartile range (IQR) (3–11) face-to-face consultations with GPs ( 13 ).
Both groups
Aged 18 years old or over; written informed consent to the study.
Exclusion criteria
Participants were excluded by GPs in the practice if they had a diagnosis of an acute life-threatening or catastrophically disabling physical illness, e.g. cancer, stroke or an acute serious mental illness such as schizophrenia, bipolar disorder, anorexia nervosa or dementia because these patients would not be referred for psychological treatment for FA in routine clinical practice. However, participants who had any of these conditions for longer than 2 years and were in remission or stable were included. Contacts for routine health care checks with nursing staff such as blood sugar or blood pressure monitoring, urine checks for drug misuse, routine blood tests, dressing changes or health promotion, e.g. smoking cessation, weight control, vaccination, were not included in the count of consultations nor were telephone contacts or contacts with staff other than GPs or practice nurses.
Recruitment of participants
Practices identified FA and NA patient groups from their practice lists by an electronic search using study inclusion and exclusion criteria. All potential FAs and NAs meeting the criteria were sent a letter from the practice that included a study invite letter addressed from the GP practice, a participant information sheet and consent to contact form. Written and oral informed consent was obtained at interview from both FAs and NAs. Case comparisons were made with NAs matched by practice, gender and age (within 5 years) from batches of NAs selected at random by the practices themselves until the required numbers of NAs from each practice were recruited.
Clinical characteristics
Baseline assessments consisted of four measures.
Structured Clinical Interview for DSM-IV
The research version of a standardized psychiatric interview was used to determine whether participants met axis 1 DSM-IV psychiatric disorder ( 14 , 15 ) in the preceding 2 years. All interviews were conducted by the research team who were trained and supervised on administering the Structured Clinical Interview for DSM-IV (SCID) by a psychiatrist. In addition to DSM-IV criteria, the abridged criteria for somatization disorder were applied ( 16 ) and an additional diagnosis of health anxiety disorder was created by replacing the criterion for specific disease conviction in the DSM-IV diagnosis of hypochondriasis with persistent worrying about acquiring a serious physical illness. Thus, the criteria for health anxiety disorder together with hypochondriasis are broadly in line with DSM-V illness anxiety disorder ( 17 ).
Health Anxiety Inventory
Health Anxiety Inventory Short Week Adapted ( 18 ) is a 14-item self-rated tool to measure health anxiety over the preceding week. A cut-off score of 15 indicates people who would be accepted by IAPT in England for psychological treatment of health anxiety (18 indicates severe health anxiety). Avoidance scores are calculated by asking respondents to rate their likelihood of avoiding 10 health situations, scores ranging from 0 (would not avoid it) to 8 (always avoid it). Reassurance seeking scores are calculated based on how often the individual seeks reassurances from a range of nine sources, scores ranging from never (0) to daily (8).
EQ-5D-3L
EuroQoL ( 19 ) is a descriptive system for health-related quality of life. The measure is self-completed and defines health states using five dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression), each with three levels of severity (no problems, some or moderate problems, extreme problems). In addition, respondents are asked to rate their health on a visual analogue scale (VAS) ranging from 0 to 100, where a score of 0 indicates their worst and a score of 100 indicates their best imaginable health state. Using a valuation from a nationally representative sample, it is possible to attach preference weights to individuals’ responses ( 20 ). This enables the elicitation of a single index value on a scale anchored at 0 and 1, where 1 is ‘full health’ and 0 is a health state of equivalent value to being dead.
Client Service Receipt Inventory
Client Service Receipt Inventory ( 21 ) involves an interview with the participant to collect data on health service use in the preceding 3 months. Information is collated for primary and secondary care use and prescribed medication.
Physical health
Details of long-term conditions of all participants were obtained from electronic primary health care records using a published classification ( 22 ). In addition, body mass index (BMI) scores were extracted from medical records.
Statistical analysis
Case and controls were compared using univariate analyses on Stata version 13 with statistical significance level set at P < 0.05. Paired t -tests were carried out to compare normally distributed data and Wilcoxon signed-rank tests were conducted for skewed variables. McNemar’s test was used to test for group differences in binary variables and symmetry tests were used to test for group differences in categorical variables. We adopted a descriptive approach using univariate statistics to describe the nature of the clinical characteristics of FAs, their health profile and quality of life in order to inform the planning of interventions.
Results
Figure 1 shows the flow of patients through the study. Recruitment became more efficient in the remaining practices after the pilot was conducted. The research team checked all data provided by practices and excluded 16 pairs of cases who had too few contacts to be FAs and too many to be NAs. Of the 472 FAs who were approached to take part in the study, 71 were recruited with a median of 37 (IQR 32–45, range 30–90) face-to-face contacts with GP or nurse. GP practices approached 422 NA patients (four practices sent three invite letters for every FA recruited and the pilot practice sent out 10 invite letters for every FA recruited) to recruit 71 controls. The median number of face-to-face GP or nurse consultations for NAs was 7 (IQR 5–12, range 0–21). In each group, there were 55 (77%) women. The mean ages for FAs and NAs, respectively, were 57 years (SD 19) (range 20–89) and 56 years (SD 18) (range 20–86).
Figure 1.
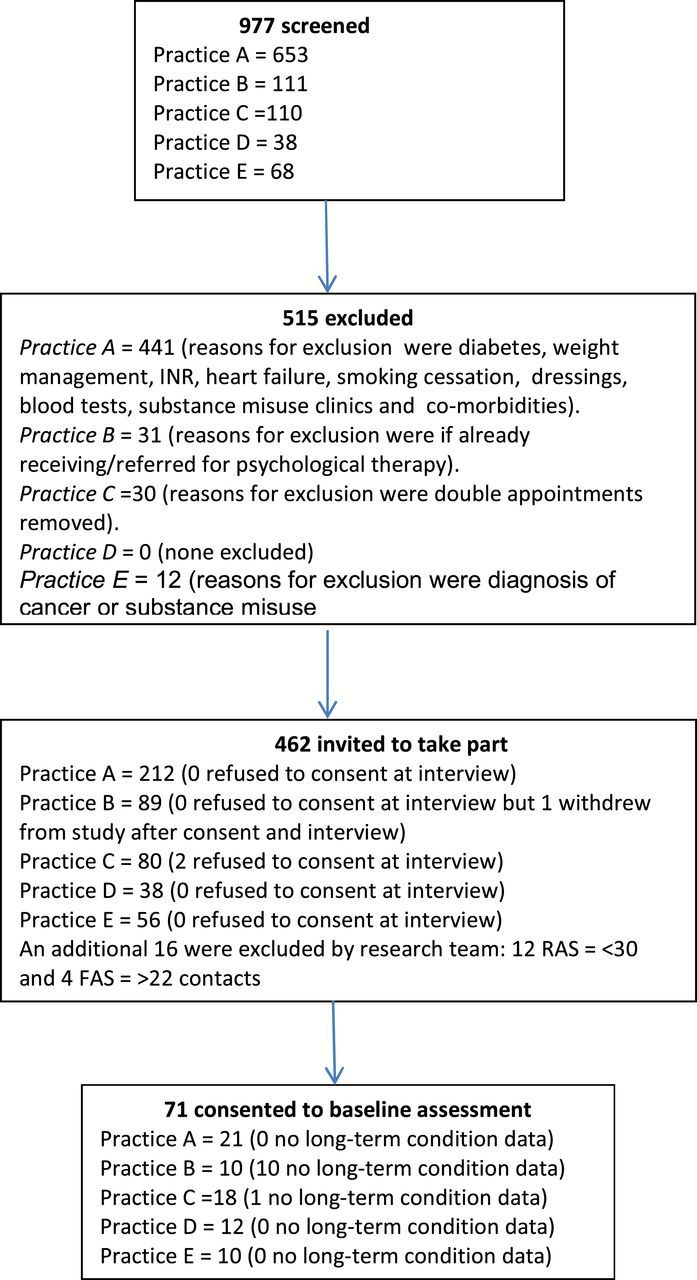
Flow of participants recruiting persistent FAs into study by practice
Table 2 shows self-reported service use in the 3 months preceding the baseline interview and socio-demographic factors for FAs and NAs. FAs reported significantly higher rates of face-to-face consultations with the GP and more prescribed medication, particularly central nervous (59%), cardiovascular (41%) and gastrointestinal systems (37%) drugs according to British National Formulary categories. Thirty-five (49%) FAs had visited the hospital at least once in the preceding 3 months compared to 27 (38%) NAs; these differences were not statistically significant. FAs were significantly less likely than NAs to be married and more likely to be without an educational qualification but slightly higher rates of unemployment and lower income were not statistically significant.
Table 2.
Socio-demographic characteristics of 71 persistent FAs and 71 NAs in the preceding 3 months
| Variable | FA median (range) or n (%) | NA median (range) or n (%) | P value |
|---|---|---|---|
| Service use | |||
| No. of GP appointments | 4 (0–30) | 1 (0–6) | <0.001 a |
| No. of medications | 5 (0–18) | 1 (0–14) | <0.001 a |
| No. of secondary care contacts | 0 (0–10) | 0 (0–6) | 0.15 a |
| No. of emergency care contacts | 0 (0–3) | 0 (0–2) | 0.17 a |
| Marital status | |||
| Married/partner | 37 (52) | 48 (68) | 0.03 b |
| Single/divorced/widow | 34 (48) | 23 (32) | |
| Highest educational qualifications | |||
| None | 28 (39) | 14 (20) | 0.009 c |
| Degree | 7 (10) | 16 (22) | |
| Other | 36 (51) | 41 (58) | |
| Occupational status | |||
| Unemployed | 11 (15) | 7 (10) | 0.36 c |
| Employed | 24 (34) | 30 (42) | |
| Retired | 29 (41) | 33 (46) | |
| Carer | 6 (8) | 1 (1) | |
| Missing | 1 (1) | 0 (0) | |
| Monthly net income | |||
| £0–£500 | 15 (21) | 9 (13) | 0.10 c |
| £500–£1000 | 14 (20) | 25 (35) | |
| £1000+ | 23 (32) | 28 (39) | |
| Missing | 19 (27) | 9 (13) | |
a Wilcoxon signed-rank tests for service use.
b McNemar’s test for marital status.
c Symmetry tests for highest educational qualifications, occupational status and monthly net income.
The median (range) of SCID_DSM-IV disorder was 2 (0–11) for FAs and 0 (0–4) ( P < 0.001) for NAs in the preceding 2 years. The number (percentage) of FAs with zero, one, two or three or more mental disorders were 17 (25), 8 (12), 10 (15) and 33 (49); the respective figures for NAs were 50 (74), 10 (15), 6 (9) and 2 (3). Although there were wide confidence intervals, FAs were 5 times more likely than NAs to have major depression, 24 times more dysthymia, 22 times more any anxiety disorder and 17 times more somatoform disorder ( Table 3 ).
Table 3.
Two-year prevalence of mental disorder in 71 persistent FAs and 71 NAs
| SCID criteria | FA, n (%) | NA, n (%) | Odds ratio (95% CI) | P value a |
|---|---|---|---|---|
| Major depressive episode | 31 (46) | 14 (21) | 5.3 (1.8–21.0) | <0.001 |
| Dysthymia | 25 (37) | 2 (3) | 24.0 (3.9–987.0) | <0.001 |
| Any depression diagnosis | 34 (50) | 14 (21) | 6.0 (2.1–23.8) | <0.001 |
| Panic disorder | 14 (21) | 1 (2) | 0.19 (0.08–0.30) b | <0.001 |
| Social phobia | 8 (12) | 0 | 0.12 (0.03–0.21) b | 0.003 |
| Specific phobia | 9 (13) | 1 (2) | 8.0 (1.1–355.0) | 0.02 |
| Post-traumatic stress disorder | 8 (12) | 0 | 0.12 (0.03–0.21) b | 0.002 |
| Generalized anxiety disorder | 22 (32) | 3 (4) | 20.0 (3.2–829.0) | <0.001 |
| Any anxiety diagnosis | 28 (41) | 7 (10) | 22.0 (3.6- 908.0) | <0.001 |
| Somatization disorder | 10 (15) | 2 (3) | 9 (1.3–394.5) | 0.011 |
| Abridged somatization disorder | 27 (40) | 2 (3) | 13.5 (3.4–117.2) | <0.001 |
| Hypochondriasis | 10 (15) | 0 | 0.12 (0.05–0.25) b | 0.002 |
| Health anxiety disorder | 10 (15) | 0 | 0.12 (0.05–0.25) b | 0.002 |
| Any somatoform disorder | 36 (53) | 4 (6) | 17.0 (4.4–146.1) | <0.001 |
CI, confidence interval. Note diagnoses are not mutually exclusive, n = 68 in both FAs and NAs.
a McNemar’s test for all binary SCID criteria variables.
b Risk difference (95% CIs).
In addition, Table 4 shows that FAs had significantly more health anxiety (including both reassurance seeking and avoidance) than NAs with a mean above the threshold for severe health anxiety, a higher BMI with a mean at the obese level for adults (30) and more long-term conditions with a median of 3 for FAs and 1 for NAs. Table 4 lists the most frequent long-term physical conditions all of which were significantly more common in FAs than NAs, including physical health conditions with a well-defined pathophysiology, e.g. hypertension, asthma and diabetes and ill-defined conditions, e.g. such chronic pain requiring the prescription of four or more different analgesics and irritable bowel syndrome. Table 4 shows that FAs rated their health status substantially worse than NAs on the EQ-5D VAS, tended to report more problems in all dimensions of the EQ-5D and had a lower index score. For all dimensions except self-care, FAs were most likely to report having moderate problems, while NAs were most likely to report having no problems.
Table 4.
Health status, BMI, long-term conditions and health anxiety in 71 persistent FAs and 71 NAs
| Variable | FA | NA | P value | Odds ratio (95% CI) |
|---|---|---|---|---|
| EQ-5D-3L | ||||
| Index value: median (IQR) | 0.66 (0.19–0.80) | 1.00 (0.80–1.00) | <0.001 a | N/A |
| VAS score: median (IQR) | 50 (40–65) | 85 (70–95) | <0.001 a | N/A |
| Dimensions b | L1 | L2 | L3 | L1 | L2 | L3 | ||
| Mobility | 30 | 41 | 0 | 56 | 15 | 0 | <0.001 a | N/A |
| Self-care | 51 | 20 | 0 | 69 | 1 | 1 | <0.001 a | N/A |
| Usual activities | 29 | 34 | 8 | 62 | 7 | 2 | <0.001 a | N/A |
| Pain/discomfort | 11 | 41 | 19 | 48 | 20 | 3 | <0.001 a | N/A |
| Anxiety/depression | 31 | 34 | 6 | 61 | 8 | 2 | <0.001 a | N/A |
| HAI | ||||
| Score of items 1–14: mean (95% CI) | 18.5 (15.9–21.2) | 6.6 (5.3–7.8) | <0.001 c | N/A |
| Reassurance seeking: mean (95% CI) | 25.0 (20.9–29.1) | 14.0 (10.5–1765) | <0.001 c | N/A |
| Avoidance: median (IQR) | 6.0 (0–17) | 0 (0–4) | 0.0024 a | N/A |
| BMI: median (IQR) | 30.1 (25.7–31.6) | 26.2 (24.6–28.8) | 0.020 a | N/A |
| Long-term conditions d : median (range) | 3 (0–8) | 1 (0–5) | <0.001 a | N/A |
| Hypertension e , n (%) | 29 (48) | 14 (23) | 0.002 | 4.8 (1.6–19.2) |
| Painful condition e , n (%) | 22 (37) | 7 (12) | 0.004 | 3.5 (1.4–10.6) |
| Asthma (currently treated) e , n (%) | 17 (28) | 7 (12) | 0.033 | 2.7 (1.0–8.3) |
| Irritable bowel syndrome e , n (%) | 16 (27) | 7 (12) | 0.020 | 4.0 (1.1–22.1) |
| Diabetes e , n (%) | 9 (15) | 2 (3) | 0.012 | 8.0 (1.1–355.0) |
CI, confidence interval.
a Wilcoxon signed-rank tests for EQ-5D dimensions, index value and VAS, Health Anxiety Inventory (HAI) avoidance scores, BMI and long-term conditions.
b Number of participants reporting level 1 (L1; no problems), level 2 (L2; some problems) and level 3 (L3; extreme problems) for each dimension.
c Paired t -tests for HAI item 1–14 scores and reassurance seeking scores.
d Long-term physical conditions from case records as defined by Barnett et al. (22), n = 60 in both FAs and NAs.
e Long-term conditions if found in 10% or more participants in either group, McNemar’s test for all binary long-term conditions.
Discussion
Summary of main findings
Persistent FAs, compared to age-, gender- and practice-matched NAs, had a complex range of clinical problems, with more mental disorders, particularly persistent mental disorder such as dysthymia, anxiety disorder and somatoform disorder, more long-term pathological (hypertension, asthma, diabetes) and more ill-defined long-term physical conditions (painful conditions associated with the use of four or more analgesics, irritable bowel syndrome) in the last 2 years, with greater health anxiety, a higher BMI and lower quality of life. On average, FAs had three mental health conditions each and three long-term physical or ill-defined conditions in the last 2 years in contrast to NAs who had one long-term physical, mental or ill-defined condition.
Comparison with previous literature
The results confirm and extend previous medical record-based studies indicating that FAs are more likely to have problems with anxiety, health anxiety, long-term and ill-defined physical conditions and BMI above 30 than NAs; the previous literature indicates that FAs may also have more traumatic life events in the preceding 3 years and a history of physical abuse in women ( 6 , 8 , 9 , 23 , 24 ). This study also shows that FAs experience high rates of persistent mental disorder such as dysthymia. FAs’ EQ-5D index values were considerably lower than population norms ( 25 ) but similar to other samples with long-term physical and ill-defined conditions such as chronic obstructive airways disease and irritable bowel syndrome ( 26 ).
Study strengths and limitations
This study has provided additional, in-depth data on the clinical characteristics of FAs compared to age- and gender-matched NAs from the same practice. The recruitment of 15% FA cases underlines the challenges of engaging this complex group of patients in research. The case–control design allowed an unbiased comparison between FAs and NAs across practices with different approaches to the organization of care and serving different populations. A strength of the design is that we recruited a sample of NAs within a broad range of normal attendance so that we did not recruit a sample of exceptionally healthy controls. The upper limit of attendance in the NA group was three quarters of the minimum attendance in the FA group so the NA group also has some persistent mental and physical disorders over 2 years. A limitation of our study is that there are no data available to compare responders to non-responders to take part in the study because the research team had no access to non-responder data.
Previous research studies, with additional research infrastructure support, have used the proportionate method for defining the top decile of attenders at each practice with different cut-offs for male and female in young adulthood, middle and old age ( 12 ). By not setting age and gender cut-offs, our sample might be biased to an older and more female sample, potentially increasing the prevalence of long-term physical conditions that become more common with age and the prevalence of some mental disorders more common in women, such as depression. However, the proportionate method may similarly increase the prevalence of other common problems seen in younger people such as substance misuse, possibly further increasing the diversity of health presentations among FAs. A limitation of the study was the exclusion of telephone contacts, which is now an important method of primary care intervention with all patients; these were excluded because we found that they were not systematically recorded.
We recognize the relatively modest sample size and cross-sectional study design may explain why differences in unemployment, income and secondary health contacts were not statistically significant, and the wide confidence intervals for mental health and other long-term conditions. The study was exploratory so no formal power calculation was performed. A further potential limitation of this study is that recruitment took part in larger than average practices. There was a very low recruitment rate in the pilot practice (only 11%), but recruitment improved for the remaining four practices (24%) as practices became more able to identify appointments due to routine monitoring and health checks. Although only a low proportion of FAs and NAs responded to the study, there is enough similarity with anonymized record-based studies involving other varied practice samples ( 6 , 8 , 9 , 23 , 24 ) to believe that our results are representative of FAs in general.
Implications for research and clinical practice
A method to identify FAs that could be operationalized in routine care without further increasing workload for GPs and other practice staff has been used. Therefore, the results would be not only generalizable but could be easily replicated for both research and service needs within the UK. Nevertheless, even setting such simple cut-off criteria entails work for practice staff, e.g. to establish whether patients were seen face-to-face by GPs or practice nurses rather than by telephone or for new problems rather than for routine health care checks. The approach enabled the practices to use preset criteria in a manageable sample rather than count and check face-to-face or other contacts in records of all patients in the practice to calculate age- and gender-adjusted rates of FA in that practice.
The study data have several implications for developing psychological interventions for FAs in primary care. The health status of FAs is comparable to people with long-term conditions who receive more structured care, often with specialist input. Care of FAs may justify additional interventions to help GPs to manage and improve care for FAs, and impact their complex co-morbidities, but further intervention development and evaluation in randomized controlled trials are required to test their clinical and cost-effectiveness. The clinical complexity of FAs with multiple and diverse and often persistent physical and mental health problems, exacerbated periodically by major depression, suggests that psychological therapists will require a high level of expertise and need to regularly communicate with the GP to enhance the consistency and effectiveness of interventions. They will need to liaise with GPs about the complex interplay of different symptoms, medication side effects and any ongoing investigations or referrals. The current IAPT approach to provide short-term psychological interventions with little ongoing communication with the GP is unlikely to be sufficient. The results suggest a potential role for GPs in both helping FAs to understand the complexity of their problems, and brokerage utilizing care from IAPT and other social, mental and physical health providers as required as outlined by Bellón et al. ( 27 ). GPs may need supervision and support from experienced primary care practitioners or mental health to plan such proactive care.
Declaration
Funding: the study was funded as part of the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) Nottinghamshire, Derbyshire and Lincolnshire, funded by a central grant from NIHR and further funding from Nottinghamshire Healthcare Trust, University of Nottingham and other National Health Service Trusts who were partners in NIHR CLAHRC. RM, JK, SP, CA, BG, MS and SM continue to be funded by NIHR CLAHRC East Midlands during the analysis and write up of this study. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Ethical approval: the study was approved by the Nottingham Multicentre Research ethics Committee and research governance approval was obtained from Nottingham City, Nottinghamshire and Bassetlaw Primary Care Trusts.
Conflict of interest: none.
References
- 1. Neal RD, Heywood PL, Morley S, Clayden AD, Dowell AC . Frequency of patients’ consulting in general practice and workload generated by frequent attenders: comparisons between practices . Br J Gen Pract 1998. ; 48 : 895 – 8 . [PMC free article] [PubMed] [Google Scholar]
- 2. Smits FT, Brouwer HJ, Zwinderman AH, et al. Morbidity and doctor characteristics only partly explain the substantial healthcare expenditures of frequent attenders: a record linkage study between patient data and reimbursements data . BMC Fam Pract 2013. ; 14 : 138 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bermingham SL, Cohen A, Hague J, Parsonage M . The cost of somatisation among the working-age population in England for the year 2008-2009 . Ment Health Fam Med 2010. ; 7 : 71 – 84 . [PMC free article] [PubMed] [Google Scholar]
- 4. Smits FT, Brouwer HJ, van Weert HC, Schene AH, ter Riet G . Predictability of persistent frequent attendance: a historic 3-year cohort study . Br J Gen Pract 2009. ; 59 : e44 – 50 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smits FT, Brouwer HJ, Zwinderman AH, et al. Predictability of persistent frequent attendance in primary care: a temporal and geographical validation study . PLoS One 2013. ; 8 : e73125 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDonald R, Cheraghi-Sohi S, Bayes S, Morriss R, Kai J . Competing and coexisting logics in the changing field of English general medical practice . Soc Sci Med 2013. ; 93 : 47 – 54 . [DOI] [PubMed] [Google Scholar]
- 7. Smits FT, Brouwer HJ, ter Riet G, van Weert HC . Epidemiology of frequent attenders: a 3-year historic cohort study comparing attendance, morbidity and prescriptions of one-year and persistent frequent attenders . BMC Public Health 2009. ; 9 : 36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tomenson B, McBeth J, Chew-Graham CA, et al. Somatization and health anxiety as predictors of health care use . Psychosom Med 2012. ; 74 : 656 – 64 . [DOI] [PubMed] [Google Scholar]
- 9. Smits FT, Brouwer HJ, Zwinderman AH, et al. Why do they keep coming back? Psychosocial etiology of persistence of frequent attendance in primary care: a prospective cohort study . J Psychosom Res 2014. ; 77 : 492 – 503 . [DOI] [PubMed] [Google Scholar]
- 10. Morriss R, Kai J, Atha C, et al. Persistent frequent attenders in primary care: costs, reasons for attendance, organisation of care and potential for cognitive behavioural therapeutic intervention . BMC Fam Pract 2012. ; 13 : 39 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Department for Communities and Local Government . The English Indices of Deprivation 2010 . Department for Communities and Local Government; , 2011. . https://www.gov.uk/government/publications/english-indices-of-deprivation-2010 (accessed on 30 September 2015 ). [Google Scholar]
- 12. Howe A, Parry G, Pickvance D, Hockley B . Defining frequent attendance: evidence for routine age and sex correction in studies from primary care settings . Br J Gen Pract 2002. ; 52 : 561 – 2 . [PMC free article] [PubMed] [Google Scholar]
- 13. Morriss R, Lindson N, Coupland C, Dex G, Avery A . Estimating the prevalence of medically unexplained symptoms from primary care records . Public Health 2012. ; 126 : 846 – 54 . [DOI] [PubMed] [Google Scholar]
- 14. First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS . Structured Clinical Interview for DSM-IV (SCID-I) . New York, NY: : American Psychiatric Press; , 1997. . [Google Scholar]
- 15. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition . Washington, DC: : American Psychiatric Association; , 1994. . [Google Scholar]
- 16. Escobar JI, Manu P, Matthews D, et al. Medically unexplained physical symptoms, somatization disorder and abridged somatization: studies with the Diagnostic Interview Schedule . Psychiatr Dev 1989. ; 7 : 235 – 45 . [PubMed] [Google Scholar]
- 17. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition . Washington, DC: : American Psychiatric Association; , 2013. . [Google Scholar]
- 18. Salkovskis PM, Rimes KA, Warwick HM, Clark DM . The Health Anxiety Inventory: development and validation of scales for the measurement of health anxiety and hypochondriasis . Psychol Med 2002. ; 32 : 843 – 53 . [DOI] [PubMed] [Google Scholar]
- 19. Euroqol Group . EuroQol: a new facility for the measurement of health related quality of life . Health Policy 1990. ; 16 : 199 – 208 . [DOI] [PubMed] [Google Scholar]
- 20. MVH Group . The Measurement and Valuation of Health: Final Report on the Modelling of Valuation Tariffs . York: : Centre for Health Economics; , 1995. . [Google Scholar]
- 21. Beecham J, Knapp M . Costing psychiatric interventions . In: Thornicroft G. (ed.). Measuring Mental Health Needs . London: : Gaskell; , 2001. , pp. 200 – 24 . [Google Scholar]
- 22. Barnett K, Mercer SW, Norbury M, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study . Lancet 2012. ; 380 : 37 – 43 . [DOI] [PubMed] [Google Scholar]
- 23. Department of Health . Talking Therapies: A Four-Year Plan of Action . London: : Department of Health; , 2011. . [Google Scholar]
- 24. Koskela TH, Ryynanen OP, Soini EJ . Risk factors for persistent frequent use of the primary health care services among frequent attenders: a Bayesian approach . Scand J Prim Health Care 2010. ; 28 : 55 – 61 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kind P, Hardman G, Macran S . UK Population Norms for EQ-5D . Centre for Health Economics; , 1999. . http://www.york.ac.uk/media/che/documents/papers/discussionpapers/CHE Discussion Paper 172.pdf (accessed on 30 September 2015 ). [Google Scholar]
- 26. Brazier J, Roberts J, Tsuchiya A, Busschbach J . A comparison of the EQ-5D and SF-6D across seven patient groups . Health Econ 2004. ; 13 : 873 – 84 . [DOI] [PubMed] [Google Scholar]
- 27. Bellón JA, Rodríguez-Bayón A, de Dios Luna J, Torres-González F . Successful GP intervention with frequent attenders in primary care: randomised controlled trial . Br J Gen Pract 2008. ; 58 : 324 – 30 . [DOI] [PMC free article] [PubMed] [Google Scholar]


