Abstract
Background
Extended endoplasmic reticulum (ER) stress may initiate apoptotic pathways in cancer cells, and ER stress has been reported to possibly increase tumor death in cancer therapy. We previously reported that caspase-8 played an important role in compound K-induced apoptosis via activation of caspase-3 directly or indirectly through Bid cleavage, cytochrome c release, and caspase-9 activation in HL-60 human leukemia cells. The mechanisms leading to apoptosis in A549 and SK-MES-1 human lung cancer cells and the role of ER stress have not yet been understood.
Methods
The apoptotic effects of compound K were analyzed using flow cytometry, and the changes in protein levels were determined using Western blot analysis. The intracellular calcium levels were monitored by staining with Fura-2/AM and Fluo-3/AM.
Results
Compound K-induced ER stress was confirmed through increased phosphorylation of eIF2α and protein levels of GRP78/BiP, XBP-1S, and IRE1α in human lung cancer cells. Moreover, compound-K led to the accumulation of intracellular calcium and an increase in m-calpain activities that were both significantly inhibited by pretreatment either with BAPTA-AM (an intracellular Ca2+ chelator) or dantrolene (an RyR channel antagonist). These results were correlated with the outcome that compound K induced ER stress-related apoptosis through caspase-12, as z-ATAD-fmk (a specific inhibitor of caspase-12) partially ameliorated this effect. Interestingly, 4-PBA (ER stress inhibitor) dramatically improved the compound K-induced apoptosis.
Conclusion
Cell survival and intracellular Ca2+ homeostasis during ER stress in human lung cancer cells are important factors in the induction of the compound K-induced apoptotic pathway.
Keywords: apoptosis, calcium, compound K, ER stress, lung cancer cells
1. Introduction
Endoplasmic reticulum (ER) is not only a reservoir for intracellular Ca2+, but also a home for folding and post-translational modifications of secretory proteins [1], [2]. ER stress occurs when ER is subjected to adverse situations such as hypoxia, nutrient deprivation, failure of posttranslational modifications, imbalance in Ca2+ homeostasis, or an increased accumulation of unfolded proteins [3]. ER stress causes the unfolded protein response (UPR) to enhance the protein folding capacity and decrease protein synthesis, and UPR activation as a result of ER stress initiates intracellular signaling pathways for cell protection.
The ER stress response is mainly regulated by three ER transmembrane proteins: activating transcription factor-6 (ATF-6), inositol requiring kinase-1 (IRE-1), and protein kinase-like ER kinase (PERK) [4]. Under normal conditions, these transmembrane proteins remain inactive and are bound to ER-resident chaperone glucose-regulated protein-78 (GRP78/BiP). The UPR is regulated by an ER stress sensor IRE1α [5]. IRE1α, a major ER stress transducer, is a serine/threonine protein kinase/endoribonuclease that, upon activation, initiates the splicing of X-box binding protein-1 (XBP-1) mRNA. Spliced XBP-1 mRNA encodes a transcriptional activator that leads to the transcription of chaperone protein-encoding genes, whose products have a role in ER protein folding [6]. PERK, another ER stress transducer, is a transmembrane kinase that phosphorylates eukaryotic translation initiation factor 2 subunit α (eIF2α), thereby reducing protein synthesis and counteracting ER protein overload [7]. Then, the activated cytoplasmic fragment of ATF-6 binds to the ER stress response element in the nucleus to activate the transcription of transcription factor genes such as XBP-1 and ER chaperone genes such as GRP78, as well as the expression of CCAAT/enhancer-binding protein-homologous protein (CHOP/GADD153) [3]. Critical or continued ER stress provokes ATF-6, PERK and IRE-1 apoptotic signaling and induces CHOP expression. Furthermore, caspase-12 has been involved in ER stress-mediated apoptosis, and caspase-12 knockdown mice are resistant to ER stress-induced apoptosis [8].
The intracellular Ca2+ level controls cellular processes, such as transcription, exocytosis, apoptosis, and proliferation [9], and its concentration is tightly regulated by multiple pumps, channels, and binding proteins [10]. The Ca2+ release from the ER is mediated through the ryanodine receptors (RyRs) and ER-resident inositol trisphosphate receptors (IP3Rs) [11]. However, even with tight regulation of Ca2+ release from the ER, the depletion of ER Ca2+ and the overload of cytosolic Ca2+ can occur due to several stimuli. The disrupted Ca2+ homeostasis and unchecked increases in cytosolic Ca2+ can induce apoptosis via the activation of ER-resident caspases and the activation of processes in the cytoplasm [12], [13], [14].
Ginseng, the root and rhizome of Panax ginseng Meyer, has been widely adapted in traditional medicine in East Asia. Ginsenosides are major bioactive components in ginseng and describe a various group of steroidal saponins. More than 20 ginsenosides have been reported to possess a variety of biological properties, including neuroprotective, anticancer, and antiinflammatory activities [15]. The two major subtypes of ginsenosides have been termed protopanaxadiols and protopanaxatriols, which after ingestion can give rise to novel metabolites in the body [12], [13]. 20-O-β-D-glucopyranosyl-20(S)-protopanaxadiol (compound K) (Fig. 1A) is a major metabolite of several protopanaxadiol-type ginsenosides, including Rb1, Rb2, and Rc, by intestinal bacteria through the multistage cleavage of sugar moieties [16]. It has been investigated in terms of its antidiabetic [17], [18], [19], antiinflammatory [20], [21], [22], and anticancer [23], [24] effects.
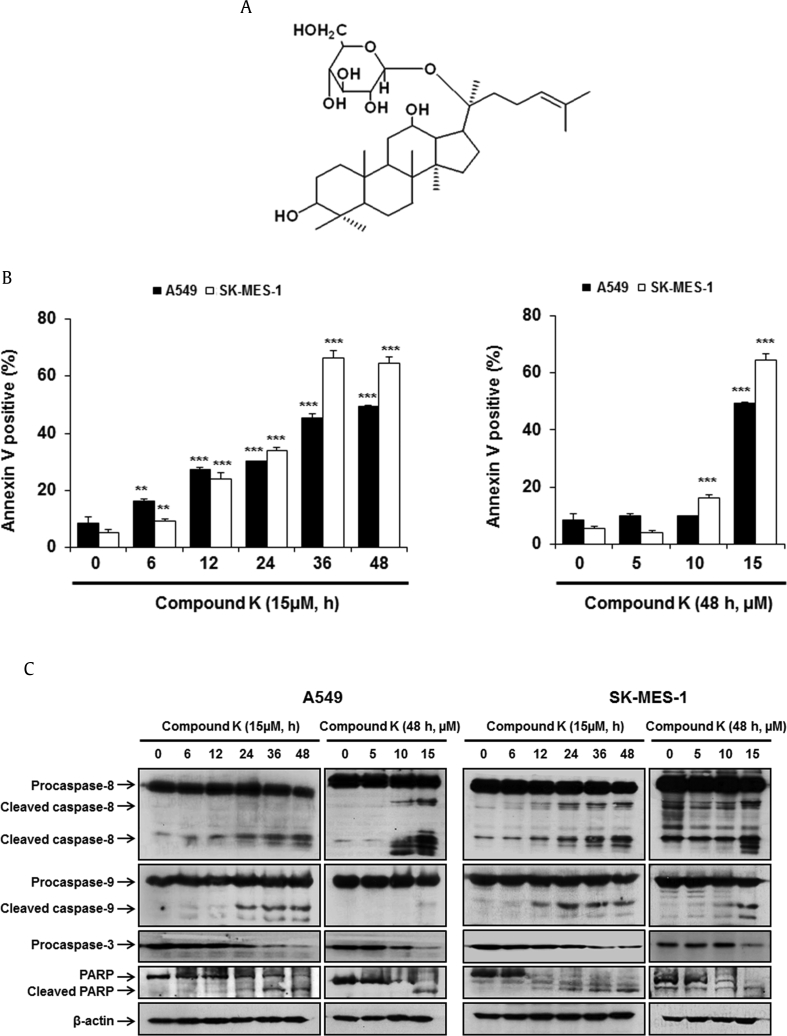
Fig. 1.
Compound K induced apoptosis in human lung cancer cells. (A) The chemical structure of compound K. (B) Cells were treated with various concentrations (5μM, 10μM, 15μM) of compound K for the indicated times. Cells were costained with PI and FITC-conjugated annexin V, and the translocation of phosphatidylserine was detected by flow cytometry. (C) Compound K induced apoptosis via caspase activation and downregulation of antiapoptotic proteins in A549 and SK-MES-1 cells. β-Actin was used as an internal control. (D) The effect of the broad-spectrum caspase inhibitor (z-VAD-fmk) on apoptosis was determined by costaining with PI and FITC-conjugated annexin V, and the translocation of phosphatidylserine was detected by flow cytometry. (E) c-FLIPL, XIAP, Bcl-2, and Bcl-xL were analyzed by Western blotting and β-actin was used as an internal control. Data are presented as means ± SD of three independent experiments. ** p < 0.01, *** p < 0.001 versus the compound K-treated group. Bcl-2, B-cell lymphoma 2; Bcl-xL, B-cell lymphoma-extra large; c-FLIPL, cellular FLICE-like inhibitory protein long isoform; PARP, poly (ADP-ribose) polymerase; XIAP, X-linked IAP.
Although a previous study suggested a role for ER stress in the induction of apoptosis in HT-29 colon cancer cells [25], the role of Ca2+ released from ER and caspase-12 in compound K-induced apoptosis in human lung cancer cells has not yet been reported. We report that compound K induces Ca2+ release from ER via the RyR channel, leading to the activation of m-calpain, caspase-12, and caspase-3. In addition, there is an increase in the levels of the endoplasmic reticular stress markers, IRE1α, XBP-1S, and GRP78.
2. Materials and methods
2.1. Chemicals and reagents
Compound K (purity > 98%) used in the present study was isolated from P. ginseng, and its structural identities were determined spectroscopically (1H and 13NMR, IR, MS) as previously described [21]. RPMI 1640 medium, DMEM medium, fetal bovine serum (FBS), penicillin, streptomycin, and Fura-2/AM were obtained from Life Technologies Inc (Chicago, IL, USA). 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl-tertazolium bromide (MTT), dimethyl sulfoxide (DMSO), RNase A, phenylmethylsulfonylfluoride (PMSF), propidium iodide (PI), 4-phenylbutyrate (4-PBA), 2-aminoethoxydiphenyl borate (2-APB), dantrolene, BAPTA-AM, ethylene glycol tetraacetic acid (EGTA), and CGP37157 were purchased from Sigma Chemical Co. The following antibodies for caspase-3, caspase-7, caspase-9, poly (ADP-ribose) polymerase (PARP), XBP-1, c-FLIPL, Bid, Bcl-2, Bcl-xL, p-eIF2α, GADD 153, m-calpain, and β-actin were purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA, USA). The antibodies for X-linked inhibitor of apoptosis protein (XIAP), caspase-8, and caspase-12 were purchased from BD Biosciences, Pharmingen (San Jose, CA, USA). The antibodies for IRE1α and GRP78 were purchased from Cell Signaling Technology (Denvers, MA, USA). z-VAD-fmk, z-ATAD-fmk were purchased from Calbiochem (San Diego, CA, USA).
2.2. Cell culture and sample treatment
Human lung adenocarcinoma A549 and human lung squamous cell carcinoma SK-MES-1 were obtained from the Korea Cell Line Bank (Seoul, Republic of Korea). A549 was grown at 37°C in RPMI-1640 medium and supplemented with 10% FBS, penicillin (100 units/mL), and streptomycin sulfate (100 μg/mL) in a humidified atmosphere of 5% CO2. SK-MES-1 was grown at 37°C in DMEM medium and was supplemented with 10% FBS, penicillin (100 units/mL) and streptomycin sulfate (100 μg/mL) in a humidified atmosphere of 5% CO2. Cells were incubated with 15μM of compound K for various times (6 h, 12 h, 24 h, 36 h, or 48 h) and 48 h for various concentrations (5μM, 10μM, 15μM) in 2% FBS contained medium.
2.3. Cytotoxicity test
The cytotoxicity was assessed through an MTT assay. Briefly, the cells (0.5 × 105 cells/mL) were seeded in each well containing 100 μL of the RPMI or DMEM medium in a 96-well plate. After 24 h, various concentrations of compound K were added. After 48 h, 20 μL of MTT [5 mg/mL stock solution in phosphate-buffered saline (PBS)] were added, and the plates were incubated for an additional 4 h. The medium was discarded, and formazan blue, which formed in the cells, was dissolved with 200 μL dimethyl sulfoxide. The optical density was measured at 540 nm.
2.4. Quantification of apoptosis via flow cytometry
For PI and Annexin V double staining, cells were suspended with 100 μL of binding buffer (10mM HEPES/NaOH, 140mM NaCl, 2.5mM CaCl2, pH 7.4) and were stained with 5 μL of FITC-conjugated Annexin V and 5 μL of PI (50 μg/mL) for 30 min at room-temperature in a dark place and then 400 μL of binding buffer was added for analysis via fluorescence-activated cell sorting cater-plus flow cytometry (Becton Dickinson Co., Heidelberg, Germany).
2.5. Protein extraction and Western blot analysis
Cells were collected via centrifugation at 200× g for 10 min at 4°C. The cells were then washed twice with ice-cold PBS, and were centrifuged at 200× g for 5 min. The obtained cell pellet was then resuspended in 1× protein lysis buffer (Intron, Seoul, Korea). Equal amounts of cell lysates were separated via sodium dodecyl sulfate-polyacrylamide gel and were transferred to nitrocellulose membranes for Western blot analysis using the indicated primary antibodies. Horseradish peroxidase-conjugated secondary antibodies were detected using an enhanced chemiluminescence (Amersham, Buckinghamshire, England) detection system.
2.6. Calcium quantification
A549 and SK-MES-1 cells grown on cover glass were incubated overnight. Cytosolic free Ca2+ was measured using the Ca2+-sensitive fluorescent indicator dye Fura-2/AM. Cells grown on a matrigel-coated cover-slide bottom dish were washed three times with PBS and were incubated in the dark for 30 min at room temperature with Fura-2/AM (final concentration 1μM) in PBS. The cells were washed again with PBS three times and were analyzed by being illuminated with an alternating light of 340 nm and 380 nm from a rotating filter wheel.
2.7. Measurement of calcium concentration
Free cytosolic calcium was measured using Ca2+-indicator dye Fluo-3/AM (cell membrane permeable fluorescent dye). After exposure to 30mM of IPA for various times, the cells were harvested and washed twice with HBSS (130mM NaCl, 2.5mM KCl, 1.2mM MgCl2, 10mM HEPES, 10mM glucose, 2mM CaCl2, pH 7.4). The cells were resuspended, and then incubated with the Fluo-3/AM (3mM) for 30 min. The free cytosolic Ca2+ levels, seen as a fluorescent signal, were then assessed via flow cytometry with an FL1 channel.
2.8. Statistical analysis
The results are expressed as the mean ± S.D. of triplicate experiments. Statistically significant values were compared using ANOVA and Dunnett's post hoc test. Statistical analysis was performed using SigmaPlot software version 10.0 (Systat Software, Inc., San Jose, CA, USA). A p value < 0.05 was considered to be statistically significant.
3. Results
3.1. Compound K induced caspase-dependent apoptosis in human lung cancer cells
We examined the effect of compound K on the cell viabilities using MTT assays in human lung cancer cells. Compound K was shown to have cytotoxicity on human lung adenocarcinoma A549 and squamous lung carcinoma SK-MES-1 cells (IC50: 17.78μM and 16.53μM, respectively). To further investigate whether the cytotoxic effect of compound K was associated with the induction of apoptosis, we estimated the translocation of phosphatidylserine using Annexin V and PI double staining. The percentage of Annexin V-positive cells was found to increase in a time- and concentration-dependent manner after treatment with compound K in A549 and SK-MES-1 cells (Fig. 1B).
To establish the mechanism associated with compound K-induced apoptosis, we examined the activation of caspase-8, -9, -3 and cleavage of PARP (an endogenous substrate of caspase-3) in A549 and SK-MES-1 cells. Compound K increased procaspase-8, -9, -3 and PARP-1 cleavage in a time- and concentration-dependent manner (Fig. 1C). To investigate the involvement of caspases in compound K-induced apoptosis, A549 and SK-MES-1 cells were pretreated with z-VAD-fmk (a broad caspase inhibitor) for 1 h and were then treated with compound K for 48 h. As shown in Figs. 1D and 1E, z-VAD-fmk significantly suppressed compound K-induced apoptosis in A549 and SK-MES-1 cells. These observations indicate that compound K-induced apoptosis involved the caspase-dependent pathway in A549 and SK-MES-1 cells. As expected, the expressions of antiapoptotic proteins c-FLIPL, X-linked IAP (XIAP), Bcl-2, and Bcl-xL were substantially decreased with compound K treatment in both cell lines in a time- and concentration-dependent manner (Fig. 1E), indicating concomitant activation of both the extrinsic and the intrinsic pathways of apoptosis.
3.2. Compound K induced ER stress and led to activation of the UPR
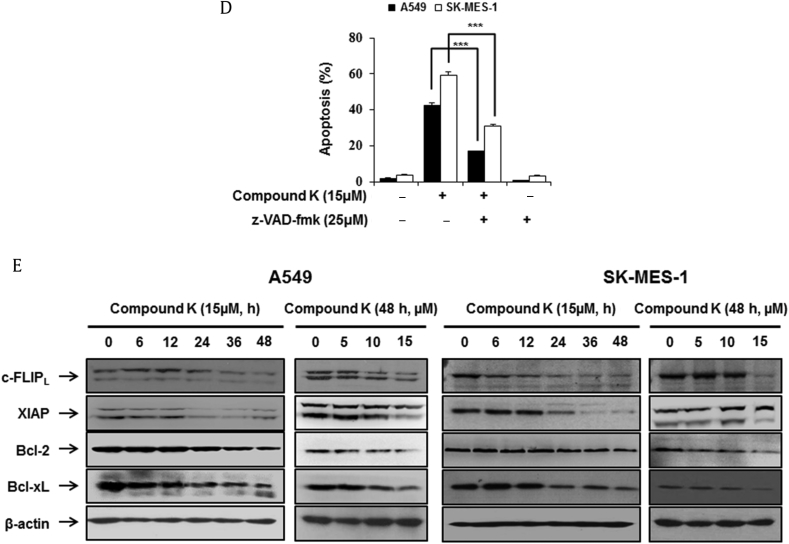
ER stress is implicated in the induction of apoptosis as well as autophagy [18]. To assess whether the compound K-induced apoptosis was linked to ER stress, we evaluated the effect of the compound K on ER stress markers. The activation of IRE1α has been shown to promote the splicing of a 26 nucleotide intron from the XBP1 mRNA and give rise to its spliced variant XBP-1S [26]. A previous study suggested the activation of XBP1 was a characteristic feature of ER stress [5]. Treatment of A549 and SK-MES-1 cells for various times with compound K (5–15μM) showed a sustained increase in the levels of IRE1α and XBP-1S in both cell lines (Fig. 2). We then proceeded to examine the other two arms of ER stress signaling (ATF-6/p-eIF2α and PERK/CHOP). An immunoblot analysis of compound K-treated cells demonstrated a significant increase of p-eIF2α in both cell lines. However, an increase in the protein levels of CHOP in A549 and SK-MES-1 cells was not observed in either of the cell lines (Fig. 2). The upregulation of molecular chaperones further confirmed the induction of ER stress in compound K-treated cells; this included increased GRP78 level, which is sensitive to changes in the ER redox state (Fig. 2).
Fig. 2.
Compound K induced ER-stress and activation of unfolded protein response. Cells were treated with various concentrations (5μM, 10μM, 15μM) of compound K (48 h) and various times (0 h, 6 h, 12 h, 24 h, 36 h, 48 h) with compound K (15μM). GRP78, IRE1α, XBP-1S, p-eIF2α, and CHOP were analyzed by Western blotting and β-actin was used as an internal control. eIF2α, eukaryotic translation initiation factor 2 subunit α; GADD153(CHOP), growth arrest and DNA damage 153(C/EBP homologous protein); GRP78(Bip), glucose-regulated protein-78(Binding immunoglobulin protein); IRE1α, inositol requiring kinase-1 α; XBP-1, X-box binding protein-1.
3.3. Compound K induced apoptosis via a caspase-12-dependent pathway
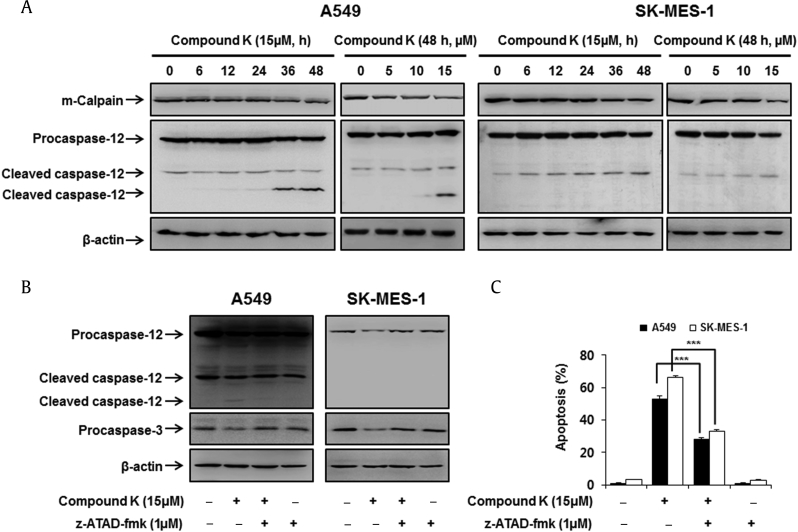
Caspase-12 is the first caspase reported to localize to the ER, and it has been proposed as a key mediator of ER stress-induced apoptosis [8]. Caspase-12 is cleaved and activated specifically during ER stress, but not by death receptor- or mitochondrial-mediated apoptotic signals. Subsequently, activated caspase-12 may stimulate the activation of executor caspases. This has prompted us to assess whether ER stress is induced after compound K treatment by evaluating the levels of caspase-12 cleavage by Western blotting in A549 and SK-MES-1 human lung cancer cells. We found both a time- and concentration-dependent increase in caspase-12 cleavage (Fig. 3A). In addition, the specific caspase-12 inhibitor z-ATAD-fmk suppressed the cleavage of caspase-12, as well as the cleavage of caspase-3 (Fig. 3B), confirming that compound K-induced apoptosis was caspase-12-dependent in those cells.
Fig. 3.
Compound K induced apoptosis via caspase-12 activation. (A) Cells were treated with various concentrations (5μM, 10μM, 15μM) of compound K (48 h) and various times (0 h, 6 h, 12 h, 24 h, 36 h, 48 h) with compound K (15μM). Caspase-12 and m-calpain were analyzed by Western blotting and β-actin was used as an internal control. (B) Cells were pretreated with 1μM z-ATAD-fmk for 1 h and then treated with 15μM of compound K for 48 h. Caspase-12 and procaspase-3 were analyzed by Western blotting and β-actin was used as an internal control. (C) The effect of the caspase-12 inhibitor (z-ATAD-fmk) on apoptosis was determined by costaining with PI and FITC-conjugated annexin V, and the translocation of phosphatidylserine was detected by flow cytometry. Data are presented as means ± SD of three independent experiments. *** p < 0.001 versus the compound K-treated group.
Caspases-12 is unique to ER and caspase-3 is the joint executor caspase for ER stress and apoptosis pathways. Thus, our result implied a crosstalk between ER stress and the extrinsic and/or intrinsic pathways of apoptosis, with caspase-12 being the upstream signaling molecule to caspase-3. Further, as shown in Fig. 3C, z-ATAD-fmk partially inhibited compound K-induced apoptosis, which implied that compound K induced apoptosis in human lung cancer cells via both caspase-12 and extrinsic and/or intrinsic signaling pathways [8].
3.4. Compound K increased the intracellular calcium levels and led to m-calpain activation
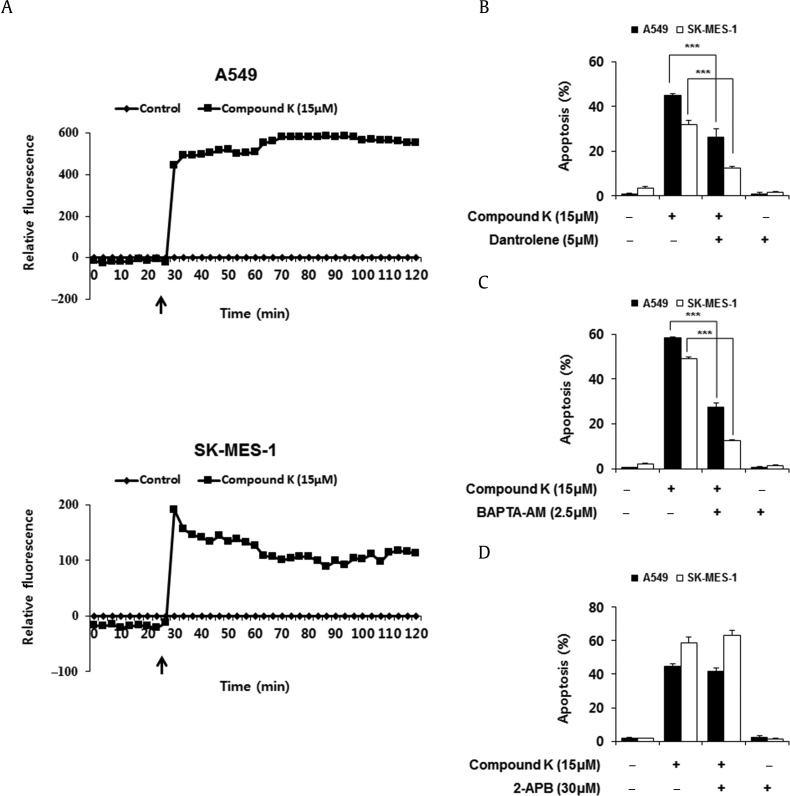
To investigate whether the compound K-induced apoptosis involved changes in intracellular Ca2+ levels ([Ca2+]i), we measured the change in intensity of the intracellular Ca2+-sensitive dye Fura-2/AM in A549 and SK-MES-1 cells before and after treatment. Treatment with compound K resulted in a significant increase in [Ca2+]i in A549 and SK-MES-1 cells (Fig. 4A).
Fig. 4.
Compound K induced apoptosis via Ca2+ mediated caspase-12 activation. (A) Cytosolic Ca2+ levels were measured by Fura-2/AM fluorescence dye. Compound K treated 30 min after the beginning of the reading as indicated by the arrow. (B, C, D) Effect of the RyR channel antagonist (dantrolene), IP3R channel antagonist (2-APB), or Ca2+ chelator (BAPTA-AM) on apoptosis was determined by testing the costaining with PI and FITC-conjugated annexin V, and the translocation of phosphatidylserine was detected by flow cytometry. Data are presented as means ± SD of three independent experiments. (E, F) Cells were pretreated with 5μM dantrolene or 2.5μM BAPTA-AM for 1 h and then treated with 15μM of compound K for 48 h. m-Calpain, caspase-12, and procaspase-3 were analyzed by Western blotting and β-actin was used as an internal control. (G) Cells were pretreated with 30μM 2-APB for 1 h and were then treated with 15μM of compound K for 48 h. Procaspase-3 were analyzed by Western blotting and β-actin was used as an internal control. *** p < 0.001 versus the compound K-treated group. 2-APB, 2-aminoethoxydiphenyl borate.
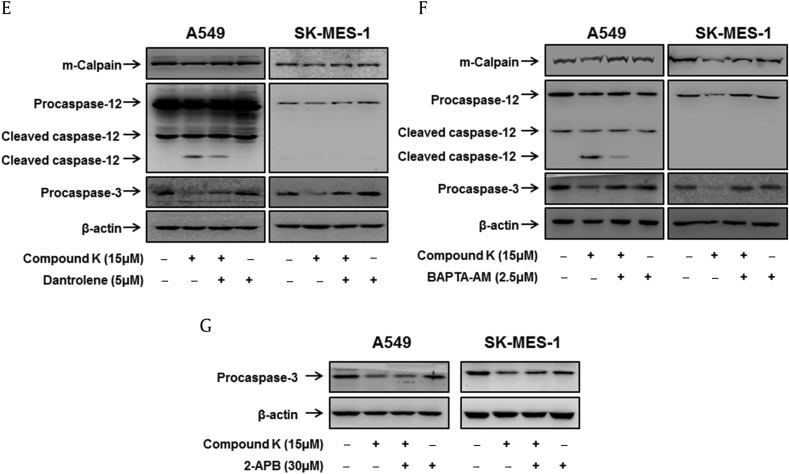
Since caspase-12 activation has been reported to be possibly mediated by calcium-dependent cysteine protease, m-calpain, in response to calcium release from the ER during ER stress [27], we examined whether compound K influenced the m-calpain activation by Western blotting. Fig. 3A shows that compound K promoted the cleavage of m-calpain to its active fragments in a time- and concentration-dependent manner. With the ER being the most important intracellular Ca2+ store [1], regulation of [Ca2+]i by the ER is mainly mediated by Ca2+ uptake into the ER through SERCA Ca2+ pumps and Ca2+ release through Ca2+ channels, such as IP3Rs or RyRs [8], [28], [29]. To determine whether compound K-induced [Ca2+]i in A549 and SK-MES-1 cells were caused by ER Ca2+-related channels, we treated the cells with Ca2+ channel blockers dantrolene or 2-aminoethoxydiphenyl borate (2-APB) or BAPTA-AM (intracellular Ca2+ chelator) to inhibit RyRs and IP3Rs, respectively. As shown in Figs. 4B–4D, dantrolene or BAPTA-AM treatment markedly attenuated compound K-induced apoptosis, whereas 2-APB did not. Next, we measured the m-calpain, caspase-12, and caspase-3 activation of cells exposed to compound K with or without BAPTA-AM or dantrolene to understand the effects of variations in cytoplasmic calcium on compound K-induced ER stress. As shown in Figs. 4E–4G, the chelation of cytosolic Ca2+ by treatment with BAPTA-AM or dantrolene suppressed the compound K-induced cleavage of m-calpain, caspase-12, and caspase-3, whereas 2-APB did not activate caspase-3. Taken together, these data suggested that RyR-mediated Ca2+ release is involved in compound K-induced ER stress and apoptotic cell death.
3.5. Inhibition of ER stress enhanced compound K-induced apoptosis
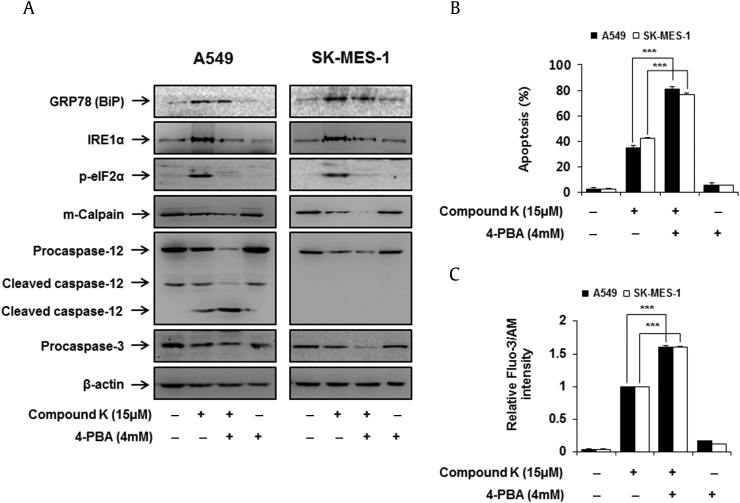
Next, we wanted to examine the relevance of ER stress in compound K-induced apoptosis in human lung cancer A549 and SK-MES-1 cells. We used 4-PBA (ER stress inhibitor) to alleviate ER stress in these lung cancer cells treated with compound K. Pretreatment with 4-PBA reduced the expression of compound K-induced ER-specific proteins, such as GRP78, IRE1α, and p-eIF2α (Fig. 5A). We then examined the expression of apoptosis-associated proteins, including cleaved caspase-12, m-calpain, and caspase-3. The results indicate that compound K-induced activation of caspase-12, m-calpain, and caspase-3 were clearly upregulated by 4-PBA (Fig. 5A). Using Annexin V and PI double staining, we found that inhibition of ER stress significantly enhanced the cell apoptosis of the compound K-treated A549 and SK-MES-1 cells at 24 h (Fig. 5B). In a manner consistent with the induction of apoptosis, inhibition of ER stress significantly increased compound K-induced [Ca2+]i levels by 4-PBA (Fig. 5C). These results demonstrated that inhibition of ER stress enhanced apoptosis via increase of [Ca2+]i levels in A549 and SK-MES-1 cells treated with compound K. Taken together, these results indicate that induction of ER stress participated in protecting the cell against compound K-induced apoptosis.
Fig. 5.
Effects of 4-PBA on compound K-induced ER stress, [Ca2+]i and apoptosis. (A) Cells were pretreated with 4mM 4-PBA for 1 h and were then treated with 15μM of compound K for 48 h. GRP78, IRE1α, p-eIF2α, m-calpain, caspase-12, and procaspase-3 were analyzed by Western blotting and β-actin was used as an internal control. (B) Effect of the 4-PBA on apoptosis was determined by testing the costaining with PI and FITC-conjugated annexin V, and the translocation of phosphatidylserine was detected by flow cytometry. (C) Cytosolic Ca2+ levels were measured by fluo-3 AM fluorescence dye and were measured using a flow cytometry. 4-PBA was pretreated for 1 h and then treated with compound K. Data are presented as means ± SD of three independent experiments. *** p < 0.001 versus the compound K-treated group. 4-PBA, 4-phenylbutyrate; eIF2α, eukaryotic translation initiation factor 2 subunit α; GRP78(Bip), glucose-regulated protein-78(Binding immunoglobulin protein); IRE1α, inositol requiring kinase-1 α.
4. Discussion
Apoptosis is a fundamental cellular activity that protects against cancer development by eliminating genetically altered and hyperproliferative cells. Thus, defects in apoptosis signaling pathways contribute to carcinogenesis and chemoresistance [24]. As mentioned above, there are two major apoptotic pathways, the extrinsic death receptor-mediated pathway and the intrinsic mitochondria-mediated pathway, and truncated Bid protein provides crosstalk between the two [30]. Both of these pathways are regulated by caspases, which are responsible, either directly or indirectly, for the cleavages of cellular proteins, a characteristic of apoptosis [31]. Our previous study has shown that caspase-8 plays a key role in compound K-stimulated apoptosis via activation of caspase-3 and -9 and modulation of Bcl-2 families in HL-60 human leukemia cells [21].
In the present study, we confirmed that compound K induced caspase-dependent apoptosis in A549 and SK-MES-1 human lung cancer cells. Since the Bcl-2 family proteins play a significant role in apoptosis, we studied the effect of compound K on the expression of Bcl-2 family proteins in A549 and SK-MES-1. Compound K was found to downregulate the levels of antiapoptotic proteins, including c-FLIPL, XIAP, Bcl-2, and Bcl-xL, leading to apoptotic pathways via both intrinsic and extrinsic activation. Our present results confirm those of Lee et al [17], in as much as compound K showed cytotoxic and caspase-dependent apoptosis-inducing activities in human lung cancer cells.
ER is a key organelle in protein processing and intracellular calcium storage, and it participates in crucial biosynthetic and signaling regulation functions in eukaryotic cells [29]. When there is an imbalance between ER protein folding load and capacity in different physiological and pathological conditions, unfolded proteins accumulate in the ER lumen, a condition known as ER stress. ER stress can initiate cell UPR, which regulates the expression of ER molecular chaperone GRP78/BiP, the ER stress sensor protein IRE1α, PERK, and ATF-6/p-eIF2α. Mild to moderate ER stress promotes cell survival through UPR to alleviate ER stress [32]. However, sustained and severe ER stress or inhibition of ER stress leads to cell death in some cancer types, including lung cancer [31].
Despite considerable studies on compound K-induced apoptosis in several cancer cell lines, little is known of the underlying cytotoxic mechanisms with respect to ER stress-mediated apoptosis [17], [18], [19], [33]. The present study shows that the induction of UPR-related proteins may be involved in compound K-treated lung cancer cells as: (1) compound K increased IRE1α and XBP-1S protein levels; (2) compound K induced an increase of GRP78 expression; and (3) compound K induced phosphorylation of eIF2α. However, we could not detect the expression of ER stress associated with the CHOP protein. This may be due to the low level of ER stress induced by compound K that could not induce ER stress-associated apoptosis.
There is a growing body of evidence that the release of Ca2+ from ER and consequent increase in cytosolic Ca2+ levels can play pivotal roles in regulating cell survival and apoptosis in a variety of cell types [34]. In addition, the dysregulation of ER Ca2+ homeostasis occurs as an early event during many forms of apoptosis and has been implicated in the pathophysiology of several diseases, including cancer. In the present study, the release of ER calcium stores resulted in an increase in cytosolic free Ca2+ levels, suggesting the disruption of intracellular Ca2+ homeostasis, which leads to the promotion of cell dysfunction and apoptosis in A549 and SK-MES-1 cells.
The elevation of [Ca2+]i or depletion of ER Ca2+ stores are typical ER stress responses of cells [29]. Cytosolic Ca2+ binds and activates Ca2+-dependent intracellular cysteine protease, m-calpain, caspase-12, and then the apoptotic pathway [8], [35]. The experimental evidence presented here showed that the evaluation of [Ca2+]i by efflux from ER caused by compound K triggered apoptosis through the activation of m-calpain and the caspase-12 pathway in human lung cancer cells.
The increase in [Ca2+]i may be due to an influx of Ca2+ from the extracellular medium across the plasma membrane and/or Ca2+ release from intracellular stores, predominantly from the ER. We investigated the role of extracellular Ca2+ in the increase in [Ca2+]i after compound K treatment in A549 and SK-MES-1 cells by analyzing [Ca2+]i in cells placed in a medium without Ca2+, and we found that the increase in cytosolic Ca2+ was similar in media with and without Ca2+ (data not shown). Thus, extracellular calcium does not seem to be involved in the increase in cytosolic Ca2+ in our model. Zhang et al [36] also previously demonstrated that increases in cytosolic and mitochondrial Ca2+ levels caused by compound K may be involved in Ca2+ release from ER stores into the cytosol of human colon cancer cells. We further investigated whether the increase in [Ca2+]i after compound K treatment in A549 and SK-MES-1 cells was also due to the release of Ca2+ from the ER through the IP3R and/or RyR channels, which have been implicated in apoptotic Ca2+ signaling in several models [37]. We thus treated the cells with inhibitors of these receptors, 2-APB and dantrolene, respectively. Pretreatment of dantrolene inhibited [Ca2+]i levels and attenuated compound K-induced apoptosis, whereas 2-APB did not. Furthermore, we used a permeant Ca2+ chelator, BAPTA-AM, to investigate the possible role of [Ca2+]i in the progression of compound K-induced apoptosis. Preincubation with BAPTA-AM also significantly suppressed compound K-induced apoptosis through inactivation of m-calpain, procaspase-12, and caspase-3. Therefore, we noticed that increased [Ca2+]i levels through the RyR channel, instead of that from the IP3R channel, were closely associated with compound K-induced apoptosis. However, further experiments are needed to examine how ER stress and increased [Ca2+]i activates the mitochondrial apoptosis pathway.
To further determine the biological function of ER stress on compound K-induced apoptosis in A549 and SK-MES-1 cells, we blocked ER stress in human lung cancer cells by using ER stress inhibitor 4-PBA. Compound K-induced apoptosis was found to have been obviously enhanced in the human lung cancer A549 and SK-MES-1 cells treated with 4-PBA. In a situation of enhanced m-calpain and caspase-12 activation by 4-PBA, compound K-induced UPR may participate in intracellular Ca2+ modulation. Based on these results, we found that UPR was related to cell protection. Also, these results could support the premise that UPR signals induced by compound K were involved in a cell protective effect against the observed apoptosis [38].
In summary, we found that compound K could induce ER stress and apoptosis, and ER stress was observed to be involved in compound K-induced protection in A549 and SK-MES-1 human lung cancer cells. Exposure to compound K could also be concluded to cause cell death by triggering ER Ca2+ release via RyR. Thus, the obligatory signaling molecules for ER stress, UPR, and calcium release might be considered to be the molecular targets for compound K-induced apoptosis.
Conflicts of interest
The authors have no conflicts of interest.
Acknowledgments
This work was supported by the research grant from the Korea Food Research Institute (E0145202).
References
- 1.Meldolesi J., Pozzan T. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem Sci. 1998;23:10–14. doi: 10.1016/s0968-0004(97)01143-2. [DOI] [PubMed] [Google Scholar]
- 2.Ma Y., Hendershot L.M. The unfolding tale of the unfolded protein response. Cell. 2001;107:827–830. doi: 10.1016/s0092-8674(01)00623-7. [DOI] [PubMed] [Google Scholar]
- 3.Kitamura M. Endoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus faces. Am J Physiol Renal Physiol. 2008;295:F323–F334. doi: 10.1152/ajprenal.00050.2008. [DOI] [PubMed] [Google Scholar]
- 4.Wang S., Kaufman R.J. The impact of the unfolded protein response on human disease. J Cell Biol. 2012;197:857–867. doi: 10.1083/jcb.201110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroder M., Kaufman R.J. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 7.Harding H.P., Zhang Y., Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 8.Szegezdi E., Fitzgerald U., Samali A. Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann N Y Acad Sci. 2003;1010:186–194. doi: 10.1196/annals.1299.032. [DOI] [PubMed] [Google Scholar]
- 9.Carafoli E. Calcium signaling: a tale for all seasons. Proc Natl Acad Sci U S A. 2002;99:1115–1122. doi: 10.1073/pnas.032427999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorlach A., Klappa P., Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 11.Bezprozvanny I. The inositol 1,4,5-trisphosphate receptors. Cell Calcium. 2005;38:261–272. doi: 10.1016/j.ceca.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 12.Squier M.K., Sehnert A.J., Sellins K.S., Malkinson A.M., Takano E., Cohen J.J. Calpain and calpastatin regulate neutrophil apoptosis. J Cell Physiol. 1999;178:311–319. doi: 10.1002/(SICI)1097-4652(199903)178:3<311::AID-JCP5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 13.Wang H.G., Pathan N., Ethell I.M., Krajewski S., Yamaguchi Y., Shibasaki F., McKeon F., Bobo T., Franke T.F., Reed J.C. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B.A., Yuan J. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 15.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa H., Sung J.H., Matsumiya S., Uchiyama M. Main ginseng saponin metabolites formed by intestinal bacteria. Planta Med. 1996;62:453–457. doi: 10.1055/s-2006-957938. [DOI] [PubMed] [Google Scholar]
- 17.Lee I.K., Kang K.A., Lim C.M., Kim K.C., Kim H.S., Kim D.H., Kim B.J., Chang W.Y., Choi J.H., Hyun J.W. Compound K, a metabolite of ginseng saponin, induces mitochondria-dependent and caspase-dependent apoptosis via the generation of reactive oxygen species in human colon cancer cells. Int J Mol Sci. 2010;11:4916–4931. doi: 10.3390/ijms11124916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim A.D., Kang K.A., Zhang R., Lim C.M., Kim H.S., Kim D.H., Jeon Y.J., Lee C.H., Park J., Chang W.Y. Ginseng saponin metabolite induces apoptosis in MCF-7 breast cancer cells through the modulation of AMP-activated protein kinase. Environ Toxicol Pharmacol. 2010;30:134–140. doi: 10.1016/j.etap.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Kim D.Y., Yuan H.D., Chung I.K., Chung S.H. Compound K, intestinal metabolite of ginsenoside, attenuates hepatic lipid accumulation via AMPK activation in human hepatoma cells. J Agric Food Chem. 2009;57:1532–1537. doi: 10.1021/jf802867b. [DOI] [PubMed] [Google Scholar]
- 20.Kim D.Y., Park M.W., Yuan H.D., Lee H.J., Kim S.H., Chung S.H. Compound K induces apoptosis via CAMK-IV/AMPK pathways in HT-29 colon cancer cells. J Agric Food Chem. 2009;57:10573–10578. doi: 10.1021/jf902700h. [DOI] [PubMed] [Google Scholar]
- 21.Cho S.H., Chung K.S., Choi J.H., Kim D.H., Lee K.T. Compound K, a metabolite of ginseng saponin, induces apoptosis via caspase-8-dependent pathway in HL-60 human leukemia cells. BMC Cancer. 2009;9:449. doi: 10.1186/1471-2407-9-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Micoud F., Mandrand B., Malcus-Vocanson C. Comparison of several techniques for the detection of apoptotic astrocytes in vitro. Cell Prolif. 2001;34:99–113. doi: 10.1046/j.1365-2184.2001.00201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann S.H., Hengartner M.O. Programmed cell death: alive and well in the new millennium. Trends Cell Biol. 2001;11:526–534. doi: 10.1016/s0962-8924(01)02173-0. [DOI] [PubMed] [Google Scholar]
- 24.Vandewynckel Y.P., Laukens D., Geerts A., Bogaerts E., Paridaens A., Verhelst X., Janssens S., Heindryckx F., Van Vlierberghe H. The paradox of the unfolded protein response in cancer. Anticancer Res. 2013;33:4683–4694. [PubMed] [Google Scholar]
- 25.Park J.W., Woo K.J., Lee J.T., Lim J.H., Lee T.J., Kim S.H., Choi Y.H., Kwon T.K. Resveratrol induces pro-apoptotic endoplasmic reticulum stress in human colon cancer cells. Oncol Rep. 2007;18:1269–1273. [PubMed] [Google Scholar]
- 26.Rasheva V.I., Domingos P.M. Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis. 2009;14:996–1007. doi: 10.1007/s10495-009-0341-y. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa T., Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 29.Mekahli D., Bultynck G., Parys J.B., De Smedt H., Missiaen L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004317. a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilieva E.V., Kichev A., Naudi A., Ferrer I., Pamplona R., Portero-Otin M. Mitochondrial dysfunction and oxidative and endoplasmic reticulum stress in argyrophilic grain disease. J Neuropathol Exp Neurol. 2011;70:253–263. doi: 10.1097/NEN.0b013e31820f8765. [DOI] [PubMed] [Google Scholar]
- 31.Dolai S., Pal S., Yadav R.K., Adak S. Endoplasmic reticulum stress-induced apoptosis in Leishmania through Ca2+-dependent and caspase-independent mechanism. J Biol Chem. 2011;286:13638–13646. doi: 10.1074/jbc.M110.201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hetz C. The UPR as a survival factor of cancer cells. More than folding proteins? Leuk Res. 2009;33:880–882. doi: 10.1016/j.leukres.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Chae S., Kang K.A., Chang W.Y., Kim M.J., Lee S.J., Lee Y.S., Kim H.S., Kim D.H., Hyun J.W. Effect of compound K, a metabolite of ginseng saponin, combined with gamma-ray radiation in human lung cancer cells in vitro and in vivo. J Agric Food Chem. 2009;57:5777–5782. doi: 10.1021/jf900331g. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman R.J. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 35.Rao R.V., Hermel E., Castro-Obregon S., del Rio G., Ellerby L.M., Ellerby H.M., Bredesen D.E. Coupling endoplasmic reticulum stress to the cell death program. Mechanism of caspase activation. J Biol Chem. 2001;276:33869–33874. doi: 10.1074/jbc.M102225200. [DOI] [PubMed] [Google Scholar]
- 36.Zhang R., Chung Y., Kim H.S., Kim D.H., Chang W.Y., Hyun J.W. 20-O-(beta-D-glucopyranosyl)-20(S)-protopanaxadiol induces apoptosis via induction of endoplasmic reticulum stress in human colon cancer cells. Oncol Rep. 2013;29:1365–1370. doi: 10.3892/or.2013.2270. [DOI] [PubMed] [Google Scholar]
- 37.Deniaud A., Sharaf el dein O., Maillier E., Poncet D., Kroemer G., Lemaire C., Brenner C. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 38.Haga N., Saito S., Tsukumo Y., Sakurai J., Furuno A., Tsuruo T., Tomida A. Mitochondria regulate the unfolded protein response leading to cancer cell survival under glucose deprivation conditions. Cancer Sci. 2010;101:1125–1132. doi: 10.1111/j.1349-7006.2010.01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]