Abstract
Euphorbia hirta commonly known as Tawa-Tawa is a plant used in folklore medicine in the Philippines for the treatment of dengue. Though, E. hirta has been extensively investigated for numerous bioactivities, limited studies have been conducted on the antidengue activity. Thus, the present study provides a comprehensive review of studies conducted on the antidengue activity of E. hirta. A systematic literature survey was carried out in scientific databases, PubMed®, Scopus, and Google Scholar, for research carried on the antidengue activity of E. hirta. The literature search identified a total of 867 articles: databases PubMed = 6, Scopus SciVerse® = 423, and Google Scholar = 437; one additional article was identified by searching reference lists. Eight full papers were entitled to the review; out of those, two studies focused on ethnobotanical surveys, three on animal experiments, one on human trial, and two on in vitro antiviral activities, and one was computational study. The available evidence conclusively demonstrates the potential of E. hirta against dengue as it holds significant antiviral and platelet increasing activities. However, the number of studies conducted to validate its antidengue activity was found to be inadequate. Hence, well-controlled clinical trials and contemporary pharmacological approaches including activity guided fractionation and elucidation of the mode of action are encouraged to establish the use of E. hirta for dengue.
1. Introduction
Dengue is a viral disease that impose the greatest human and economic burden in tropical and subtropical regions [1]. Dengue is caused by four 4 distinct, yet closely related, serotypes (DEN-1, DEN-2, DEN-3, and DEN-4) belonging to family Flaviviridae, genus Flavivirus [1]. It is transmitted to humans by Aedes mosquitoes, mainly Aedes aegypti, an urban breeding mosquito [1].
Dengue is endemic in over 100 countries including Asia, the Pacific, the Americas, Africa, and the Caribbean [2]. In recent decades, the incidences of dengue have increased rapidly, currently reaching pandemic levels [3]. It is estimated that around 50 million cases occur each year and more than 2.5 billion of people are at risk of infection [3]. The disease not only is onerous to healthcare but also adversely impacts the economy caused by illness, premature death, and increased healthcare costs [4].
Dengue possesses a wide clinical manifestation ranging from nonsevere to severe forms [5]. The symptoms of dengue range from a mild flu-like syndrome (known as dengue fever [DF]) to the most severe forms of the disease, which are characterized by coagulopathy, increased vascular permeability, and plasma leakage (dengue hemorrhagic fever [DHF]) which eventually leads to dengue shock syndrome (DS) [5]. Dengue associated mortality is usually reflected in the increase in the progression of DF patients developing DHF/DS [6].
The severity of dengue is amplified by the lack of effective treatment [7]. Usually, the clinical symptoms of dengue are managed through fluid balance, a supplement of electrolytes, and blood clotting parameters [8]. Anti-D immune globulin therapy in some instances is used for severe thrombocytopenia [9], but this is expensive making it challenging for developing nations to afford such a treatment regimen. The first dengue vaccine, Dengvaxia, has been licensed in 2015 for clinical practice [10]. However, this may not ensure the protection from infection of all serotypes [7].
Along with the allopathic care, most people living in tropical and subtropical countries depend on folk/traditional medicine to alleviate dengue infection; in fact herbal medicines have been invaluable therapeutic agents going back to the earliest human civilizations [7]. Currently, there is a resurgent interest in herbal medicine among the public considering its safety and cost-effectiveness. There have been approximately 30 different plant species found to have the potential to combat dengue, such as Andrographis paniculata [Hempedu Bumi (Malaysia)], Alternanthera philoxeroides (alligator weed), Carica papaya (papaya), Cladosiphon okamuranus (brown seaweed), and Momordica charantia [bitter melon, Peria (Malaysia)] [7]. Among these, C. papaya leaf juice has been widely used as remedy against dengue in many Asian countries [11–13]. In this regard, extensive research has been carried out to establish the platelet activating [13], white blood cell increasing properties [14], and membrane stabilization [15] potential of C. papaya leaf extracts.
Euphorbia hirta is another plant used in folk medicine to cure dengue fever by people in rural areas of the Philippines [7]. The leaves of E. hirta, locally known as “Tawa-Tawa” or gatas–gatas, are used to make a decoction that believe to alleviate viral infection and associated fever symptoms [7]. E. hirta belonging to Euphorbiaceae family is a hairy herb grown in open grasslands, roadsides, and pathways. It is a popular folkloric plant in other countries like India, Sri Lanka, Malaysia, Java, and Vietnam [16]. Previously, the antiviral, antibacterial, antimalarial, antifungal, anti-inflammatory, anthelmintic, and antitumor properties of the plant have been validated [16]. Particularly for dengue, a commercial formulation (capsule) has been developed and the local communities in the Philippines consume it as a treatment for dengue [17].
Despite the popularity of E. hirta as a folk remedy for dengue, few scientific validations have been carried out. Therefore, the present review intends to provide a comprehensive account of available scientific evidence to validate the effectiveness of E. hirta against dengue. It is anticipated that this work will provide the platform for future investigations on E. hirta. Further, this attempt warrants exploring the bioactive compounds and possible mechanisms of action and will pave the way to develop a new antidengue drug lead from E. hirta.
2. Methodology
2.1. Search Strategy
A systematic review of published studies on the use of Euphorbia hirta against dengue was undertaken in accordance with the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) [18]. A comprehensive search of the literature was conducted in the following databases: PubMed (US National Library of Medicine, USA), SciVerse Scopus (Elsevier Properties SA, USA), and Google Scholar for studies published before 31 October 2017. The following medical subject headings and keywords, “Euphorbia hirta dengue”, “Tawa Tawa dengue”, “gatas–gatas dengue”, were included in the search. Results were limited to studies in English, while conference proceedings and commentaries were excluded.
All the papers obtained from searching the databases with above search criteria were pooled together and duplicates were removed. The remaining articles were initially screened by reading the “title” and thereafter the “abstracts.” Studies not satisfying the inclusion criteria (Section 2.2) were excluded at these stages. The remaining articles were screened in the final stage by reading the full-text and those not meeting inclusion criteria were excluded. Additional articles were obtained manually using the reference lists of included articles.
2.2. Inclusion/Exclusion Criteria
The following inclusion criteria were used: (a) ethnobotanical studies based on use of E. hirta against dengue, (b) in vivo studies investigating the platelet increasing, white blood cell increasing and membrane stabilization potential of E. hirta, tested on laboratory animals such as mice, rats, and rabbits, (c) in vitro cell culture studies conducted against dengue viruses, (d) molecular docking studies involving the interaction between phytochemicals of E. hirta and dengue virus proteins, and (e) studies published before 31 October 2017.
Studies were excluded based on the following exclusion criteria: (a) different species of Euphorbia, (b) other bioactivities other than dengue or related pathologies, and (c) reviews written on E. hirta.
3. Results
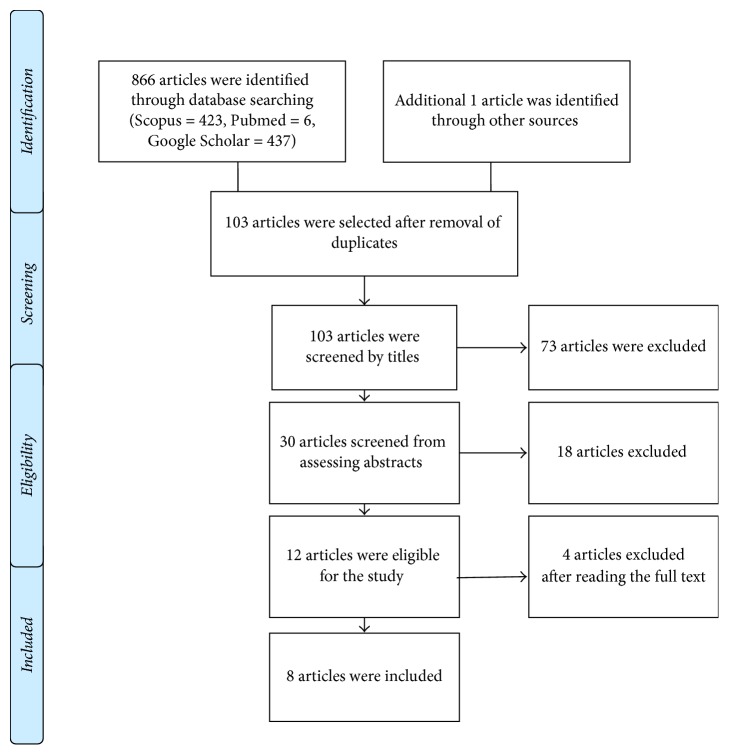
The literature search identified the following number of articles in the respective databases: PubMed (n = 6) and SciVerse Scopus (n = 423), and Google Scholar (n = 437). One additional article was identified by manually searching the reference lists and forward citations of included papers. After removing duplicates, the total number of articles included in the present review was eight. Figure 1 presents the search strategy used in selecting the articles.
Figure 1.
Schematic diagram representing the search strategy.
This systematic review pools the available scientific data on the effectiveness of E. hirta on dengue infection. Out of 8 studies, two studies were ethnobotanical surveys, 3 were animal experimentations, 1 study was from human trials, 2 were in vitro antidengue/antiviral studies, and 1 was from computations modelling (Table 1).
Table 1.
Summary of studies conducted on antidengue activity of Euphorbia hirta.
| Number | Study | Experimental model | Part of the plant/preparation method | Outcome |
|---|---|---|---|---|
| 1 | [19] | Ethnopharmacological survey | Decoction of leaves or bark | Predominately 60–80 years of age mostly females with primary and secondary education were aware of the use of E. hirta for dengue. |
|
| ||||
| 2 | [20] | Descriptive ethnobotanical survey | Expressed juice of E. hirta | A survey conducted using questionnaires in Agoo, La Union, Philippines revealed Tawa-Tawa is the most sought medicinal plant for dengue. |
| In vivo assay on rabbits (Aspirin-induced thrombocytopenia) | Expressed juice of E. hirta | A significant increase of platelet count after 24 hours of administration of E. hirta juice into thrombocytopenic rabbits. | ||
|
| ||||
| 3 | [21] | In vivo assay on rats (ethanol (i.p injection) induced thrombocytopenic model) | Decoction of fresh whole plant | A significant increase of platelet counts after 14 days of administration of E. hirta, further reporting decreased bleeding time and clotting time of rats. |
|
| ||||
| 4 | [22] | In vivo assay using rats (thrombocytopenia was induced using Anagrelide) | Water extract of leaves | Mean platelet count was increased by 80% following E. hirta treatment for 9 days. |
|
| ||||
| 5 | [23] | Clinical study using dengue patients admitted to Sir Ganga Ram Hospital, Lahore. | Herbal water | Over 70% patients exhibited a platelet increase. Marked recovery in fever and flu like symptoms following 24 hours of administration of E. hirta. |
|
| ||||
| 6 | [25] | In vitro assay for DENV-2 serotype | Ethanol extract of leaves | Virus inhibition by 34.7%. |
|
| ||||
| 7 | [24] | In vitro assay | Tea of E. hirta and ethyl acetate/methanol and ethyl acetate partitioning. | The ethyl acetate fraction of whole plant of E. hirta extracted using methanol and dichloromethane significantly reduced (85%) the plaque forming capacity of dengue virus serotype 1. Nine compounds were isolated from the fraction. |
|
| ||||
| 8 | [26] | Molecular docking of phytochemicals with 2FOM-dengue proteases, 2P40-methyl transferase of dengue |
Leaves of E. hirta | Quercetin exhibited strongest binding with dengue virus. Thus, E. hirta can be indicated as effective against dengue virus. |
3.1. Ethnobotanical Survey
The two ethnobotanical surveys conducted on E. hirta have been carried out in different parts of the Philippines. A survey conducted in 3 different indigenous communities in the island municipality of Anda, Mt. Balungao, and Mt. Colisao municipal park found that mostly females 60–80 years of age with primary and secondary education generally use E. hirta extract to alleviate the symptoms of dengue and related bleeding episodes [19]. It was found that both topical application and oral treatment are recommended for symptom management of dengue [19]. Decoctions of leaves and bark are popularly administrated for dengue [19].
According to a descriptive survey conducted using questionnaires in Agoo, La Union, the Philippines, out of several plant species (Tawa-Tawa, Papaya, and Malunggay), Tawa-Tawa/E. hirta was the most popular medicinal plants consumed by patients who suffered from dengue fever [20].
3.2. Platelet Augmentation Activity
3.2.1. Animal Models
Three studies have been conducted to investigate the platelet augmentation potential of E. hirta. All of them have established a significant platelet increasing activity following the administration of E. hirta leaf extract/decoction. These three studies have employed thrombocytopenic agents to lower the platelet counts prior to the administration of E. hirta extracts/juice.
Apostol et al. [21] had used ethanol (intraperitoneal injection) induced thrombocytopenic Sprague-Dawley rats to evaluate the platelet increasing activity of E. hirta. The results revealed that fourteen-day administration of 100 mg/kg of the lyophilized decoction of E. hirta had significantly increased platelet counts in rats [21]. Similarly, the bleeding time which was increased by the i.p. injection of ethanol was lowered by the E. hirta decoction. Simultaneously, the clotting time was decreased in E. hirta-treated rats compared to that of ethanol-induced thrombocytopenic rats [21].
A significant increase of mean platelet count was also observed following the oral treatment for 9 days of water extract of E. hirta leaves in Sprague-Dawley rats who were orally treated with Anagrelide (given for 15 days) for the induction of thrombocytopenia [22].
Similarly, in another study, when the expressed juice of E. hirta was administered in thrombocytopenic rabbit (by aspirin oral treatment), platelet counts were markedly increased after 24 hours [20].
3.2.2. Clinical Study
A clinical study conducted with dengue patients (both age groups 30–55 and 14–25) admitted to the hospital Sir Ganga Ram Hospital Lahore showed that the oral treatment with herbal water of E. hirta had increased the platelet and total leukocyte counts after 24 hours. A significant platelet increase was also observed in the patients of 30–55 age group following the treatment with E. hirta, while the increment was not significant in the 14–25 age group, compared with the control group [23]. Similarly, the decrease in hematocrit values was not significant. Moreover, 70% patients had recovered from fever and flu-like symptoms [23].
3.3. Antidengue/Antiviral Activity
According to Tayone et al. [24], the ethyl acetate fraction of the whole plant of E. hirta extracted using methanol and dichloromethane significantly reduced (85%) the plaque forming capacity of dengue virus serotype 1 from ~1400 to ~200 PFU. Further purification of ethyl acetate fraction revealed the presence of 9 compounds. It was assumed that these compounds individually or synergistically may have contributed to antidengue activity [24].
Similarly, in another study, the ethanol extract of E. hirta demonstrated 34.7% inhibition of virus serotype 2 (DENV-2) under in vitro conditions [25].
3.4. Computer Modelling: Molecular Docking
Phytochemicals found in E. hirta, specifically quercetin, myricetin, rutin, kaempferol, gallic acid and protocatechuic acid, were used for docking study using Maestro (Glide) and Lead IT (FlexX) software applications. The dengue protease (2FOM) and dengue methyl transferase (2P40) have been selected as targets for binding assays. Out of the phytochemicals, quercetin exhibited the strongest binding with dengue targets. Hence, this study indicates that the E. hirta is effective against dengue virus [26].
4. Discussion
Euphorbia hirta is one of the most widely used medicinal plant in the Philippines as a treatment for dengue [19]. The ethnobotanical survey conducted in Pangasinan where dengue continues to be prevalent revealed most people, especially women, used this preparation as a supportive therapy for dengue [19]. Moreover, E. hirta was identified as the widely consumed remedy taken by dengue patients in Agoo, La Union [20]. These indicated the folkloric belief of E. hirta as a treatment against dengue. It was stated that both topical applications and oral treatments are recommended in traditional practice [20]. Particularly, the decoctions of leaves and aerial parts of the plant are commonly used for preparation. The ready availability of E. hirta in home gardens has increased its use as a home remedy [20].
Though E. hirta is used as a folkloric plant in India [16], Pakistan [27], and Sri Lanka [28] for common ailments (fever and infection), no studies have been carried out in these countries to investigate its use against dengue. Hence, more ethnobotanical surveys are warranted to overly validate the effectiveness of E. hirta as an antidengue medicinal plant in other parts of the world.
Three animal studies conducted using rats and rabbits established that the subacute administration of E. hirta leaves/whole plant increases the platelet counts [20–22]. E. hirta has significantly increased the platelet counts in rats treated with ethanol to induce thrombocytopenia [21]. Intraperitoneal injection of ethanol may cause portal hypertension and hypersplenism leading to thrombocytopenia. Hence, the platelet increment by the E. hirta is allied to replenishment of platelet from the spleen. Furthermore, reduction of bleeding time in treated rats, compared to that of control rats, indicates an increase of platelet counts [21]. This reduction in bleeding time by E. hirta would be advantageous in managing DHF with the severity of bleeding episodes. Further, this study established an alteration of clotting time by E. hirta in treated rats compared with the control rats. This indicates the potential of modulating the coagulation pathways. Collectively, the hemostatic potential of E. hirta was validated by this study and these properties are prudent in managing dengue infection.
Coloma et al. [20] reported significant platelet augmentation in thrombocytopenic rabbits by E. hirta following 24 hours of treatment. However, this comparative analysis revealed that the platelet augmentation potential of E. hirta was lower than Carica papaya leaf extract [20].
E. hirta was also effective in increasing platelet counts in dengue patients of 30–55 age group [23]. Importantly, in around 70% of patients, flu-like symptoms were markedly reduced following E. hirta treatment which was attributed to the anti-inflammatory properties of the plant [23, 29]. Hence, in-depth analysis of immunomodulatory properties is fortified to establish the potential E. hirta on the alleviation of clinical symptoms of dengue. Since this study has enrolled patients only during the initial 72 hours of illness, additional prospective cohort studies are warranted to establish the clinical utility of E. hirta [23].
The phytochemical analysis of E. hirta has demonstrated the presence of alkaloids, carbohydrates, glycosides, saponins, phytosterols, phenolic compounds, and flavonoids [21]. Phenolics and flavonoids are well-known compounds with platelet increasing potential [21]. Even though E. hirta has a small amount of phenolic compounds compared to the other plants used for dengue (e.g., Carica papaya), it may be sufficient to exert an effect promoting quality and quantity of platelets. If the phenolic compounds contain sufficient hydroxyls and another group (such as carboxyl) to form strong complexes-forming properties of tannins, then that could exert a positive effect in the platelet count in blood [20]. Moreover, flavonoids can improve megakaryocytes to produce sufficient numbers of platelets and to modulate the platelet counts [20]. In addition, it was assumed that the anti-inflammatory potential of flavonoids may have contributed to the alleviation of flu-like symptoms in dengue patients following the treatment with E. hirta [23].
Although C. papaya is more effective in platelet increasing activity, the antidengue potential of the plant is less established. C. papaya has exhibited moderate or low inhibitory action against DENV2 growth in in vitro conditions [30]. Conversely, the ethanol extract of E. hirta under in vitro condition demonstrated an 85% inhibition of plaque forming capacity of dengue virus serotype 1 (DENV-1) [24] and 34.7% inhibition of virus serotype 2 (DENV-2) [25]. Further purification of ethyl acetate fractions of the plant revealed 9 compounds which may exert an antidengue effect individually or synergistically [24]. The molecular docking studies using these compounds, the antidengue activity of E. hirta could be attributed to quercetin as it showed a stronger affinity towards the dengue protease (2FOM) and dengue methyl transferase (2P40) [26]. However, antidengue/antiviral assays should be performed on these pure compounds to validate these theoretical investigations.
According to the present survey, the existing evidence supports the platelet increasing and antiviral activity of E. hirta extracts. However, these studies are inadequate to establish a clinical use of the plant. Particularly, the mechanisms of platelet increasing activity have to be identified. Further, the effect of E. hirta on the vascular leakage, which is the main pathological indicator of severe dengue [1], may be corroborated to justify its utility in severe dengue conditions.
Herbal medicines have a prudent use in dengue as a supportive therapy as effective antiviral drugs and vaccines for all stereotypes have not yet been developed. There are several commercial herbal formulations including C. papaya leaf capsule and Tawa-Tawa capsule in the market as over-the-counter drugs for dengue. However, it is important that these preparations are properly standardized and scientifically validated [31]. Though some studies have established that E. hirta is less toxic in murine models [32], it is essential to investigate its toxicity in the human context [33].
5. Conclusions
According to the present survey, it is reiterated that the E. hirta is a potential therapy against dengue as it holds a significant antiviral and platelet increasing activities. However, relatively few studies have been carried out to accept the clinical application of E. hirta as an antidengue therapy. To overly validate the traditional claim, more studies on contemporary pharmacological approaches including isolation of active compounds and elucidating the mode of action of antidengue activities are warranted. Further, well-controlled double-blind clinical trials are required to reevaluate the efficacious and side effects in order to establish its place in clinical applications.
Acknowledgments
Sincere thanks are due to Dr. Rafael A. Espiritu, Department of Chemistry, De La Salle University, Manila, Philippines, for critically reading the manuscript and suggesting substantial improvements.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Gubler D. J. Dengue and dengue hemorrhagic fever. Clinical Microbiology Reviews. 1998;11(3):480–496. doi: 10.1128/cmr.11.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Dengue and severe dengue. Fact Sheet No. 117. 2017
- 3.Stanaway J. D., Shepard D. S., Undurraga E. A., et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. The Lancet Infectious Diseases. 2016;16(6):712–723. doi: 10.1016/s1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shepard D. S., Undurraga E. A., Halasa Y. A. Economic and Disease Burden of Dengue in Southeast Asia. PLOS Neglected Tropical Diseases. 2013;7(2) doi: 10.1371/journal.pntd.0002055.e2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martina B. E. E., Koraka P., Osterhaus A. D. M. E. Dengue virus pathogenesis: an integrated view. Clinical Microbiology Reviews. 2009;22(4):564–581. doi: 10.1128/CMR.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guha-Sapir D., Schimmer B. Dengue fever: new paradigms for a changing epidemiology. Emerging Themes in Epidemiology. 2005;2, article 1 doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abd Kadir S. L., Yaakob H., Mohamed Zulkifli R. Potential anti-dengue medicinal plants: A review. Journal of Natural Medicines. 2013;67(4):677–689. doi: 10.1007/s11418-013-0767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NaTHNaC. Dengue Fever. Health Protection Agency. Natural Travel Health Network and Centre; 2009. Dengue Fever. Health Protection Agency. Natural Travel Health Network and Centre. [Google Scholar]
- 9.De Castro R. A. C., De Castro J.-A. A., Barez M. Y. C., Frias M. V., Dixit J., Genereux M. Thrombocytopenia associated with dengue hemorrhagic fever responds to intravenous administration of anti-D (RH0-D) immune globulin. The American Journal of Tropical Medicine and Hygiene. 2007;76(4):737–742. [PubMed] [Google Scholar]
- 10.Agnandji S. T. Malaria vaccine: WHO position paper-January. The New England Journal of Medicine. 2011;365(20):1863–1875. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 11.Gammulle A., Ratnasooriya W. D., Jayakody J. R. A. C., Fernando C., Kanatiwela C., Udagama P. V. Thrombocytosis and anti-inflammatory properties and toxicological evaluation of Carica papaya mature leaf concentrate in a murine model. International Journal of Medicinal Plants Research. 2012;1(2):21–30. [Google Scholar]
- 12.Patil S., Shetty S., Bhide R., Narayanan S. Evaluation of platelet augmentation activity of Carica papaya leaf aqueous extract in rats. Journal of Pharmacognosy and Phytochemistry. 2013;1(5) [Google Scholar]
- 13.Subenthiran S., Choon T. C., Cheong K. C., et al. Carica papaya leaves juice significantly accelerates the rate of increase in platelet count among patients with dengue fever and dengue haemorrhagic fever. Evidence-Based Complementary and Alternative Medicine. 2013;2013:7. doi: 10.1155/2013/616737.616737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jayasinghe C. D., Gunasekera D. S., De Silva N., Jayawardena K. K. M., Udagama P. V. Mature leaf concentrate of Sri Lankan wild type Carica papaya Linn. modulates nonfunctional and functional immune responses of rats. BMC Complementary and Alternative Medicine. 2017;17(1, article no. 230) doi: 10.1186/s12906-017-1742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranasinghe P., Ranasinghe P., Kaushalya M. Abeysekera W. P., et al. In vitro erythrocyte membrane stabilization properties of Carica papaya L. leaf extracts. Pharmacognosy Research. 2012;4(4):196–202. doi: 10.4103/0974-8490.102261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar S., Malhotra R., Kumar D. Euphorbia hirta: Its chemistry, traditional and medicinal uses, and pharmacological activities. Pharmacognosy Reviews. 2010;4(7):58–61. doi: 10.4103/0973-7847.65327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. http://malaya.com.ph/business-news/living/tawa-tawa-dengue-cure-capsule.
- 18.Moher D., Liberati A., Tetzlaff J., Altman D. G., Prisma Group Preferred reporting items for systematic reviews and meta-analyses. the PRISMA statement. medicine. 2009;6(7)e1000097 [PMC free article] [PubMed] [Google Scholar]
- 19.de Guzman G. Q., Dacanay A. T. L., Andaya B. A., Alejandro G. J. D. Ethnopharmacological studies on the uses of Euphorbia hirta in the treatment of dengue in selected indigenous communities in pangasinan (Philippines) Journal of Intercultural Ethnopharmacology. 2016;5(3):239–243. doi: 10.5455/jice.20160330124637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coloma A. J. L., Casilla A. S. B., Estolero B. H. J. M., Ulalan M. G. E., Veloso G. D., MAN R. Thrombocytotic Efficacy of Tawa-Tawa, Papaya and MalunggayAmong Aspirin-Induced Laboratory Rabbits. ICHAMS Health Care Journal. 5(1):8–16. [Google Scholar]
- 21.Apostol J. G., Gan J. V., Raynes R. J., Sabado A. A., Carigma A. Q., Santiago L. A. Platelet-increasing effects of Euphorbia hirta Linn. (Euphorbiaceae) in ethanol-induced thrombocytopenic rat models. International Journal of Pharmaceutical Frontier Research. 2012;2:1–11. [Google Scholar]
- 22.Arollado E. C., Pena I. G., Dahilig V. R. Platelet augmentation activity of selected Philippine plants. International Journal of Pharmaceutical Frontier Research. 2013:121–123. [Google Scholar]
- 23.Mir M., Khurshid R., Aftab R. Management of thrombocytopenia and flu-like symptoms in dengue patients with herbal water of Euphorbia hirta. Journal of Ayub Medical College. 2012;24(3-4):6–9. [PubMed] [Google Scholar]
- 24.Tayone W. C., Tayone J. C., Hashimoto M. Isolation and structure elucidation of potential Anti-Dengue metabolites from Tawa-Tawa (Euphorbia hirta Linn.) Walailak Journal of Science and Technology. 2014;11(10):825–832. [Google Scholar]
- 25.Saptawati L., Febrinasari R. P., Yudhani R. D., et al. In vitro study of eight Indonesian plants extracts as anti Dengue virus. Health Science Journal of Indonesia. 2017;8(1) [Google Scholar]
- 26.Siva Ganesh M., Awasthi P., Timiri A. K., Ghosh M. A novel approach for rationale selection of medicinal plants against viruses via molecular docking studies.
- 27.Hussain K., Nisar M. F., Majeed A., Nawaz K., Bhatti K. H. Ethnomedicinal survey for important plants of Jalalpur Jattan, district Gujrat, Punjab, Pakistan. Ethnobotanical Leaflets. 2010;7(11) [Google Scholar]
- 28.Ediriweera E. R. H. S. S. A Review on Medicinal uses of Weeds in Sri Lanka. Tropical Agricultural Research and Extension. 2010;10:11–16. doi: 10.4038/tare.v10i0.1865. [DOI] [Google Scholar]
- 29.Lanhers M.-C., Fleurentin J., Dorfman P., Mortier F., Pelt J.-M. Analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta. Planta Medica. 1991;57(3):225–231. doi: 10.1055/s-2006-960079. [DOI] [PubMed] [Google Scholar]
- 30.Joseph B., Sankarganesh P., Ichiyama K., Yamamoto N. In vitro study on cytotoxic effect and anti-DENV2 activity of Carica papaya L. leaf. Frontiers in Life Science. 2015;8(1):18–22. doi: 10.1080/21553769.2014.924080. [DOI] [Google Scholar]
- 31.Choudhary N., Sekhon B. S. An overview of advances in the standardization of herbal drugs. Journal of Pharmaceutical Education and Research. 2011;2(2):p. 55. [Google Scholar]
- 32.Yuet Ping K., Darah I., Chen Y., Sreeramanan S., Sasidharan S. Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. BioMed Research International. 2013;2013:14. doi: 10.1155/2013/182064.182064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Mel Y., Perera S., Ratnaweera P. B., Jayasinghe C. D. Novel insights of toxicological evaluation of herbal medicine: Human based toxicological assays. Asian Journal of Pharmacy and Pharmacology. 2017;3(2):41–49. [Google Scholar]