Abstract
Background
The impact of fungicide azoxystrobin, applied as foliar spray, on the physiological and biochemical indices and ginsenoside contents of ginseng was studied in ginseng (Panax ginseng Mey. cv. “Ermaya”) under natural environmental conditions. Different concentrations of 25% azoxystrobin SC (150 g a.i./ha and 225 g a.i./ha) on ginseng plants were sprayed three times, and the changes in physiological and biochemical indices and ginsenoside contents of ginseng leaves were tested.
Methods
Physiological and biochemical indices were measured using a spectrophotometer (Shimadzu UV-2450). Every index was determined three times per replication. Extracts of ginsenosides were analyzed by HPLC (Shimadzu LC20-AB) utilizing a GL-Wondasil C18 column.
Results
Chlorophyll and soluble protein contents were significantly (p = 0.05) increased compared with the control by the application of azoxystrobin. Additionally, activities of superoxide dismutase, catalase, ascorbate peroxidase, peroxidase, and ginsenoside contents in azoxystrobin-treated plants were improved, and malondialdehyde content and O2− contents were reduced effectively. Azoxystrobin treatments to ginseng plants at all growth stages suggested that the azoxystrobin-induced delay of senescence was due to an enhanced antioxidant enzyme activity protecting the plants from harmful active oxygen species. When the dose of azoxystrobin was 225 g a.i./ha, the effect was more significant.
Conclusion
This work suggested that azoxystrobin played a role in delaying senescence by changing physiological and biochemical indices and improving ginsenoside contents in ginseng leaves.
Keywords: azoxystrobin, ginsenoside contents, leaves, Panax ginseng, physiological and biochemical indices
1. Introduction
Azoxystrobin is the name of the compound methyl (E)-2-{2[6-(2-cyanophenoxy) pyrimidin-4-yloxy] phenyl}-3-methoxyacrylate [1]. Azoxystrobin retains the methyl β-methoxyacrylate group of the naturally occurring strobilurins, by inhibiting mitochondrial respiration by blocking the transfer of electrons in the mitochondrial b,c1 complex for mitochondrial electrons [2], [3]. Strobilurins are first discovered from wood-decaying Basidiomycete species [4]. Strobilurins have been proved good in yield increase and improving the quality of agricultural produce [5], [6]. In earlier studies, wheat treated with azoxystrobin showed significant increases in production [7], [8]. Enhanced postharvest, delayed senescence, and water conservation are some of the positive physiological changes of strobilurin treatment that have been reported [9], [10], [11], [12].
Ginseng (Panax ginseng Meyer) is a valuable medicinal plant that has been used extensively in many countries for more than 5,000 yr [13]. Ginseng is known for its ginsenosides that have ecological and pharmacological benefits [14]. A large number of ginsenosides are a major constituent found in different tissues of ginseng [15], [16]. Ginseng is generally harvested after a cultivation period of >4 yr [17], [18]. Studies have shown that the contents of these active components in ginseng increase every year during the fast-growth period and remain relatively stable during the slow-growth period [19], [20].
Peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD) are widely distributed enzymes in plants [21]. As we all know, leaf senescence is an important oxidative process [22]. Active oxygen is involved in biochemical and physiological changes during leaf senescence [23]. The enzymatic antioxidant system is a protective mechanism [24]. Nowadays, many research works have revealed the reaction pattern (alkaline stress, senescence, acid stress, and salt stress) [25], [26], [27], [28]. Strobilurin fungicides have been shown to have antioxidative properties [29]. Most of these investigations focused on the effect of azoxystrobin [30].
Azoxystrobin is a systemic fungicide. We investigated its impact on the physiological and biochemical indices, and ginsenoside contents of ginseng leaves.
2. Materials and methods
2.1. Plant material and azoxystrobin application
During the growing season of 2013, ginseng (Panax ginseng Mey. cv. “Ermaya”) was grown on Baishan Experimental Farmland [Experimental Farmland of Jilin Province in China (E126°18′, N41°42′; henceforth abbreviated as “BS”] and Huanren Experimental Farmland [Experimental Farmland of Liaoning Province in China (E124°47′, N41°32′); henceforth abbreviated as “HR”). When plants were at the phenological growth stage ([PGS]) 800/909 (all fruits green) [31], 5-yr-old ginseng plants were chosen and divided into three plots (each plot 15 m2 in size). At [PGS] 800/909 (all fruits green), 25% azoxystrobin SC plus water control were sprayed. The application equipment was a 3WBD-16 power sprayer. Air temperature at azoxystrobin SC application was 27.2–29.1°C, and the weather was sunny or cloudy. Wind velocity was 0.5–0.7 m/s. azoxystrobin SC (25%) was sprayed three times at 7 d intervals. The following azoxystrobin doses were used: 25% azoxystrobin SC 150 g a.i./ha and 225 g a.i./ha. The experiment was repeated in the same fields, but not in the same plots, in the growing season of 2014.
2.2. Sampling preparation
At [PGS] 801/909 (beginning of fruit reddening), [PGS] 805/909 (50% of fruit red), [PGS] 809/909 (fruit fully ripe), [PGS] 902/909 (beginning of leaf yellowing), and [PGS] 903/909 (most of the leaves yellowish and drooping), five samples of ginseng were taken from each plot every time. One part of leaves was frozen in liquid N2 and stored at −86°C in a refrigerator (cryogenic, DW-HL388) until extraction for enzyme analyses. The other part of leaves was used for chlorophyll (CHL), soluble protein (SP), malondialdehyde (MDA), superoxide radicals (O2−), hydrogen peroxide (H2O2), and ginsenoside content assays immediately.
2.3. Measurement of CHL and SP contents
CHL was extracted from leaf blade discs (3 per replication), by homogenizing in acetone and ethanol solution (1:1, v:v); absorbance of the leaf extract was measured at 664 nm [10] using a spectrophotometer (Shimadzu UV-2450). SP content was assayed by the method of Bradford [32] using bovine serum albumin as a standard and expressed as mg/g fresh weight (FW). Absorbance of the sample was measured using a spectrophotometer.
2.4. Measurement of H2O2, O2−, and MDA contents
H2O2 was assayed following the modified method of Bonasia et al [10]. The absorbance was immediately measured at 450 nm using a spectrophotometer. The O2– assay followed the modified method of Lu et al [33]. The absorbance was measured at 530 nm using a spectrophotometer. MDA content was measured by the thiobarbituric acid method (TBA) method and expressed as μmol/g FW [34].
2.5. Measurement of antioxidative enzyme activity
SOD activity was determined according to the modified method of Ferreira et al [35]. SOD activity was expressed as U/g FW, and measured at 560 nm by a spectrophotometer. POD activity was assayed by measuring the oxidation of guaiac-based phenol in the presence of H2O2 [36]. It was defined as an absorbance change of 0.01 in 1 min at 470 nm using a spectrophotometer. CAT activity was determined using the method of Zhang et al [7]. It was followed by measuring the absorbance change at 240 nm using a spectrophotometer. Ascorbate peroxidase (APX) activity was measured by the method of Rahman and Punja [37], which was measuring the absorbance change at 290 nm using a spectrophotometer. Every index was determined three times per replication.
2.6. Extraction and analysis of ginsenosides
Extraction of ginsenosides was based on the method described by Palazón et al [38]. Extracts of ginsenosides were analyzed by HPLC (Shimadzu LC20-AB) utilizing a GL-Wondasil C18 (250 × 4.6 mm × 5 μm) column. Ginsenosides Rb1 (98.5%), Re (98.5%), and Rg1 (98.5%) were purchased from Jilin University in China.
2.7. Statistical analysis
Statistical analysis was carried out with the analysis of statistically significant (SPSS for Windows, version 18.0) and means were separated with the least significant difference test at p = 0.05, to determine whether azoxystrobin had a significant effect on leaf senescence and ginseng ginsenoside contents [39].
3. Results
Similar results were recorded for each dose treatment or water control from the two experiments in 2013 and 2014. Statistical analysis revealed that there was no significant difference between the two sets of data (p = 0.05). Thus, data from the two experiments were combined.
3.1. Effect of azoxystrobin on CHL and SP contents
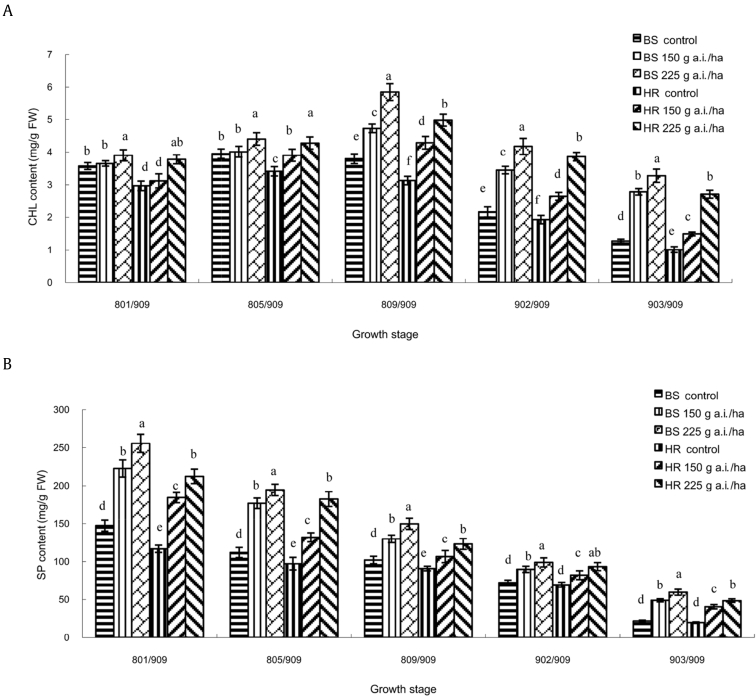
The overall levels of CHL content in ginseng leaves increased until [PGS] 809/909 and then declined with further aging of plants (Fig. 1A). It showed that CHL contents of azoxystrobin-treated plants were higher than those of the control plants at all growth stages in both BS and HR of 150 g a.i./ha and 225 g a.i./ha. On the 3rd day of [PGS] 809/909 (fruit fully ripe), CHL contents of 150 g a.i./ha azoxystrobin-treated plants were 1.26 times those of the control plants in BS and 1.47 times in HR, and CHL content of 225 g a.i./ha azoxystrobin-treated plants was 1.53 times higher than that of the control plants in BS and 1.63 times higher in HR. At [PGS] 903/909, CHL contents of 150 g a.i./ha azoxystrobin-treated plants were 2.2 times those of the control plants in BS and 1.5 times in HR, and CHL content of 225 g a.i./ha azoxystrobin-treated plants was 2.58 times higher than that of the control plants in BS and 2.68 times higher in HR. Thus, CHL content was higher with the application of 25% azoxystrobin SC 225 g a.i./ha than with 150 g a.i./ha, and it was higher in BS than in HR. Moreover, at [PGS] 903/909, CHL contents of azoxystrobin-treated plants were higher than those of the control plants at [PGS] 902/909 in both BS and HR. It suggested that azoxystrobin played a role in improving CHL contents in ginseng leaves; the leaves of ginseng treated with 225 g a.i./ha azoxystrobin reached 903/909 at 120 d after 800/909, while the control reached at 140 d.
Fig. 1.
Effect of azoxystrobin on the contents of (A) CHL and (B) SP in ginseng leaves. Data are mean ± SE of three replicates. Letters under x-axis refer to the difference at significance level p = 0.05 among different treatments. Data are not significantly different for values followed by the same letter. BS, Baishan Experimental Farmland; CHL, chlorophyll; FW, fresh weight; HR, Huanren Experimental Farmland; SP, soluble protein; SE, standard error.
The overall levels of SP content decreased steadily with the aging of ginseng leaves (Fig. 1B). SP content was significantly (p = 0.05) increased compared with the control on treatment with azoxystrobin 150 g a.i./ha and 225 g a.i./ha at all growth stages in both BS and HR. However, the enhancement in CHL content of ginseng leaves of azoxystrobin-treated plants started significantly earlier than the CHL content, from [PGS] 801/909. At all growth stages, SP content of azoxystrobin 225 g a.i./ha was significantly (p = 0.05) higher than that of 150 g a.i./ha in both BS and HR. It showed a similar result with CHL content that SP content was higher in BS than in HR.
3.2. Effect of azoxystrobin on MDA content and active oxygen species
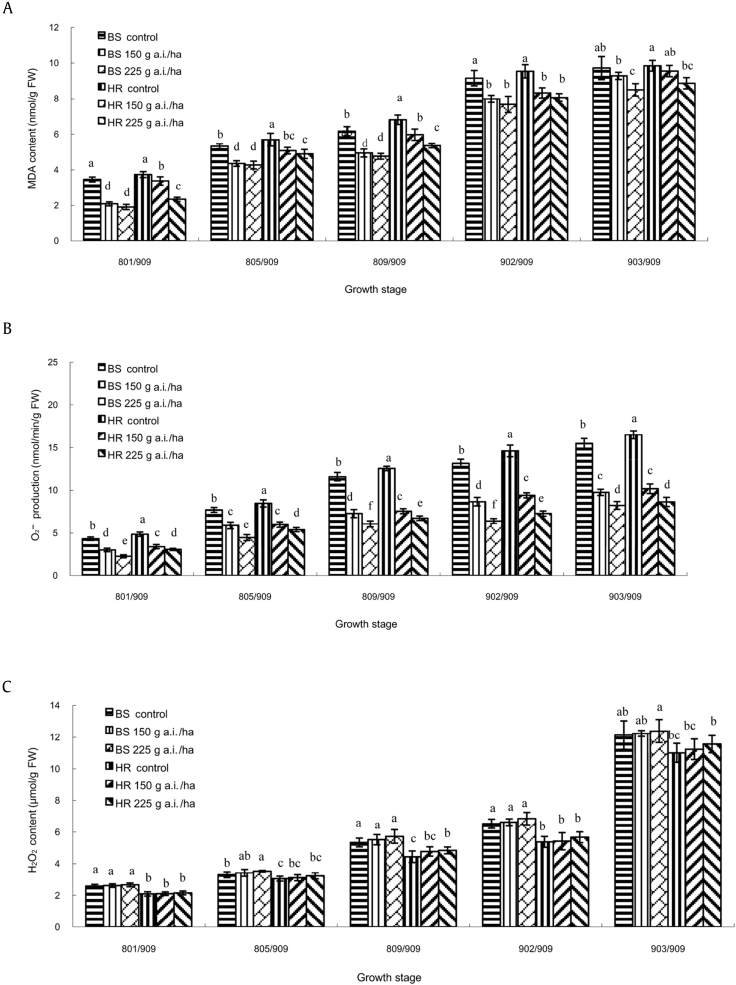
The MDA levels in ginseng leaves steadily increased with aging of plants with or without azoxystrobin treatments (Fig. 2A). Azoxystrobin-treated plants delayed the increase of MDA. Statistical analysis revealed that at [PGS] 903/909, MDA contents of azoxystrobin-treated plants were lower than those of the control plants at [PGS] 902/909 in both BS and HR. There was a significant (p = 0.05) decrease by the treatment of azoxystrobin 225 g a.i./ha at all growth stages in both BS and HR. Furthermore, application of 25% azoxystrobin SC 225 g a.i./ha was more effective than that of 150 g a.i./ha, and more effective in BS than in HR.
Fig. 2.
Effect of azoxystrobin on the contents of (A) MDA, (B) O2−, and (C) H2O2 in ginseng leaves. Data are mean ± SE of three replicates. Letters under x-axis refer to the difference at significance level p = 0.05 among different treatments. Data are not significantly different for values followed by the same letter. H2O2, hydrogen peroxide; FW, fresh weight; MDA, malondialdehyde; O2−, superoxide radicals; SE, standard error.
In parallel with MDA, O2− levels in the plants, with or without azoxystrobin treatments, steadily enhanced with the aging of the plants (Fig. 2B). Azoxystrobin-treated plants were significantly (p = 0.05) lower than control at all growth stages in both BS and HR of 150 and 225 g a.i./ha, and the dose of 225 g a.i./ha was more effective. Superoxide radical content at [PGS] 903/909 of 25% azoxystrobin SC 225 g a.i./ha and 150 g a.i./ha was less than that of water control plants at [PGS] 809/909, and nearly same as that of water control plants at [PGS] 809/909. The results strongly showed that azoxystrobin can effectively decrease O2− content.
The effect of azoxystrobin on H2O2 was less compared with water control (Fig. 2C). The overall levels of f H2O2 content almost kept increasing steadily until [PGS] 902/909 and strongly increased at [PGS] 903/909. In all five stages, H2O2 content was not significantly (p = 0.05) changed compared with the control by the application of azoxystrobin 150 g a.i./ha and 225 g a.i./ha in both BS and HR.
3.3. Effect of azoxystrobin on antioxidative enzyme activity
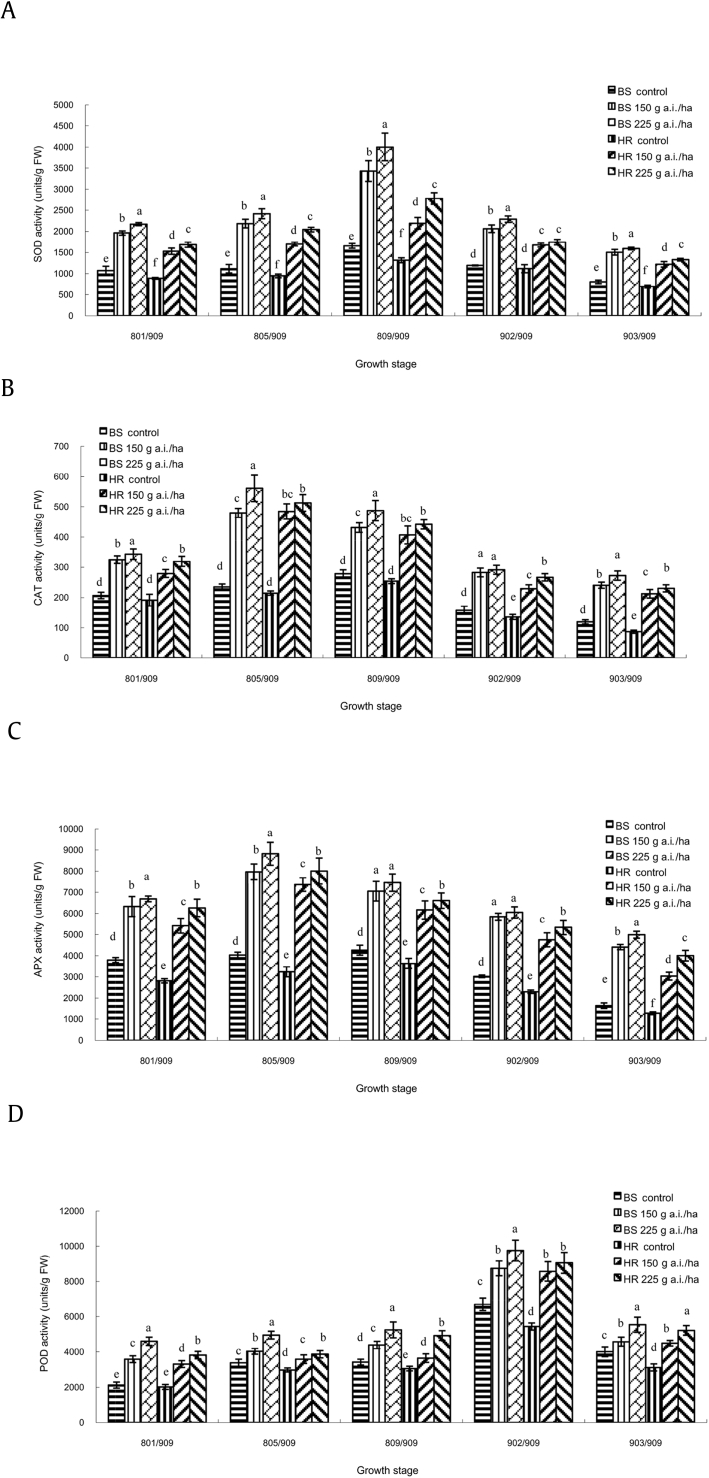
The overall levels of SOD activity, with or without azoxystrobin treatments, first slightly increased until [PGS] 809/909 and then slightly declined until [PGS] 903/909 (Fig. 3A). When compared with the control, the activity of SOD in azoxystrobin-treated plants was about one to three times higher than that in untreated plants, particularly at [PGS] 809/909. When the dose of azoxystrobin was 225 g a.i./ha, the effect was more significant; it was 2.58 times higher in BS and 2.12 times higher in HR than in untreated plants.
Fig. 3.
Effect of azoxystrobin on the contents of (A) SOD, (B) CAT, (C) APX, and (D) POD in ginseng leaves. Data are mean ± SE of three replicates. Letters under x-axis refer to the difference at significance level p = 0.05 among different treatments. Data are not significantly different for values followed by the same letter. APX, ascorbate peroxidase; CAT, catalase; FW, fresh weight; POD, peroxidase; SE, standard error; SOD, superoxide dismutase.
The effect on CAT activity in ginseng leaves with or without fungicide treatments was different (Fig. 3B). The CAT activity in the control plants increased slightly until [PGS] 809/909 and then declined slightly until [PGS] 903/909. However, the CAT activity of azoxystrobin-treated plants did not increase until [PGS] 805/909. It reached the highest level earlier than water treatments. It showed a significantly (p = 0.05) strong increase in CAT content compared with the control—2.38 times higher in BS and 2.33 times higher in HR by the treatment of azoxystrobin 225 g a.i./ha at [PGS] 805/909. Moreover, CAT contents of azoxystrobin-treated plants at [PGS] 903/909 were higher than those of the control plants at [PGS] 902/909 in both BS and HR, and nearly same as that of the control plants at [PGS] 809/909. It suggested that CAT content was also high at [PGS] 903/909 with treatments of azoxystrobin 150 g a.i./ha and 225 g a.i./ha both in BS and HR.
In parallel with CAT, APX content was significantly (p = 0.05) increased by the treatment of azoxystrobin 150 g a.i./ha and 225 g a.i./ha at all growth stages, both in BS and HR (Fig. 3C). It was observed that APX content showed the same trend as CAT content.
Unlike SOD, CAT, and APX, POD content steadily increased with aging of ginseng plants with or without azoxystrobin treatments (Fig. 3D). It was significantly (p = 0.05) elevated by the treatment of azoxystrobin 150 g a.i./ha and 225 g a.i./ha at all stages in both BS and HR. Statistical analysis revealed that POD content was significantly (p = 0.05) higher for azoxystrobin 225 g a.i./ha than for 150 g a.i./ha, and it was higher in BS than in HR.
3.4. Effect of azoxystrobin on the of ginsenoside contents of ginseng leaves
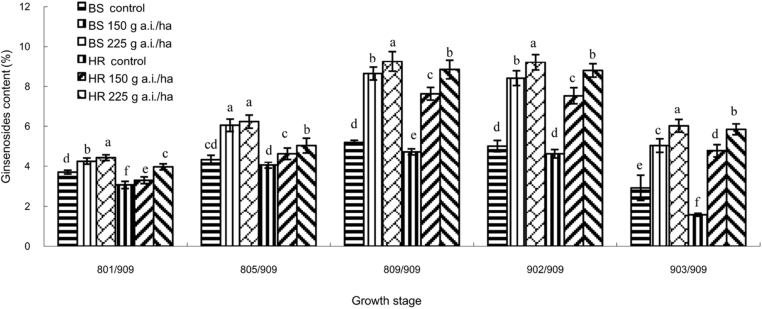
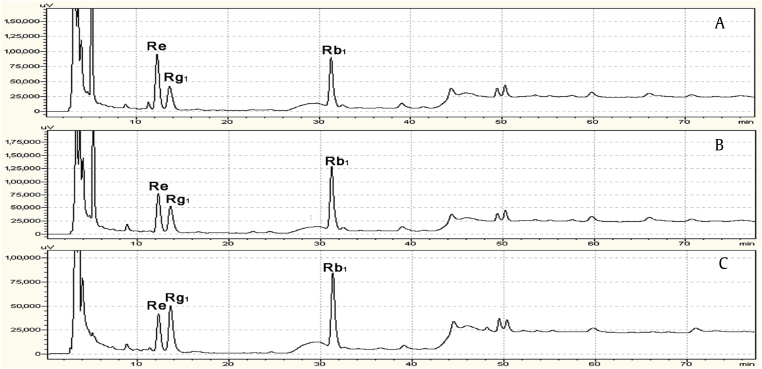
The total content of ginsenosides (Re, Rb1, and Rg1) in ginseng leaves was significantly (p = 0.05) increased by the effect of azoxystrobin 150 g a.i./ha and 225 g a.i./ha at all growth stages in both BS and HR (Fig. 4). Especially at these two stages ([PGS] 809/909 and [PGS] 902/909), the effect was more significant; it was one to two times higher than in untreated plants. A chromatogram (Fig. 5) showed that there was different elevated among ginsenosides Re, Rb1, and Rg1 at [PGS] 809/909 in BS in 2014. The chromatogram showed that the content of ginsenoside Re in ginseng leaves was increased with the increase in the dose of azoxystrobin application. It was highest for azoxystrobin 150 g a.i./ha (Fig. 5A), and when the dose of azoxystrobin was 225 g a.i./ha (Fig. 5B), it was higher than the control (Fig. 5C) but lower than that at 150 g a.i./ha. However, there was no marked difference in the content of ginsenoside Rg1 in ginseng leaves with or without azoxystrobin treatments. Unlike ginsenosides Re and Rg1, the content of ginsenoside Rb1 was highest with azoxystrobin 225 g a.i./ha (Fig. 5B), and there was no marked difference in the content of ginsenoside Rb1 in ginseng leaves with azoxystrobin 150 g a.i./ha (Fig. 5A) and without azoxystrobin treatment (Fig. 5C). The same result was obtained in HR.
Fig. 4.
Effect of azoxystrobin on the total content of ginsenosides (Re, Rb1, and Rg1) in ginseng leaves. Data are mean ± SE of three replicates. Letters under x-axis refer to the difference at significance level p = 0.05 among different treatments. Data are not significantly different for values followed by the same letter. SE, standard error.
Fig. 5.
Chromatogram of ginseng samples at [PGS] 809 (fruit fully ripe) in Baishan in 2014. (A) Azoxystrobin treatment 150 g a.i./ha. (B) Azoxystrobin treatment 225 g a.i./ha. (C) Control. [PGS], phenological growth stage.
4. Discussion and conclusion
P. ginseng is one of the most important medicinal plants in China. It is primarily cultivated in Jilin Province and Liaoning Province in China. In this study, modern strobilurin fungicide azoxystrobin was preferred because it was one of the earliest registration fungicides of ginseng in China, which had widely been used for controlling Alternaria panax whetz of ginseng. Information on the physiological and biochemical effects of azoxystrobin on ginseng was lacking. Furthermore, previous works mostly focused on the biochemical effect of azoxystrobin on wheat [7], [8] and the net rate of photosynthesis enhancement of strobilurins in wheat plants [40]. In the present work, we primarily determined the effect of azoxystrobin alone on the physiological and biochemical changes of ginseng plants under field conditions. Results obtained indicated a significant (p = 0.05) impact of foliar application of azoxystrobin on the delay of ginseng leaf senescence and enhancing antioxidant enzyme activity protecting plants from harmful active oxygen species (AOS) of ginseng leaves. Ginsenoside contents in leaves of azoxystrobin-treated plants were improved. There was no report earlier than the present results showing the physiological and biochemical impact of azoxystrobin on ginseng leaves.
The decreased CHL content and SP content are very early symptoms of senescence [41]. Strobilurin application extends the life of leaves by retention of higher CHL content [42]. In our present study, the CHL and SP contents in ginseng leaves with or without azoxystrobin treatment gradually decreased from [PGS] 805/909 to [PGS] 903/909, with ginseng plants becoming senescent both in BS and HR. The retardation of senescence in the leaves of ginseng by azoxystrobin is characterized by the delay of degradation of CHL and SP contents. Thus, the contents of CHL and SP at [PGS] 903/909 still remained at a high level; especially the CHL content of azoxystrobin-treated plants at [PGS] 903/909 was not significantly declined and was higher than that of the control plants at [PGS] 902/909 in both BS and HR. These results suggested that azoxystrobin application delayed leaf senescence in ginseng. Furthermore, the contents of CHL and SP in BS were higher than those in HR with or without azoxystrobin treatment, which might be because the temperature, humidity, and soil composition in BS were more suitable for growing ginseng plants compared with HR.
Senescence is considered a process associated with AOS [43]. H2O2 is dismutated from O2− by SOD. To counter the AOS stress, plants have evolved antioxidative strategies in which antioxidant enzymes play an important role [44], [45]. APX, CAT, and PODs are key enzymes in the active-oxygen scavenging system that can quench O2− and H2O2, and inhibit the accumulation of AOS [34].
The present study demonstrated the changes in SOD activity and O2− levels in aging ginseng leaves. This is the first report showing that azoxystrobin delayed the senescence in ginseng leaves and prevented the accumulation of O2− at all stages in two experimental farmlands in both 2013 and 2014. Azoxystrobin at the recommended field rate of 225 g a.i./ha was more effective in delaying ginseng leaf senescence than 150 g a.i./ha.
In our present study, with aging of ginseng plants, O2− levels in azoxystrobin treatment stably enhanced rather than water treatment strongly increased, the H2O2 content still remained relatively low until [PGS] 903/909. Moreover, CAT and APX activities of azoxystrobin treatments did not increase until [PGS] 805/909. They reached the highest level earlier than water treatments and SOD at [PGS] 809/909, which were consistent with the tendency of SOD. This indicated that H2O2 generated by SOD was removed by the induced activities of CAT and APX. After [PGS] 809/909, the CAT and APX activities in azoxystrobin-treated ginseng leaves declined rapidly, but the H2O2 content still remained relatively low until [PGS] 903/909. However, from [PGS] 809/909 the POD activity in azoxystrobin-treated ginseng leaves increased significantly. These results suggested that after [PGS] 809/909, POD played a key role in decomposing H2O2. In addition, POD activity is positively correlated with stress resistance, and it is also a key enzyme for synthetic lignin, which is a secondary metabolite in plant growth and development. POD biosynthesis can be induced upon various conditions, such as wounding and metabolic stress [46], [47]. Although azoxystrobin was unable to change the decreasing tendency of CAT, APX, and POD activities, azoxystrobin treatments can delay the senescence-related decrease of CAT, APX and POD activities in ginseng leaves and help them remain at a relatively high level compared with water control treatment. Consequently, it also indicated that azoxystrobin was effective in delaying ginseng plant senescence. At the dose of 225 g a.i./ha, the effect of azoxystrobin was more significant.
MDA is considered another important symptom of leaf senescence and can be initiated by free radicals. The acceleration of senescence of ginseng leaves was always associated with an increase of MDA and membrane leakiness. In the present study, we also found an increased level of MDA being paralleled by the enhancement of AOS production, and decrease of antioxidative enzyme activity and the contents of SP and CHL during aging of ginseng leaves, indicating the causal involvement of AOS in initiating lipid peroxidation of membranes. This indicated that 225 g a.i./ha azoxystrobin was more effective than 150 g a.i./ha azoxystrobin in delaying the membrane leakiness of ginseng leaves due to plant senescence.
In our present study, we investigated the impact of azoxystrobin on ginsenoside contents of ginseng leaves. Ginsenosides Re, Rb1, and Rg1 were chosen because amounts of these components in ginseng are relatively high. We found a tendency of ginsenosides contents being paralleled by the trend of CHL during aging of ginseng leaves. Changes were specifically attributable to Re and Rb1. The contents of ginsenosides were significantly decreased with ginseng leaf senescence. All functional products of ginseng available in the Chinese market use ginseng leaves as raw materials for the extraction of ginsenosides. The cost of ginseng roots is very high, while that of ginseng leaves is low. Therefore, the study on ginsenoside contents in ginseng leaves is valuable.
In conclusion, azoxystrobin appeared to delay the senescence of ginseng plants and increase the ginsenoside contents of ginseng leaves. This information helps understand the other role of azoxystrobin except for the control for A. panax whetz, and provides insight into the mechanism by which azoxystrobin delays the senescence of ginseng plants and increases the contents of ginsenosides. Enhancement of CHL content, SP content, and antioxidant enzyme of activity protecting the plants from harmful AOS is the additional physiological effect of azoxystrobin that contributes to the enhancement of ginsenoside contents of ginseng leaves. This work suggests that azoxystrobin plays a role in delaying senescence by changing physiological and biochemical indices of ginseng plants.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Rodrigues E.T., Lopes I., Pardal M.A. Occurrence, fate and effects of azoxystrobin in aquatic ecosystems: a review. Environ Int. 2013;53:18–28. doi: 10.1016/j.envint.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Herms S., Seehaus K., Koehle H., Conrath U. A strobilurin fungicide enhances the resistance of tobacco against tobacco mosaic virus and Pseudomonas syringae pv tabaci 1. Plant Physiol. 2003;130:120–127. doi: 10.1104/pp.004432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauter H., Steglich W., Anke T. Strobilurins: evolution of new class of active substances. Angew Chem Int Ed. 1999;38:1328–1349. doi: 10.1002/(SICI)1521-3773(19990517)38:10<1328::AID-ANIE1328>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Anke T., Oberwinkler F., Steglich W., Schramm G. The strobilurins—new antifungal antibiotics from the basidiomycete Strobiluris tenacellus. J Antibiot. 1977;30:806–810. doi: 10.7164/antibiotics.30.806. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett D.W., Clough J.M., Godwin J.R., Hall A.A., Hamer M., Dobrzanski B.P. The strobilurin fungicides. Pest Manag Sci. 2002;58:649–662. doi: 10.1002/ps.520. [DOI] [PubMed] [Google Scholar]
- 6.Margot P., Huggenberger F., Amrein J., Weiss B. CGA279202: a new broad-spectrum strobilurin fungicide. Pest Dis. 1998;2:375–382. [Google Scholar]
- 7.Zhang Y.J., Zhang X., Chen C.J., Zhou M.G., Wang H.C. Effects of fungicides JS399-19, azoxystrobin, tebuconazole, and carbendazim on the physiological and biochemical indices and grain yield of winter wheat. Pestic Biochem Physiol. 2010;98:151–157. [Google Scholar]
- 8.Wu Y.X., Von-Tiedemann A. Physiological effects of azoxystrobin and epoxiconazole on senescence and the oxidative status of wheat. Pestic Biochem Physiol. 2001;71:1–10. [Google Scholar]
- 9.Vincelli P., Dixon E. Resistance to QoI (strobilurin like) fungicides in isolates of Pyricularia grisea from perennial ryegrass. Plant Dis. 2002;86:235–240. doi: 10.1094/PDIS.2002.86.3.235. [DOI] [PubMed] [Google Scholar]
- 10.Bonasia A., Conversa G., Lazzizera C., Elia A. Pre-harvest nitrogen and azoxystrobin application enhances postharvest shelf-life in butterhead lettuce. Postharvest Biol Technol. 2013;85:67–76. [Google Scholar]
- 11.Ruske R.E., Gooding M.J., Jones S.A. The effects of triazole and strobilurin fungicide programmes on nitrogen uptake, partitioning, remobilization and grain N accumulation in winter wheat cultivars. J Agric Sci Technol. 2003;140:395–407. [Google Scholar]
- 12.Joshi J., Sharma S., Guruprasad K.N. Foliar application of pyraclostrobin fungicide enhances the growth, rhizobial-nodule formation and nitrogenase activity in soybean. Pestic Biochem Physiol. 2014;114:61–66. doi: 10.1016/j.pestbp.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 14.Leung W. vol. 1. Chemical Industry Press; Beijing: 2010. The State Pharmacopoeia Commission of the People's Republic of China; pp. 16–18. [Google Scholar]
- 15.Li T.S.C., Mazza G., Cottrell A.C., Gao L. Ginsenosides in roots and plants of American ginseng. J Agric Food Chem. 1996;44:717–720. [Google Scholar]
- 16.Tani T., Kubo M., Katsuki T., Higashino M., Hayashi T., Arichi S. Histochemistry. II. Ginsenosides in ginseng (Panax ginseng) root. J Nat Prod. 1981;44:401–407. [Google Scholar]
- 17.Roh S.W., Kim H.H., Ku Y.C., Jo J.S., Pyon J.Y. Occurrence and distribution of weeds in ginseng gardens in Korea. Korean J Weed Sci. 2002;22:350–358. [Google Scholar]
- 18.Furuya T., Yoshikawa T., Orihara Y., Oda H. Saponin production in cell suspension cultures of Panax ginseng. Planta Med. 1983;48:83–87. doi: 10.1055/s-2007-969892. [DOI] [PubMed] [Google Scholar]
- 19.Lin W.N., Lu H.Y., Lee M.S., Yang S.Y., Chen H.J., Chang Y.S., Chang W.T. Evaluation of the cultivation age of dried ginseng radix and its commercial products by using 1H-NMR fingerprint analysis. Am J Chin Med. 2010;38:205–218. doi: 10.1142/S0192415X10007762. [DOI] [PubMed] [Google Scholar]
- 20.Yun T.K., Lee Y.S., Lee Y.H., Kim S.I., Yun H.Y. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. J Korean Med Sci. 2001;16:6–18. doi: 10.3346/jkms.2001.16.S.S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troitskaya L.A., Komov V.P., Kirillova N.V. Peroxidase turnover in ginseng strains under standard conditions and temperature stress. Plant Physiol. 1999;155:281–284. [Google Scholar]
- 22.Johe G.S. Oxygen stress and superoxide dismutase. Plant Physiol. 1993;101:7–12. doi: 10.1104/pp.101.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foyer C.H., Lelandais M., Kunert K.J. Photooxidative stress in plants. Plant Physiol. 1994;92:696–717. [Google Scholar]
- 24.Zkiewicz M.D., Ska-Polit E.S., Krupa Z. Copper-induced oxidative stress and antioxidant defence in Arabidopsis thaliana. Biometals. 2004;17:379–387. doi: 10.1023/b:biom.0000029417.18154.22. [DOI] [PubMed] [Google Scholar]
- 25.Liu X.A., Vance-Baird W.M. Identification of a novel gene, HAABRC5, from Helianthus annuus (Asteraceae) that is upregulated in response to drought, salinity and abscisic acid. Am J Bot. 2004;91:184–191. doi: 10.3732/ajb.91.2.184. [DOI] [PubMed] [Google Scholar]
- 26.Chaves M.M., Maroco J., Pereira J. Understanding plant responses to drought—from genes to the whole plant. Plant Biol. 2003;30:239–264. doi: 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- 27.Shinozaki K., Shinozaki K.Y., Seki M. Regulatory network of gene expression in the drought and cold stress responses. Curr Opin Plant Biol. 2003;6:410–417. doi: 10.1016/s1369-5266(03)00092-x. [DOI] [PubMed] [Google Scholar]
- 28.Kreps J.A., Wu Y.J., Chang H.S. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002;130:2129–2141. doi: 10.1104/pp.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cromey M.G., Butler R.C., Mace M.A., Cole A.L.J. Effects of the fungicides azoxystrobin and tebuconazole on Didymella exitialis, leaf senescence and grain yield in wheat. Crop Prot. 2004;23:1019–1030. [Google Scholar]
- 30.Bertelsen J.R., Neergaard E.D., Petersen V.S. Fungicidal effects of azoxystrobin and epoxiconazole on phyllosphere fungi, senescence and yield of winter wheat. Plant Pathol. 2001;50:190–205. [Google Scholar]
- 31.Proctor J.T.A., Dorais M., Bleiholder H., Willis A., Hack H., Meier V. Phenological growth stages of North American ginseng (Panax quinquefolius) Ann Appl Biol. 2003;143:311–317. [Google Scholar]
- 32.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Lu W., Xu X.M., Zhang R.X., Dai X.B. Effect of adding acetic acid on improvement of determination of superoxide anion content in plants. Food Chem. 2004;27:82–84. [Google Scholar]
- 34.Zhao H., Dai T.B., Jing Q., Jiang D. Leaf senescence and grain filling affected by post-anthesis high temperatures in two different wheat cultivar. Plant Growth Regul. 2007;51:149–158. [Google Scholar]
- 35.Ferreira L.C., Scavroni J., Silva J.R.V., Cataneo A.C., Martins D., Boaro C.S.F. Copper oxychloride fungicide and its effect on growth and oxidative stress of potato plants. Pestic Biochem Physiol. 2014;112:63–69. doi: 10.1016/j.pestbp.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y.X., Von-Tiedemann A. Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ Pollut. 2002;116:37–47. doi: 10.1016/s0269-7491(01)00174-9. [DOI] [PubMed] [Google Scholar]
- 37.Rahman M., Punja Z.K. Biochemistry of ginseng root tissues affected by rusty root symptoms. Plant Physiol Biochem. 2005;43:1103–1114. doi: 10.1016/j.plaphy.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Palazón J., Cusidó R.M., Bonfill M., Mallol A., Moyano E., Morales C., Piñol M.T. Elicitation of different Panax ginseng transformed root phenotypes for an improved ginsenoside production. Plant Physiol Biochem. 2003;41:1019–1025. [Google Scholar]
- 39.Ma R., Sun L.W., Chen X.N., Jiang R., Sun H., Zhao D.Q. Proteomic changes in different growth periods of ginseng roots. Plant Physiol Biochem. 2013;67:20–32. doi: 10.1016/j.plaphy.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Grossmann K., Kwiatkowski J., Caspar G. Regulation of phytohormone levels, leaf senescence and transpiration by the strobilurin kresoxim-methyl in wheat (Triticum aestivum) J Plant Physiol. 1999;154:805–808. [Google Scholar]
- 41.Merzlyak M.K., Hendry G.A.F. Free radical metabolism, pigment degradation and lipid peroxidation in leaves during senescence. Proc Sec A Math R Soc Edinburgh. 1994;102:459–461. [Google Scholar]
- 42.Mc C.C., Mercer P.C., Cooke L.R., Fraaije B.A. Effects of a strobilurin based spray programme on disease control, green leaf area, yield and development of fungicide-resistance in Mycosphaerella graminicolain Northern Ireland. Crop Prot. 2007;26:1272–1280. [Google Scholar]
- 43.Li Z.Z., Niu W., Qiao X.W., Ma L.P. Anti-oxidant response of Cucumis sativus L. to fungicide carbendazim. Pestic Biochem Physiol. 2007;89:49–59. [Google Scholar]
- 44.Hadrami A.E., Kone D., Lepoivre P. Effect of juglone on active oxygen species and antioxidant enzymes in susceptible and partially resistant banana cultivars to black leaf streak disease. J Plant Physiol. 2005;113:241–254. [Google Scholar]
- 45.Pompeu G.B., Gratão P.L., Vitorello V.A., Azevedo R.A. Antioxidant isoenzyme responses to nickel-induced stress in tobacco cell suspension culture. Agric Sci China. 2008;65:548–552. [Google Scholar]
- 46.Caño-Delgado A., Penfield S., Smith C., Catley M., Bevan M. Reduced cellulose synthesis invokes lignification and defense responses in Arabidopsis thaliana. Plant J. 2003;34:351–362. doi: 10.1046/j.1365-313x.2003.01729.x. [DOI] [PubMed] [Google Scholar]
- 47.Tronchet M., Balagué C., Kroj T., Jouanin L., Roby D. Cinnamyl alcohol dehydrogenases-C and D, key enzymes in lignin biosynthesis, play an essential role in disease resistance in Arabidopsis. Mol Plant Pathol. 2010;11:83–92. doi: 10.1111/j.1364-3703.2009.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]