Abstract
Background
Chlamydia psittaci is a gram-negative, obligate intracellular organism. Birds are the main reservoir, but also non-avian domestic animals and humans can be infected. In humans it mostly causes respiratory infections due to occupational exposure with varying severity. Sensitive and specific diagnostic tests are needed to define psittacosis in humans as these tests also allow rapid tracing of the animal source. However, diagnosis in humans is often based on time-consuming culture techniques and antibody detection assays as in many countries, the existing molecular diagnostic tests for psittacosis are not reimbursed by the public health insurance.
Case presentation
An 82-year old female was referred to the hospital with a non-productive cough since four weeks and since one week fever up to 39 °C, myalgia, generalized skin rash, acral edema and generalized weakness under treatment with moxifloxacin. Blood analysis showed signs of inflammation with mild eosinophilia. Chest CT showed multiple peripheral ground glass opacities with consolidation in both lungs. Pulmonary function testing only showed a mild decrease in diffusion capacity. Viral and bacterial serology were negative. As the patient kept a pet parakeet for over ten years, a nested PCR for C. psittaci was performed on a nasopharyngeal swab of the patient and on feces of the parakeet. Both returned positive for the same genotype. Genotyping was performed by a genotype-specific real-time PCR. The patient fully recovered after a ten-day course of azithromycin.
Conclusion
Due to non-specific signs during psittacosis, early detection of the infection and differentiation from hypersensitivity pneumonitis can be challenging. Culture and antibody titers for C. psittaci have a lower sensitivity than PCR-testing due to several factors. We present a case of human psittacosis (presenting as pneumonia) with diagnosis based on clinical findings confirmed by means of nested PCR. This case suggests the added value of PCR in suspect cases despite negative serology. Our current paper underlines the need for a broader implementation of PCR for early diagnosis of human psittacosis and thus early initiation of correct antibiotic treatment with reduction of morbidity and mortality.
Keywords: Chlamydia psittaci, Psittacosis, Atypical pneumonia, Polymerase chain reaction
List of abbreviations
- CRP
C-reactive protein
- CT
computed tomography
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- DRESS
drug reaction with eosinophilia and systemic symptoms
- PCR
polymerase chain reaction
- ompA
outer membrane protein A
- MOMP
major outer membrane protein
1. Case description
An 82-year old female patient was referred to the hospital with the following symptoms that had lasted for a week: fever up to 39 °C, myalgia, a generalized skin rash, edema of the hands and feet and generalized weakness. These symptoms were preceded by bronchitis with non-productive cough that developed 4 weeks before, for which she had been treated with oral amoxicillin during 1 week. At the time of admission she was taking oral moxifloxacin.
There were no other people in her environment with similar complaints. Her medical history included a cholecystectomy, a hysterectomy per vaginam, and a pyelonephritis. There were no specific familial antecedents. There was no history of nicotine, alcohol or drug abuse. There were no known allergies. She had been keeping a small parakeet (Pyrrhura molinea molinea) as a pet for over 10 years. The bird did not show any signs of illness, nor had he been ill in the past.
2. Clinical examination
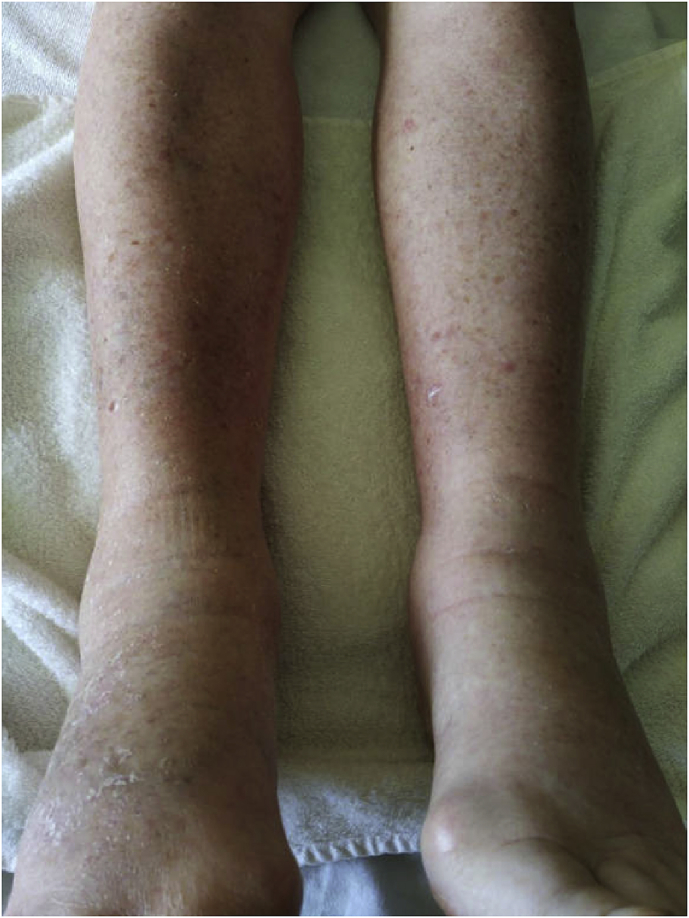
On admission, the patient had a systolic blood pressure of 140 and a diastolic blood pressure of 70 millimeter of mercury, a heart rate of 77 beats per minute, an axillar temperature of 37.8 °C and a blood oxygen level of 96% on pulse oximetry. General inspection showed generalized erythema and diffuse edema, most notably on the limbs and trunk (Fig. 1). Fine inspiratory crackles could be heard across both lower lung fields. Further clinical examination showed no abnormalities.
Fig. 1.
Edema of the lower limbs with diffuse maculopapular rash.
3. Technical investigations
Blood analysis showed an elevated C-reactive protein (CRP) (166.7 mg/L) and a sedimentation rate of 57 mm/h. There was limited leukocytosis (10300/μL) and an elevated eosinophilic count (309/μL). Liver enzymes were elevated, with an SGOT of 69 U/L, an SGPT of 88 U/L, a gamma-GT of 115 U/L and alkaline phosphatase of 414 U/L. Bilirubin levels were normal. Auto-immune serology including rheumatoid factor, anti-Cyclic Citrullinated Peptide antibodies, Anti-Nuclear Factor and Antineutrophil Cytoplasmic Antibodies were negative. Viral serology including Cytomegalovirus, measles, Influenza A and B, Varicella zoster, Adenovirus, Herpes simplex and Enteroviridae was negative. Bacterial serology including Mycoplasma pneumoniae and Chlamydia pneumoniae returned negative as well (Table 1).
Table 1.
Routine serology results showing absence of acute humoral response to viral or atypical respiratory pathogens.
| IgG | IgM | |
|---|---|---|
| Viral Serology | ||
| CMV | − | − |
| Measles | + | − |
| Adenovirus | + | − |
| Influenza A | − | − |
| Influenza B | + | − |
| Varicella Zoster | + (612 IU/mL) | − |
| Herpes Simplex | + | − |
| Epstein Barr | + (105 U/mL) | − |
| Enterovirus | − | − |
| Bacterial Serology | ||
| Mycoplasma pneumoniae | − | − |
| Chlamydia pneumoniae | − | − |
| Coxiella burnetti | − | − |
| Chlamydia psittaci | − | − |
Chest X-ray revealed a diminished transparency of the perihilar lung tissue, and on the profile incidence an ill-defined pneumonic infiltrate could be discerned at the posterobasal region (Fig. 2).
Fig. 2.
Chest X-ray, profile incidence, at first presentation showing patchy retrocardial infiltrate.
Computed Tomography (CT) scan of the chest was performed, which clearly showed multiple peripheral ground-glass opacities with consolidation in both lungs (Fig. 3). Pulmonary function tests showed a normal Forced Vital Capacity (FVC), FEV1 (Forced Expiratory Volume in 1 second) and FEV1/FVC (Tiffeneau Index) and a mild decrease in diffusion capacity (77% of expected value). Transthoracic cardiac echography and abdominal echography revealed no abnormalities. A Doppler ultrasound of the lower limbs showed only diffuse subcutaneous edema, no signs of deep venous thrombosis.
Fig. 3.
Computed tomography (CT) scan after one week of treatment with a beta-lactam antibiotic.
Urine culture, sputum culture and repeated aerobic and anaerobic blood cultures revealed no specific pathogen. Legionella antigen testing on urine was negative.
4. Differential diagnosis
In summary, this patient was admitted to the hospital with a recent history of non-productive cough progressing into a generalized condition with fever, myalgia, diffuse rash and edema of the limbs. Imaging showed diffuse patchy infiltrates on both lungs. Blood sampling revealed CRP elevation and mild eosinophilia, while routine virological, bacteriological and auto-immune serology were negative. The following entities were considered in the differential diagnosis:
4.1. Atypical pneumonia
The term 'atypical pneumonia' originates from descriptions in the early part of the last century of a community-acquired pneumonia syndrome distinct from the typical features of acute illness with fever and mucopurulent sputum. It is characterized traditionally by initial systemic complaints, relatively mild respiratory symptoms and scant sputum production. Progression to an illness of varying severity with possible extrapulmonary involvement and unresponsiveness to penicillin can occur. Among atypical pathogens, Mycoplasma pneumoniae, Legionella spp., Chlamydia pneumoniae, Chlamydia psittaci and Coxiella burnetii are considered key pathogens in this concept [1].
4.2. Hypersensitivity pneumonitis
Hypersensitivity pneumonitis, also called extrinsic allergic alveolitis, is a respiratory condition involving the lung parenchyma and more specifically the alveoli, terminal bronchioli and alveolar interstitium. The underlying cause is a delayed allergic reaction secondary to repeated and prolonged inhalation of organic dust or other substances. Hypersensitivity pneumonitis can be divided in an acute, subacute and chronic type, depending on the intensity and the frequency of exposure to the causative antigen [2]. With respect to our patient, exposure to organic dust is documented in the form of avian feces-derived dust. She was keeping a pet parakeet for over 10 years and regularly cleaning the bird's cage, which would predispose her to immune sensitization and the development of hypersensitivity pneumonitis. Her symptoms, including fever and myalgia, are compatible with the acute/subacute manifestation of hypersensitivity pneumonitis [2]. CT-graphic characteristics of an acute/subacute hypersensitivity pneumonitis include patchy or diffuse ground-glass opacities, lobular areas of decreased attenuation or air-trapping and small centrilobular nodules. Chest CT of our patient shows a similar image. In contrast, chronic hypersensitivity pneumonitis is characterized by the development of fibrotic changes with thickening of the interlobular septa, intralobular reticulation and traction bronchiectasis [2].
4.3. Paraneoplastic syndrome
Paraneoplastic syndromes include specific dermatologic and rheumatologic manifestations such as erythroderma and dermatomyositis. Dermatomyositis is an inflammatory myopathy associated with multiple skin changes which occur before the onset of proximal muscle weakness. Possible skin eruptions are a heliotrope rash on the upper eyelids, an erythematous rash on the face, neck, back, chest and shoulders and Gottron papules (small purple or red flat papules on extensor surfaces, particularly present on the elbows and joints of the hand). 10–25% of all cases of dermatomyositis are paraneoplastic. Biochemical changes include high inflammatory markers and an elevated level of creatine phosphokinase. Patients with erythroderma show an erythematous and exfoliating diffuse rash, which is often pruritic. The diagnosis can be confirmed by performing a skin biopsy [3,4].
Our patient showed a distal muscle weakness, most prominently localized in the lower limbs. She also showed a skin rash which was limited to the upper and lower limbs and some parts of the trunk. There was no pruritus. Laboratory tests did not show an elevated creatinine phosphokinase. However, the lung parenchymal changes CT are not suggestive for an underlying malignancy.
4.4. DRESS-syndrome (drug reaction with eosinophilia and systemic symptoms)
DRESS-syndrome is a severe adverse drug induced reaction which is potentially life threatening. Symptoms include a severe skin eruption, fever, hematological abnormalities (eosinophilia or atypical lymphocytosis) and internal organ involvement. Symptoms typically occur 2–6 weeks after the initiation of the drug therapy and persistence or aggravation of the symptoms despite discontinuation of the culprit drug is possible. The diagnosis of DRESS is challenging because of the diversity of the clinical manifestations concerning skin eruption and the various internal organs that can be involved. Many drugs can be responsible for the development of a DRESS-syndrome, including amoxicillin [5,6]. Our patient had been taking amoxicillin during one week when symptoms appeared, including skin rash, fever and eosinophilia. Pulmonary involvement however is not very common in classical DRESS syndrome. The timeframe was not very suggestive as the pulmonary symptoms, presumably related to the radiological anomalies, preceded the start of amoxicillin.
In our patient, imaging was compatible with an atypical infectious pneumonia, yet serology for M. pneumoniae, C. pneumoniae and viral pathogens was negative. Since there was a positive history of contact with psittacine birds, in this case her pet parakeet, further testing for C. psittaci was performed on a nasopharyngeal swab. A nested polymerase chain reaction (PCR) assay [7] specific for C. psittaci turned out positive. The PCR detects the outer membrane protein A (ompA) gene of C. psittaci. The ompA gene encodes for the major outer membrane protein (MOMP) of C. psittaci. Additionally, the nested PCR assay performed on the parakeet's feces was positive as well for C. psittaci. Serological testing in patient serum however was negative, which can be due to several factors, as discussed further. Molecular characterization of C. psittaci by an ompA genotyping real-time PCR for C. psittaci [8], revealed the presence of genotype A in both the patient and the pet bird.
5. Treatment and clinical outcome
The therapy with moxifloxacin was continued after admission to our ward. However, as clinical improvement was lacking, and given the systemic manifestations with eosinophilia as sign of generalized immune hyperactivation, a decision was made to add oral glucocorticosteroids (methylprednisolone 32 mg with progressive tapering). After one day a significant positive effect on the fatigue and myalgia was noticed. As soon as the results of the PCR came in a few days later, showing an infection with C. psittaci, a treatment with azithromycin during ten days was given afterwards to prevent relapse. The patient recovered completely from this episode with normalization of laboratory value and diffusion capacity upon outpatient evaluation 4 weeks after hospital discharge.
The parakeet was taken on by new owners and was treated with doxycycline drops in its drinking water during four weeks. Afterwards a new fecal swab was obtained and nested PCR was performed which was negative for C. psittaci.
6. Background on Chlamydia psittaci pneumonia
6.1. Microbiological features
All micro-organisms in the family of the Chlamydiaceae are gram-negative, obligate intracellular organisms. This family currently includes one genus – Chlamydia – which contains a total of eleven species [9]. Originally, C. psittaci comprised nine ompA genotypes designated A to F, E/B, M56, and WC [10]. Later on, six additional genotypes found in psittacines and wild birds and designated 1V, 6N, Mat116, R54, YP84 and CPX0308 were proposed [11]. These pathogens have adapted to a broad range of hosts, including mammals and avian species. They are characterized by a biphasic life cycle, consisting of a metabolically inactive, infectious form and a metabolically active, non-infectious form, respectively called the elementary body and the reticulate body. To characterize the different genotypes within the avian C. psittaci species, an analysis of the MOMP encoding outer membrane protein A gene (ompA) is performed. These genotypes are relatively host specific. Genotypes A and B are most often found in psittacine birds and pigeons, respectively, genotype C in ducks and geese and D in turkeys. Genotype F has been found in both psittacine birds and turkeys, and genotype E has been found in a wide variety of hosts including pigeons, ratites, ducks, turkeys and occasionally humans. Genotype E/B has been found most frequently in ducks and the WC and M56 genotypes have been isolated from cattle and muskrats. All these genotypes can be transmitted to humans and could potentially cause severe disease [[12], [13], [14], [15], [16]].
6.2. Epidemiology and transmission
Most cases of psittacosis are sporadic occupational infections, most commonly seen in young and middle-aged adults. Local outbreaks occur from time to time. In most countries cases of human psittacosis must be reported. Unfortunately figures of these reports probably vastly underestimate the real burden of this disease as not all infections present as pneumonia and remain undetected. In Belgium (comparable to other European countries), for instance, only around ten human cases are reported each year, despite a very high prevalence in poultry [12]. The disease can be found in avian species –which are the primary reservoir- but also in non-avian domestic animals and humans. As already mentioned, C. psittaci is classified in different genotypes which display some preference for certain host species [14,17]. C. psittaci infections can occur in many different bird species, spanning 30 different bird orders. Psittacidae (which include parakeets, parrots, cockatoos and lories), and Columbiformes (pigeons) seem to be most commonly affected [[17], [18], [19]].
The disease manifestations in infected birds can vary from asymptomatic shedding of micro-organisms to pneumo-enteritis. Latency of the infection with a possible reactivation in situations of stress is described [17]. The organism is excreted in feces and nasal discharges of infected birds and the organism can remain infectious in the environment for months. Transmission from birds to humans most commonly occurs through inhalation of contaminated particles. Mouth-to-beak contact or handling of infected birds' plumage or tissues can also lead to infection [17,19,20].
6.3. Typical clinical presentation
Infection with Chamydia psittaci in humans mainly causes respiratory infection. Clinical symptoms can be highly variable with involvement of different organs. The incubation period usually varies between 5 and 14 days but exceptions involving a longer incubation time have been described. The most frequently reported clinical symptoms are high fever, chills, malaise, headache, myalgia, non-productive cough and respiratory distress [12,14]. Gastro-intestinal symptoms or rash can also be seen. Rare complications include myocarditis, encephalitis, hepatitis, keratoconjunctivitis, acute respiratory distress syndrome and multiple organ failure, and C. psittaci could possibly be linked to the development of ocular lymphoma [12,14]. With the modern availability of antibiotics, lethal cases of human psittacosis have become extremely infrequent. However, severe or life-threatening disease can occur when the initial signs of psittacosis are not recognized and treatment is delayed [14]. Hematological leukopenia grade 1–2 can occur during the acute phase of the disease. Augmented CRP and liver enzyme levels in psittacosis patients seem to correlate with the severity of the infection. In most of hospitalized patients, chest X-ray shows abnormalities. Unilateral lower lobe consolidation is the most common but bilateral consolidates, multiple nodular infiltrates or miliary spreading can be found as well [21].
6.4. Diagnosis
Any positive history of contact with birds and a suggestive clinical presentation should rise suspicion for an infection with C. psittaci. A case of human psittacosis can be confirmed if the clinical findings are in concordance with psittacosis and a laboratory confirmation [12]. In the past, a laboratory confirmation was said to be obtained by at least one of the following methods: (i) isolation of the causative agent from respiratory secretions, (ii) four-fold or more increase of antibody titer between paired sera by complement fixation test or the more sensitive micro-immunofluorescence test or (iii) detection of IgM antibodies against C. psittaci using a micro-immunofluorescence test to a reciprocal titer of 16 or more. However, negative serology in hospitalized psittacosis patients is not uncommon. The current serological tests are less sensitive than PCR. Moreover serology can be negatively influenced by antibiotic use and by genetic variations in Toll-like receptors and nucleotide oligomerization domain-like receptors, leading to inadequate recognition of Chlamydia by the host immune system [22]. However, in most countries, human psittacosis is a notifiable disease. In case of a notifiable disease, specific and rapid diagnosis in humans is required in addition to source tracing to prevent further spreading of the infection. This is the reason why in the past decade, diagnostic C. psittaci PCR assays were developed and are currently being introduced in the routine clinical setting. PCR is not only more sensitive than culture and serology, it also allows genotyping, which assists in source tracing as C. psittaci is currently divided into genotypes (A-F, E/B, M56 and WC), all more or less associated with a preferred avian host [13].
6.5. Therapeutic approach and prognosis
Human cases of psittacosis are preferably treated with tetracyclines, including doxycycline or tetracycline hydrochloride. This treatment must be continued during a period of at least 10–14 days in order to be effective and to prevent relapse. When treatment with tetracyclines is contra-indicated, macrolides are probably the best alternative [12,19]. Quinolones may also be a treatment alternative, but they are less active against C. psittaci than tetracyclines and macrolides [17,19,23].
As for the avian reservoir, treatment or extermination is recommended to prevent further spreading. Isolation in a clean cage with proper husbandry is recommended and doxycycline is the antibiotic of choice. There is no clear evidence of necessary length of treatment, but 21–30 days should suffice. In relation to potential outbreaks, retesting with PCR is recommended after four weeks [19].
7. Conclusion
Due to the non-specific clinical signs that occur during an infection with C. psittaci, early identification of the disease can be challenging. However, a history of frequent contact with psittacine bird species or pigeons, along with suggestive symptoms, should trigger additional diagnostics for psittacosis in order to launch treatment in humans and contact bird(s) as soon as possible. In the context of exposure to bird droppings, differential diagnosis with hypersensitivity pneumonitis can be difficult. Our case stresses the added value of PCR and especially of the genotype-specific real-time PCR on a human respiratory sample as this test led us to the correct diagnosis, the correct treatment modality and the infection source, being the pet bird. In many countries, the existing molecular diagnostic tests for this entity are not yet reimbursed by the public health insurance. We conclude that PCR-based testing in suspect cases is valuable for early diagnosis and early initiation of correct antibiotic treatment with reduction of morbidity and mortality and should me more broadly implemented.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
We are thankful to the patient and her family for allowing us to report this clinical case.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.rmcr.2018.01.010.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Basarab M., Macrae M.B., Curtis C.M. Atypical pneumonia. Curr. Opin. Pulm. Med. 2014;20:247–251. doi: 10.1097/MCP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 2.Riario Sforza G.G., Marinou A. Hypersensitivity pneumonitis: a complex lung disease. Clin. Mol. Allergy. 2017;15:6. doi: 10.1186/s12948-017-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelosof L.C., Gerber D.E. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin. Proc. 2010;85:838–854. doi: 10.4065/mcp.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricceri F., Prignano F. Gottron papules: a pathognomonic sign of dermatomyositis. Cmaj. 2013;185:148. doi: 10.1503/cmaj.111791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cacoub P., Musette P., Descamps V., Meyer O., Speirs C., Finzi L., Roujeau J.C. The DRESS syndrome: a literature review. Am. J. Med. 2011;124:588–597. doi: 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Roberts J.R., Rhiannon J.J. Amoxicillin-induced DRESS syndrome. SGIM Forum. 2013;36:12–13. [Google Scholar]
- 7.Van Loock M., Verminnen K., Messmer T.O., Volckaert G., Goddeeris B.M., Vanrompay D. Use of a nested PCR-enzyme immunoassay with an internal control to detect Chlamydophila psittaciin turkeys. BMC Infect. Dis. 2005;5:76. doi: 10.1186/1471-2334-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geens T., Dewitte A., Boon N., Vanrompay D. Development of a Chlamydophila psittaci species-specific and genotype-specific real-time PCR. Vet. Res. 2005;36:787–797. doi: 10.1051/vetres:2005035. [DOI] [PubMed] [Google Scholar]
- 9.Sachse K., Bavoil P.M., Kaltenboeck B., Stephens R.S., Kuo C.C., Rosselló-Móra R., Horn M. Emendation of the family Chlamydiaceae: proposal of a single genus, Chlamydia, to include all currently recognized species. Syst. Appl. Microbiol. 2015;38:99–103. doi: 10.1016/j.syapm.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Harkinezhad T., Verminnen K., De Buyzere M., Rietzschel E., Bekaert S., Vanrompay D. Prevalence of Chlamydophila psittaci infections in a human population in contact with domestic and companion birds. J. Med. Microbiol. 2009;58:1207–1212. doi: 10.1099/jmm.0.011379-0. [DOI] [PubMed] [Google Scholar]
- 11.Sachse K., Laroucau K., Hotzel H., Schubert E., Ehricht R., Slickers P. Genotyping of Chlamydophila psittaci using a new DNA microarray assay based on sequence analysis of ompA genes. BMC Microbiol. 2008;8:1–12. doi: 10.1186/1471-2180-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beeckman D., Vanrompay D. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin. Microbiol. Infect. 2009;15:11–17. doi: 10.1111/j.1469-0691.2008.02669.x. [DOI] [PubMed] [Google Scholar]
- 13.Heddema E.R., Van Hannen E.J., Bongaerts M., Dijkstra F., Ten Hove R.J., De Wever B., Vanrompay D. Typing of chlamydia psittaci to monitor epidemiology of psittacosis and aid disease control in The Netherlands, 2008 to 2013. Eurosurveillance. 2015;20:1–8. doi: 10.2807/1560-7917.es2015.20.5.21026. [DOI] [PubMed] [Google Scholar]
- 14.Radomski N., Einenkel R., Müller A., Knittler M.R. Chlamydia–host cell interaction not only from a bird's eye view: some lessons from Chlamydia psittaci. FEBS Lett. 2016;590:3920–3940. doi: 10.1002/1873-3468.12295. [DOI] [PubMed] [Google Scholar]
- 15.Bavoil P., Kaltenboeck B., Greub G. In Chlamydia veritas. Pathol. Discov. 2013;67:89–90. doi: 10.1111/2049-632X.12026. [DOI] [PubMed] [Google Scholar]
- 16.Carlier L., Kempf M., Aaziz R., Jolivet-Gougeon A., Laroucau K. A severe case of pneumopathy in a duck breeder due to Chlamydia psittaci diagnosed by 16S rDNA sequencing. JMM Case Rep. 2014;1:1–5. [Google Scholar]
- 17.Fraeyman A., Boel A., Van Vaerenbergh K., De Beenhouwer H. Atypical pneumonia due to Chlamydophila psittaci: 3 case reports and review of literature. Acta Clin. Belg. 2010;65:192–196. doi: 10.1179/acb.2010.040. [DOI] [PubMed] [Google Scholar]
- 18.Lagae S., Kalmar I., Laroucau K., Vorimore F., Vanrompay D. Emerging Chlamydia psittaci infections in chickens and examination of transmission to humans. J. Med. Microbiol. 2014;63:399–407. doi: 10.1099/jmm.0.064675-0. [DOI] [PubMed] [Google Scholar]
- 19.Balsamo G., Maxted A.M., Midla J.W., Murphy J.M., Wohrle R., Edling T.M., Fish P.H., Flammer K., Hyde D., Kutty P.K., Kobayashi M., Helm B., Oiulfstad B., Ritchie B.W., Stobierski M.G., Ehnert K., Tully T.N. Compendium of measures to control Chlamydia psittaci infection among humans (psittacosis) and pet birds (avian chlamydiosis), 2017. J. Avian Med. Surg. 2017;31:262–282. doi: 10.1647/217-265. [DOI] [PubMed] [Google Scholar]
- 20.Chau S., Tso E.Y.K., Leung W.S., Fung K.S.C. Three cases of atypical pneumonia caused by Chlamydophila psittaci. Hong Kong Med. J. 2015;21:272–275. doi: 10.12809/hkmj144321. [DOI] [PubMed] [Google Scholar]
- 21.Heddema E.R., Van Hannen E.J., Duim B., De Jongh B.M., Kaan J.A., Van Kessel R., Lumeij J.T., Visser C.E., Vandenbroucke-Grauls C.M.J.E. An outbreak of psittacosis due to Chlamydophila psittaci genotype A in a veterinary teaching hospital. J. Med. Microbiol. 2006;55:1571–1575. doi: 10.1099/jmm.0.46692-0. [DOI] [PubMed] [Google Scholar]
- 22.den Hartog J.E., Morré S.A., Land J.A. Chlamydia trachomatis-associated tubal factor subfertility: immunogenetic aspects and serological screening. Hum. Reprod. Update. 2006;12:719–730. doi: 10.1093/humupd/dml030. [DOI] [PubMed] [Google Scholar]
- 23.Butaye P., Ducatelle R., De Backer P., Vermeersch H., Remon J.P., Haesebrouck F. In vitro activities of doxycycline and enrofloxacin against European Chlamydia psittaci strains from turkeys. Antimicrob. Agents Chemother. 1997;41:2800–2801. doi: 10.1128/aac.41.12.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.