Preventive application of heparinoid moisturizer from the first day of WBRT significantly increased water content and helped to improve skin desquamation and dryness compared with no treatment.
Keywords: breast cancer, radiation dermatitis, skin hydration, emollients, randomized controlled trial
Abstract
Background
The application of heparinoid moisturizer for 2 weeks following whole-breast radiotherapy (WBRT) was previously reported to significantly increase skin water content (WC) and help improve skin dryness and desquamation. The prospective open-label, randomized trial included an exploratory arm to investigate the preventive efficacy of heparinoid moisturizer for acute radiation dermatitis (ARD).
Methods
Between April 2011 and April 2013, patients receiving WBRT were assigned (1:2:2) to receive either: moisturizer for prophylaxis (group P), moisturizer starting 2 weeks after WBRT for treatment (group M), and no moisturizer (group C). This paper presents the results of comparison between the exploratory arm and no moisturizer group. Skin WC was measured prior to WBRT, on the last day of WBRT, and 2 weeks, 4 weeks and 3 months following WBRT. Signs and symptoms were also assessed.
Results
Comparing two groups, WC values were significantly higher in group P until 4 weeks following WBRT. At 2 weeks following WBRT, mean WC values in group P and C were 38.5 ± 6.1 arbitrary units (a.u.) and 30.2 ± 7.8 a.u., respectively (P < 0.001). In group C, dryness was more severe at 2 and 4 weeks following WBRT and desquamation more severe until 3 months following WBRT. However, the erythema score showed no difference between the two groups. Regarding symptoms, group C pain scores on the last day of WBRT were significantly higher than in group P (P < 0.030).
Conclusions
The preventive application of heparinoid moisturizer has the potential of reducing skin desquamation and dryness in patients receiving WBRT.
Introduction
A systematic review confirmed that post-operative radiotherapy (RT) reduces recurrence and breast cancer death after breast-conserving surgery (1,2). Radiotherapy is therefore essential for patients undergoing breast-conserving surgery.
For almost all patients undergoing breast radiotherapy, acute radiation dermatitis (ARD) is the most common reported reaction (3). ARD occurs between a few hours and a few weeks following RT and symptoms present at different degrees of type and severity: erythema, skin dryness, desquamation, moist desquamation, and sometimes ulceration. In contrast, chronic radiation dermatitis occurs between a few months and a few years after RT and leads to symptoms such as fibrosis, pigmentation, skin atrophy and telangiectasia (4). Owing to these symptoms, radiation dermatitis has a profound impact on patients’ quality of life (QOL). In addition, ARD may be the cause of premature interruption of RT which can impact negatively on the control of cancer. Therefore, managing radiation dermatitis is an important priority in caring for patients undergoing RT. Although many topical agents are currently used in clinical practice for prevention and treatment of ARD, there has been little evidence to support the effectiveness of a specific medicine (5). Many patients undergoing RT in Japan are instructed to not apply any topical agent providing that ARD is not severe.
In our previous report, the application of heparinoid moisturizer for 2 weeks following whole-breast radiotherapy (WBRT) significantly increased skin water content (WC) and helped improve skin dryness and desquamation compared with no use of moisturizer (6). The aim of this study is to assess the efficacy of heparinoid moisturizer as a prophylactic agent for ARD in patients with breast cancer receiving WBRT.
Patients and methods
Study design
The prospective open-label, randomized trial was originally planned to evaluate the efficacy of the application of heparinoid moisturizer after 2 weeks following WBRT, comparing the moisturizer treatment group vs. control. The results of this comparison were reported previously (6). The third group had been prospectively set for the exploratory aim of investigating the efficacy of moisturizer as a prophylactic agent. According to block randomization, patients receiving WBRT were assigned (1:2:2) to receive either: moisturizer for prophylaxis (exploratory, group P), moisturizer starting 2 weeks after WBRT for treatment (group M), and no moisturizer (group C). This paper presents the results of comparison between the exploratory arm and the control group.
Patients in group P were instructed to apply the heparinoid moisturizer from the first day of WBRT and to continue to use the moisturizer twice daily until 3 months after completion of WBRT. Group C patients were instructed to not apply any topical moisturizer during the study period (although patients with itchy or reddened skin could use topical corticosteroids). Randomization was stratified according to the beam energy (4 MV vs 6 MV).
Patients
Eligible patients were women aged between 30 and 65 years with histologic evidence of a primary invasive breast cancer or ductal carcinoma in situ who underwent lumpectomy at St Luke’s International Hospital. The tumor was required to be located outside the inner-upper quadrant, which was designated as a skin WC measurement site. Patients with the following characteristics were excluded: a history of RT to the thorax; wide-spreading skin disease; collagen vascular disease; sensitivity to heparinoid substance; and patients who did not keep to the instructions on how to apply the topical agents on the breast. A compliance rate of 60% was set as the lower limit for acceptance.
Radiation therapy
The treatment plan was devised with computed tomography scans in all cases. The field-in-field technique (7) was used and efforts were made to ensure that the breast treatment volume received was not less than 95%, nor more than 107%, of the prescribed dose. Conventional fractionation was used in 2 Gy 5 days a week up to 48–50 Gy with photons (4–6 MV). An additional dose of 10–18 Gy was boosted with an electron beam (4–6 MeV), if necessary, according to the patient’s age and pathological findings.
Moisturizer treatment
Hirudoid® (Maruho, Osaka, Japan), was applied to group P patients as the designated moisturizer as it is commonly used in Japan. It contains mucopolysaccharide polysulphate (at 0.3% w/w), which is structurally closely related to components of the connective tissue. The efficacy of heparinoid moisturizer has previously been demonstrated in the treatment of milder atopic dermatitis (8).
Study outcomes
Skin water content
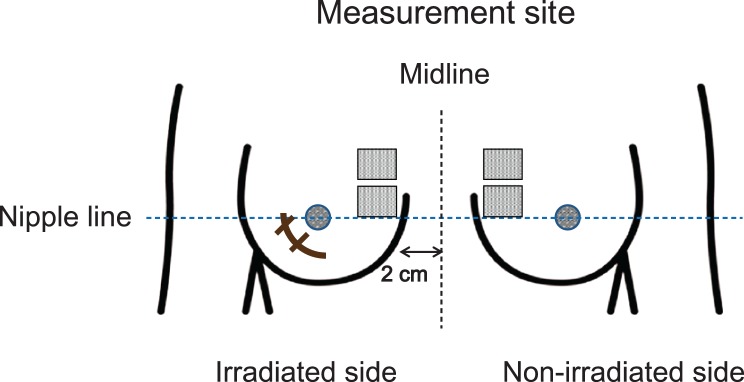
The primary outcome measure was a comparison of WC at each study point between the two groups. Skin WC was measured using the corneometer CM825® (Courage+Khazaka, Cologne, Germany). On the day of measurement, patients were instructed to not bathe in the morning and to not apply any topical agents to the bilateral breast. Following a minimum of 20 min bed rest, the skin was washed with hypoallergenic soap. After wiping gently with lint-free cloth, the skin was dried well for 20 min and WC was measured using a corneometer. The two designated areas for WC measurement were 3 × 3 cm2 for each skin area analysis in an upper-inner quadrant of the irradiated breast, at least 2 cm apart from the midline. These areas had to be at least 2 cm away from the surgical wound and 1 cm away from the edge of the boost. WC was measured a total of five times at different points in each area, and the mean value of 10 measurements was used for analysis. For controls, the corresponding area of skin of the non-irradiated breast was measured in the same manner (6) (Fig. 1).
Figure 1.
Measurement site for water content.
Skin-related signs and symptoms
Secondary outcomes of the study were to compare ARD signs and symptom between two groups.
Clinical evaluation
A dermatologist and a radiation oncologist assessed signs associated with ARD (i.e., skin dryness, desquamation and erythema) using different scoring scales. The dermatologist used atopic dermatitis severity classification of dryness and desquamation (9), while radiation oncologists assessed ARD using a simpler grading system. Erythema was assessed with the same scale. Radiation oncologists directly assessed ARD under direct vision, whereas the dermatologist, who was blinded for the treatment group, assessed ARD signs via digital photographs (Table 1).
Table 1.
Severity scoring system for acute radiation dermatitis
| Severity score | Dryness and desquamation | Erythema | |
|---|---|---|---|
| Dermatologist (D) | Radiation oncologist (RO) | D and RO | |
| 0 | None | None | None |
| 1 | Mild | Mild | Faint |
| 2 | Moderate | Moderate-Severe | Moderate |
| 3 | Severe | – | Brisk |
| 4 | Very severe | – | – |
The diary was distributed to all the participants so that they could record about daily compliance of the heparinoid moisturizer and rate the degree of itching and pain within the irradiated field by means of the visual analog scale (VAS) (a 100-mm horizontal line that was anchored at the extremes by ‘none’ and ‘severe’).
Statistical methods
Two sets of skin WC measurements between groups and study points, skin toxicity scores, VAS and demographic factors were compared for differences using the Wilcoxon signed-rank test with Bonferroni’s correction, Mann–Whitney U test, Student’s t-test, and χ2 test. All P values expressed are two-sided with statistical significance evaluated at the 0.05 alpha level. Statistical analyses were performed using SPSS software (IBM SPSS statistics 25; IBM Corp, New York, NY, USA).
Ethical considerations
The study was approved by the institutional review board. Consent was obtained from all patients and signed copies of the consent form were provided to each of these patients. The completed study is registered with UMIN-CTR5532.
Results
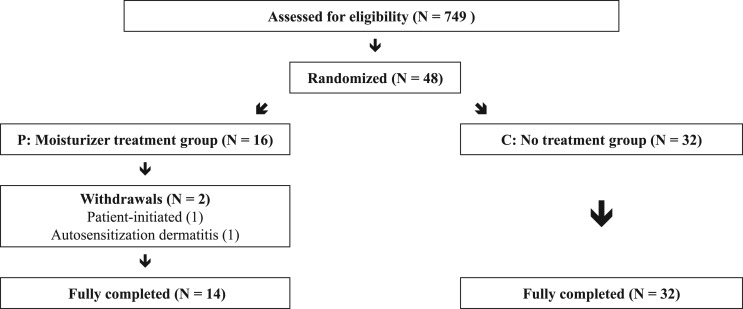
As previously mentioned, group P was an exploratory arm of the main study. As such, the required number of prophylactic group was not estimated and the enrollment was stopped in accordance with the main study. A total of 749 patients underwent RT following breast-conserving surgery between April 2011 and April 2013. Among those patients, 48 constituted the exploratory and control cohort. There were two withdrawals due to consent decline and autosensitization dermatitis. Therefore, 14 patients remained on the preventative application group P, and 32 patients on the control group C (Fig. 2). All patients in each group completed every WC measurements and clinical assessment.
Figure 2.
Consort trial flow diagram.
Despite the small number of patients, the patient and treatment characteristics were similar with respect to age, tumor location, breast volume, adjuvant therapy, boost dose, photon energy, smoking history and body mass index. Each group was almost identical in terms of the rate of topical steroid use in the treated breast (Table 2).
Table 2.
Patient and treatment characteristics
| Group P N = 14 | Group C N = 32 | P* | |
|---|---|---|---|
| Age (mean ± SD) | 46.4 ± 9.0 | 51.6 ± 7.2 | 0.073 |
| Affected breast | |||
| Right | 6 | 18 | |
| Left | 8 | 14 | 0.40 |
| Tumor location | |||
| Lateral | 13 | 28 | |
| Inner | 0 | 3 | |
| Central | 1 | 1 | 0.43 |
| Breast size: CTV (cm3) (mean ± SD) | 331.4 ± 176.7 | 330.0 ± 137.1 | 0.98 |
| V107% (%) (mean ± SD) | 2.47 ± 7.5 | 0.41 ± 0.87 | 0.32 |
| Chemotherapy before RT | 4 | 4 | 0.22 |
| Endocrine therapy | 1 | 1 | 0.52 |
| Boost | |||
| 6-16 Gy | 9 | 16 | |
| No boost | 5 | 16 | 0.37 |
| Energy | |||
| 4 MV | 10 | 24 | |
| 6 MV | 4 | 8 | 0.54 |
| Smoker | |||
| Never | 10 | 25 | |
| Former | 4 | 6 | |
| Current | 0 | 1 | 0.63 |
| Body mass index (mean ± SD) | 21.6 ± 3.5 | 21.2 ± 3.2 | 0.69 |
| Topical corticosteroids use in the treated breast during or after RT | 6 | 9 | 0.63 |
*Student’s t-test, chi-square test.
CTV (cm3), clinical target volume (=breast tissue); V107% (%), breast volume receiving 107% of prescribed dose; RT, radiotherapy.
Time-course of skin WC
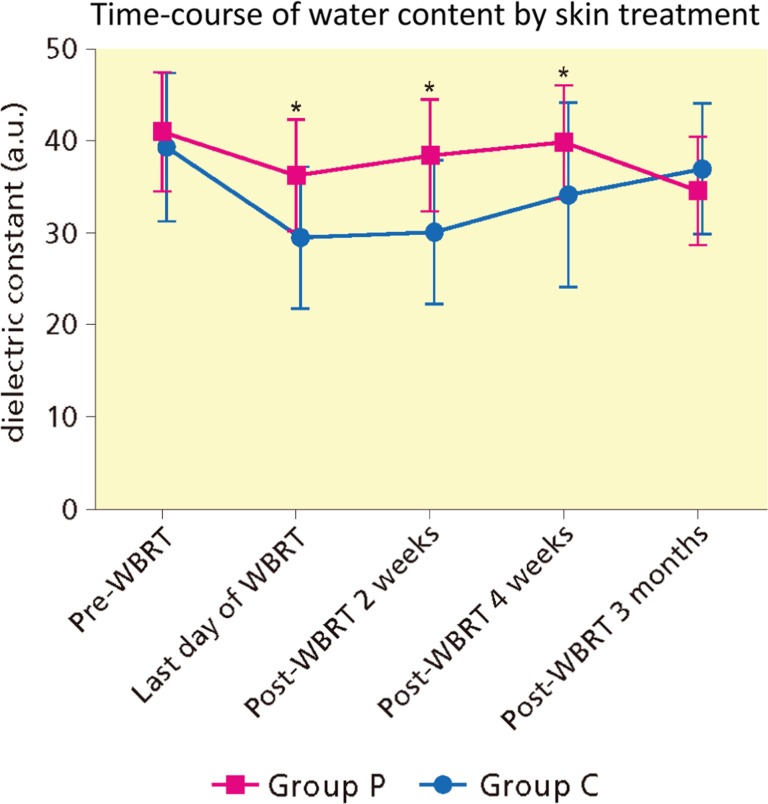
The skin WC in group C was significantly lower than the pre-WBRT level until 4 weeks following WBRT. In contrast, the skin WC in group P significantly decreased at the last day of WBRT compared with baseline, and improved at 2 weeks following RT. Thereafter, until 3 months, no significant difference with baseline was noted (Fig. 3 and Supplementary Material S1). Comparisons between two groups revealed that WC in group C was significantly lower than those in group P from the last day of WBRT until 4 weeks following WBRT. However, there was no significant difference at 3 months following WBRT (P = 0.28).7
Figure 3.
Time-course of water content by skin treatment. WBRT, whole breast radiotherapy. *Statistically significant by Mann–Whitney-U test.
Clinical assessment
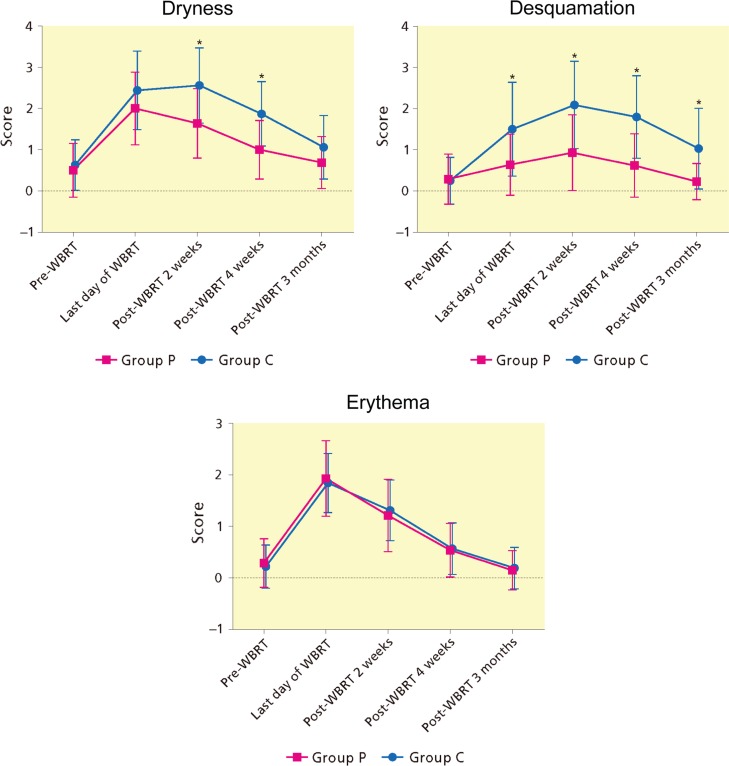
According to the skin toxicity score graded by the dermatologist, all scores were maximal at the end of RT or 2 weeks following WBRT (Fig. 4 and Supplementary Material S2). Dryness scores in group C were significantly higher from the last day until 4 weeks following WBRT compared to pre-WBRT score. The time-course in group P showed a similar and lower curve to that in group C; however, there was no difference at 4 weeks and 3 months following WBRT. Comparison between two groups revealed a significant difference at 2 weeks and at 4 weeks following WBRT. Regarding desquamation in group C, scores were significantly higher than pre-WBRT at the last day of WBRT and continued to the end of study. In contrast, there was no significant difference at every study point in group P, compared with pre-WBRT. As a result, score curves between the two groups were more separated and significant difference between two groups remained after the completion of WBRT until 3 months following WBRT. Erythema scores of two groups were almost similar through the study period. The skin toxicity score by radiation oncologists was almost identical to the score made by the dermatologist (data not shown).
Figure 4.
Time-course of grading score of ARD signs by skin treatment. ARD, acute radiation dermatitis; WBRT, whole breast radiotherapy. *Statistically significant by Mann–Whitney-U test.
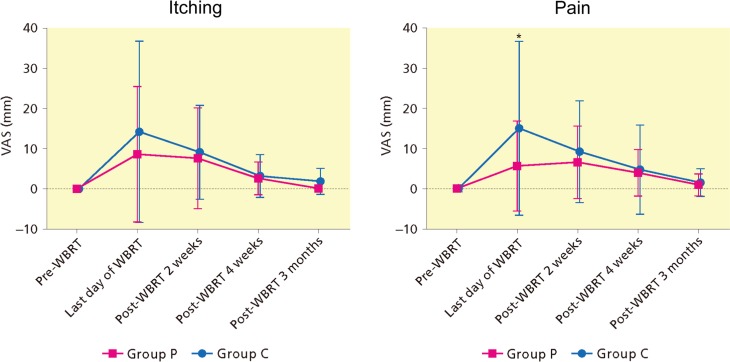
The itching and pain VAS scores, generally low, were highest at the last day or 2 weeks following WBRT, and gradually improved in both groups. Itching and pain scores in group C were higher compared with pre-WBRT from the last day until 4 weeks or 3 months following WBRT. Skin symptom score demonstrated no differences between groups except pain at the last day of WBRT (Fig. 5,Supplementary Material S3). One of 15 patients suffered from autosensitization dermatitis due to heparinoid moisturizer; this improved after the cessation of application and she was able to complete WBRT without delay.
Figure 5.
Time-course of patient reported symptoms (visual analog scale) by skin treatment. VAS, Visual Analog Scale; WBRT, whole breast radiotherapy. *Statistically significant by Mann–Whitney-U test.
Discussion
Adequate skin hydration is critical for maintaining healthy skin. In normal skin, the ability to hold water is primarily related to the epidermis and its outermost stratum corneum (SC), which plays the role of barrier to water loss (10).
The retention of water in the SC is dependent on two major components. First, natural moisturizing factor (NMF) within the corneocytes (SC cells). Secondly, the SC intercellular lipids orderly arranged to form a barrier to transepidermal water loss (TEWL) (10). In addition, in the upper chest which is one of the highest concentration area of sebaceous glands (11), the endogenous glycerol which is derived from the sebaceous gland and also from the circulation plays an important role in SC hydration. Sebaceous glands-enriched skin sites display higher SC hydration (10).
Skin is susceptible to radiation damage because the basal keratinocytes, hair follicle stem cells, and melanocytes are highly radiosensitive (4). Furthermore, sebaceous glands (12) and eccrine sweat glands (13) are also very sensitive to radiation. Radiation skin injury involves immediate damage to these cells after which the barrier function of epidermis to hold water suffers serious damage. It causes a reduction in NMF and intercellular lamellar lipids, and severe damage to sweat and sebaceous glands, leading to a loss of skin barrier function. Consequently, irradiated skin often feels drier, which causes symptoms such as discomfort, irritation, itching and pain (14).
Although ARD is a common adverse event following RT, few systematic studies for prevention and management have been published on this topic (5,14). Currently, a variety of interventions are used for topical therapy of ARD, however, randomized controlled trials (RCTs) have not yet consistently indicated the superiority of any single agent (15). In a pooled analysis and systematic review, the prophylactic application of topical corticosteroid in breast cancer patients undergoing radiotherapy appears to significantly reduce the incidence of ARD, specifically moist desquamation, compared with other treatments (16,17). However, the use of topical steroids may result in thinning of the SC, which leads to the transepidermal water loss (TEWL) and skin irritation (18). Furthermore, moist desquamation beyond skin folds and creases is infrequent using megavoltage irradiation to WBRT (19).
So, we evaluated in a randomized fashion whether heparinoid moisturizer was appropriate candidate as non-steroidal prophylactic agent or not for ARD. WC values of SC, and skin related signs and symptoms were monitored.
Cutaneous barrier function can be assessed quantitatively using bioengineering techniques, such as measurement of the capacitance of a dielectric medium (corneometer) and TEWL. Both hydrometers are deemed relevant and valid for assessment of skin moisture, however the corneometer might be more sensitive (20). The findings on ARD using TEWL measurement have conflicted in the literature (21,22). With the corneometer CM825®, the measurement depth is very small (10–20 μm of the SC) to exclude the influence of deeper skin layers like the blood vessels. The measurement accuracy has ever been evaluated in a broad multicentric study (23). In our previous report, with interindividual difference and seasonal fluctuation in moisture state taken into account, the normalized ratio of skin WC ratio between irradiated and non-irradiated field was used to assess moisture recovery. However, unexpectedly, WC values of SC as a reference in the non-irradiated fields temporally decreased over time without significant change (Supplementary Material S4). Hence, in this report, we used raw a.u. to evaluate WC changes. The distance of ≥2 cm from the field edge was insufficient. According to Epstein et al., the surface dose of contralateral breast 2–4 cm from the midline receives 3–12% of the prescribed dose (24).
The time-course of WC from pre-WBRT in the irradiated field was again analyzed. However, contrary to the previous report, there was no significant difference at the 3 months following RT, owing to analysis using a.u. The immediate decrease and following slow recovery of WC after WBRT were consistent with the time-course reported by Yamazaki et al. (25). Comparing two groups, the group P significantly preserved WC at the last day, 2 and 4 weeks after RT. However, there was no significant difference at 3 months after WBRT (Fig. 3,Supplementary Material S1). Di Franco et al. reported the preventive effect of five types of moisturizer cream for ARD in breast cancer patients. Patients began the application of cream 15 days before and stopped to use one month after WBRT. They confirmed using corneometer that skin hydration increased at the end of topical treatment. All moisturizing creams used in their study were equally valid (26). In our study, WC values at pre-RT and 4 weeks following WBRT were 41.1 ± 6.5 a.u. and 40.0 ± 6.2 a.u., respectively (P = 1.00) (Supplementary Material S1). Although the reason for the discrepancy between studies is unclear, each time we measured WC after taking 20 min bed rest, washing with soap, wiping and drying well.
There may be several reasons for the non-significant difference between groups at 3 months. First, the slow and natural recovery of WC following WBRT noted in the control group may have contributed to it. Second, it may be caused by a change in the patient’s pattern of behavior. The compliance of moisture application gradually decreased over time. Although the rate was within acceptance, it decreased slightly from 96%, 93%, 92% and 89% at the last day, 2 weeks, 4 weeks, and 3 months following WBRT, respectively. Third, at 3 months following WBRT in the late-acute phase of dermatitis, damage to the SC might be so prominent that it is unable to maintain any WC provided by the moisturizer.
The severity of signs of ARD, such as dryness and desquamation, which was evaluated by the experienced dermatologist, correlated well with WC as a marker of skin barrier function. Those signs remained milder in the moisturizer group. At 2 weeks and 4 weeks following WBRT, dryness score was significantly less in group P, compared with group C. Desquamation score was also significantly less from the last day until 3 months following RT in group P, compared with group C. In contrast, there was no difference between erythema score curves.
Four RCTs have assessed non-steroidal topical products as a prophylactic agent for ARD compared to no treatment. Williams et al. found no difference in ARD severity including erythema and desquamation with or without Aloe vera gel in breast cancer patients receiving RT (27). Fenig et al. also evaluated two topical moisturizers in patients receiving WBRT and found no advantage for either preparation compared to no treatment in terms of the proportion of Grade 3–4 dermatitis (P = 0.15) (28). In contrast, Rizza et al. examined the time-course of erythema in breast cancer patients using non-invasive instrumental reflectance spectrophotometry. Patients were randomized either to treatment (one of two topical agents, twice daily from the first day of WBRT over 8 weeks) or nontreatment groups. The application of topical moisturizers was found to significantly reduce erythema compared with the non-treated group (29). Wells et al. tested aqueous, sucralfate, or no cream in patients mainly with head and neck and breast cancer. Patients randomized to either of the two creams were advised to apply it to the treatment area twice daily, from the first day of RT. The investigators evaluated ARD with modified Radiation Therapy Oncology Group (RTOG) score and erythema with reflectance spectrophotometry (30). They analyzed data at the fifth week after the start of RT as the time at which the worst skin reactions were apparent. On an adjusted analysis, desquamation score was lower for the sucralfate and aqueous cream groups than no-cream group (P = 0.04). However, the differences of erythema score by modified RTOG grading system between three groups were clearly small (P = 0.69). Even when the reflectance spectrophotometry was used, erythema meter readings showed that sucralfate was better than aqueous cream, but it was not better than no cream (P = 0.41). An analysis of itching and pain on the patient diary card revealed only small differences between groups (P = 0.97, P = 0.77 respectively). In our study, although the use of the heparinoid moisturizer helped alleviate the pain related to ARD, the scores were generally low, suggesting that the positive influence of heparinoid moisturizer is limited.
For breast cancer patients receiving RT, dryness is the most uncomfortable symptom of ARD for which they want management (3). Previously, we reported the application of heparinoid moisturizer for 2 weeks following WBRT significantly increased WC and helped improve skin dryness and desquamation compared with no use of moisturizer. However, we believe that the preventive application for ARD is more effective than a treatment of local side effects once they appear.
Findings in this study can be generalized to patients who receive postmastectomy RT because similar doses are generally used to the same body part. However, for patients with head and neck cancer, who often receive higher doses of RT in combination with concurrent chemotherapy, efficacy may be limited.
There were several limitations to our study. First, this study had small numbers of patients and confidence intervals were wide. Nevertheless, the preventive treatment of heparinoid moisturizer significantly helped to keep WC until 4 weeks following WBRT compared with no use. Second, the symptoms were assessed with VAS alone. As symptom severity is mild, and information from VAS is limited, it is hard to evaluate the usefulness of moisturizer from the point of view of impact on patient QOL. In a Phase 3 trial of momentasone cream vs. placebo to prevent ARD in breast cancer patients, patient-reported outcome (burning, itching, and tenderness) was not correlated with CTCAE (the Common Terminology Criteria for Adverse Events) (31). We are currently recruiting patients into a new RCT to evaluate the preventive efficacy of heparinoid moisturizer using DLQI (32), which is one of the instruments to measure the effects of skin disease on patients’ QOL (trial ID: UMIN000026987).
The present study has also several strengths. First, to our knowledge, the present study is the only randomized trial to compare the time course of WC in patients with or without moisturizer during and after WBRT. The WC of SC was prospectively measured in a specific, accurate and reproducible way using the corneometer, which is useful for objectively assessing ARD for breast cancer patients (25).
Furthermore, this is the first study using a blinded method for the evaluation of ARD signs taken by digital camera. Skin appearance on digital images was evaluated by an expert dermatologist blinded to the randomly assigned group. According to Wengstrom et al., visual assessment by the RTOG scoring system correlated well with that by digital photographs (33).
In conclusion, the preventive application of heparinoid moisturizer was safe and improved WC loss during and after WBRT. It also helped improve skin desquamation and dryness. These findings suggest that the preventive application of heparinoid moisturizer from the early phase of RT is recommended for breast cancer patients receiving WBRT.
Supplementary Material
Acknowledgements
Aspects of this study were presented at the 56th ASTRO (San Francisco, USA, September, 2014). The authors are grateful to Ms M. Hirose and Ms R. Nagasaka for technical assistance. The authors are also grateful to Dr Martin Guppy for editorial assistance.
Supplementary data
Supplementary data are available at Japanese Journal of Clinical Oncology online.
Funding
This research was supported by Research Grant of St. Luke’s International Hospital.
Conflict of interest statement
The authors declare that they have no competing interests.
References
- 1. Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, et al. . Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, et al. . Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014;383:2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee J, Park W, Choi DH, et al. . Patient-reported symptoms of radiation dermatitis during breast cancer radiotherapy: a pilot study. Qual Life Res 2017;26:1713–9. [DOI] [PubMed] [Google Scholar]
- 4. Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol 2012;132:985–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh M, Alavi A, Wong R, Akita S. Radiodermatitis: a review of our current understanding. Am J Clin Dermatol 2016;17:277–92. [DOI] [PubMed] [Google Scholar]
- 6. Sekiguchi K, Ogita M, Akahane K, et al. . Randomized, prospective assessment of moisturizer efficacy for the treatment of radiation dermatitis following radiotherapy after breast-conserving surgery. Jpn J Clin Oncol 2015;45:1146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de la Torre N, Figueroa CT, Martinez K, Riley S, Chapman J. A comparative study of surface dose and dose distribution for intact breast following irradiation with field-in-field technique vs. the use of conventional wedges. Med Dosim 2004;29:109–14. [DOI] [PubMed] [Google Scholar]
- 8. Kawakami T, Soma Y. Questionnaire survey of the efficacy of emollients for adult patients with atopic dermatitis. J Dermatol 2011;38:531–5. [DOI] [PubMed] [Google Scholar]
- 9. Yoshida H, Aoki T, Furue M, et al. . English version of the interim report published in 1998 by the members of the Advisory Committee on Atopic Dermatitis Severity Classification Criteria of the Japanese Dermatological Association. J Dermatol 2011;38:625–31. [DOI] [PubMed] [Google Scholar]
- 10. Verdier-Sevrain S, Bonte F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermatol 2007;6:75–82. [DOI] [PubMed] [Google Scholar]
- 11. De Luca C, Valacchi G. Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediators Inflamm 2010;2010:321494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borak J, Leddy ET. The radiation biology of the cutaneous glands. Radiology 1936;27:365–5. [Google Scholar]
- 13. Johns H, Morris WJ, Joiner MC. Radiation response of murine eccrine sweat glands. Radiother Oncol 1995;36:56–64. [DOI] [PubMed] [Google Scholar]
- 14. McQuestion M. Evidence-based skin care management in radiation therapy: clinical update. Semin Oncol Nurs 2011;27:e1–17. [DOI] [PubMed] [Google Scholar]
- 15. Chan RJ, Larsen E, Chan P. Re-examining the evidence in radiation dermatitis management literature: an overview and a critical appraisal of systematic reviews. Int J Radiat Oncol Biol Phys 2012;84:e357–62. [DOI] [PubMed] [Google Scholar]
- 16. Meghrajani CF, Co HC, Ang-Tiu CM, Roa FC. Topical corticosteroid therapy for the prevention of acute radiation dermatitis: a systematic review of randomized controlled trials. Expert Rev Clin Pharmacol 2013;6:641–9. [DOI] [PubMed] [Google Scholar]
- 17. Haruna F, Lipsett A, Marignol L. Topical management of acute radiation dermatitis in breast cancer patients: a systematic review and meta-analysis. Anticancer Res 2017;37:5343–53. [DOI] [PubMed] [Google Scholar]
- 18. Lehmann P, Zheng P, Lavker RM, Kligman AM. Corticosteroid atrophy in human skin. A study by light, scanning, and transmission electron microscopy. J Invest Dermatol 1983;81:169–76. [DOI] [PubMed] [Google Scholar]
- 19. Shaitelman SF, Schlembach PJ, Arzu I, et al. . Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: a randomized clinical trial. JAMA Oncol 2015;1:931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blichmann CW, Serup J. Assessment of skin moisture. Measurement of electrical conductance, capacitance and transepidermal water loss. Acta Derm Venereol 1988;68:284–90. [PubMed] [Google Scholar]
- 21. Jensen JM, Gau T, Schultze J, et al. . Treatment of acute radiodermatitis with an oil-in-water emulsion following radiation therapy for breast cancer: a controlled, randomized trial. Strahlenther Onkol 2011;187:378–84. [DOI] [PubMed] [Google Scholar]
- 22. Schmuth M, Sztankay A, Weinlich G, et al. . Permeability barrier function of skin exposed to ionizing radiation. Arch Dermatol 2001;137:1019–23. [PubMed] [Google Scholar]
- 23. Heinrich U, Koop U, Leneveu-Duchemin MC, et al. . Multicentre comparison of skin hydration in terms of physical-, physiological- and product-dependent parameters by the capacitive method (Corneometer CM 825). Int J Cosmet Sci 2003;25:45–53. [DOI] [PubMed] [Google Scholar]
- 24. Epstein RJ, Kelly SA, Cook M, et al. . Active minimisation of radiation scatter during breast radiotherapy: management implications for young patients with good-prognosis primary neoplasms. Radiother Oncol 1996;40:69–74. [DOI] [PubMed] [Google Scholar]
- 25. Yamazaki H, Yoshida K, Kotsuma T, et al. . Longitudinal practical measurement of skin color and moisture during and after breast-conserving therapy: influence of neoadjuvant systemic therapy. Jpn J Radiol 2009;27:309–15. [DOI] [PubMed] [Google Scholar]
- 26. Di Franco R, Sammarco E, Calvanese MG, et al. . Preventing the acute skin side effects in patients treated with radiotherapy for breast cancer: the use of corneometry in order to evaluate the protective effect of moisturizing creams. Radiat Oncol 2013;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Williams MS, Burk M, Loprinzi CL, et al. . Phase III double-blind evaluation of an aloe vera gel as a prophylactic agent for radiation-induced skin toxicity. Int J Radiat Oncol, Biol, Phys 1996;36:345–9. [DOI] [PubMed] [Google Scholar]
- 28. Fenig E, Brenner B, Katz A, et al. . Topical Biafine and Lipiderm for the prevention of radiation dermatitis: a randomized prospective trial. Oncol Rep 2001;8:305–9. [PubMed] [Google Scholar]
- 29. Rizza L, D’Agostino A, Girlando A, Puglia C. Evaluation of the effect of topical agents on radiation-induced skin disease by reflectance spectrophotometry. J Pharm Pharmacol 2010;62:779–85. [DOI] [PubMed] [Google Scholar]
- 30. Wells M, Macmillan M, Raab G, et al. . Does aqueous or sucralfate cream affect the severity of erythematous radiation skin reactions? A randomised controlled trial. Radiother Oncol 2004;73:153–62. [DOI] [PubMed] [Google Scholar]
- 31. Neben-Wittich MA, Atherton PJ, Schwartz DJ, et al. . Comparison of provider-assessed and patient-reported outcome measures of acute skin toxicity during a Phase III trial of mometasone cream versus placebo during breast radiotherapy: the North Central Cancer Treatment Group (N06C4). Int J Radiat Oncol Biol Phys 2011;81:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol 1994;19:210–6. [DOI] [PubMed] [Google Scholar]
- 33. Wengstrom Y, Forsberg C, Naslund I, Bergh J. Quantitative assessment of skin erythema due to radiotherapy – evaluation of different measurements. Radiother Oncol 2004;72:191–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.