Abstract
An unusual case of infective endocarditis and concurrent multiple liver abscesses both caused by Streptococcus anginosus in a splenectomised patient is reported. The microorganism is a very rare cause of endocarditis and its presentation with multiple liver abscesses is highly unusual. It was initially misdiagnosed as Streptococcus sanguinis and issues relating to the different clinical presentations of S. anginosus including the rare cases of endocarditis, the role of the patient’s splenectomy and problems that may contribute to its potential laboratory misidentifications are discussed.
Keywords: Infective endocarditis, Streptococcus anginosus, Liver abscess
Background
Streptococcus anginosus is an extremely rare cause of infective endocarditis (IE), as is the presentation of IE with multiple liver abscesses caused by the same aetiological agent.
We report this association and review its clinical aspects, the pathogenicity of S. anginosus and possible association with the splenectomy and difficulties in its identification.
Case presentation
A 68-year-old man was admitted with fever and confusion. His medical history included hypertension, COPD and splenic artery aneurysm 9 years prior necessitating splenectomy. Before the operation, he had been vaccinated against Haemophilus influenzae, Streptococcus pneumoniae and Neisseria meningitidis.
Present illness included fever (>38.3°C), productive cough and weight loss over 3 weeks, associated with recent onset of confusion and increasingly aggressive behaviour. On admission, poor dental hygiene was noted, as well as crepitations over the right lower lung and bilateral leg oedema with normal jugular venous pressure. Heart sounds were normal with no murmurs. Liver was neither enlarged nor tender to palpation. There were no peripheral stigmata of IE. He was aggressive and confabulative but orientated and without focal neurological findings. Haemoglobin was 11.4 g/dL, white cell count (WCC) 22.4×109/L, C-reactive protein 130 mg/dL (n<6) and serum albumin 1.9 g/dL (globulins 2.1 g/dL) with normal urinalysis, renal function and liver enzymes. Chest X-ray showed right lower lobe (RLL) infiltrate and widespread interstitial changes. The ECG was unremarkable.
Initial working diagnosis was pneumonia and, possibly, associated encephalitis. Empiric ceftriaxone, ampicillin and acyclovir treatment was started.
Investigations
Head CT showed cortical atrophy. A lumbar puncture was performed and the cerebrospinal fluid showed 15 red blood cells, two WCC, normal glucose and protein levels and negative smear and culture. Serologies for HIV, Q fever, rickettsia and blood cryptococcal antigen were all negative. Chest and abdominal CT showed RLL consolidation with air bronchogram and surprisingly demonstrated hypodense liver lesions up to 62 mm, initially reported as suggestive of metastases (figure 1). No other biliary or abdominal abnormality was found.
Figure 1.
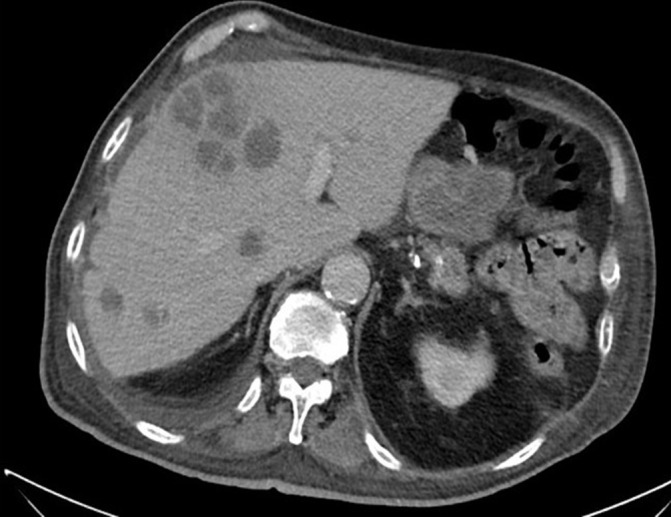
The patient’s CT near admission revealing multiple large hypodense liver lesions. The largest was aspirated under CT guidance yielding pus that was positive for Streptococcus anginosus using molecular methods.
All blood cultures grew Streptococcus sanguinis as identified by VITEK 2 system (bioMérieux, Marcy l’Etoile, France). Transthoracic echocardiography was unremarkable. Transoesophageal echocardiography showed a distorted aortic valve with small irregular mobile elements on the right coronary cusp and on the non-coronary cusp, consistent with vegetations. The patient denied any recent dental or other procedures. Colonoscopy, done at a later stage to rule out a possible colonic neoplasm as a portal of entry of streptococcal bacteraemia, was normal.
CT-guided aspiration of the largest liver abscess yielded 10 mL of pus. Culture was negative but 16S gene sequencing identified S. anginosus. This raised concerns that the initial identification of haematogenous S. sanguinis was incorrect. The blood culture isolate was retested using 16S gene sequencing and indeed, the same S. anginosus was identified in the blood and in the liver abscess.
Differential diagnosis
Before aspiration, the liver imaging was considered to be liver metastases—but this was inconsistent with the relatively short history of the patient’s illness, the fever, pneumonia and positive blood cultures. The differential diagnosis of liver abscesses includes pyogenic and amoebic abscess. These cannot be reliably distinguished by imaging studies alone. The proof was in the aspiration of pus and its analysis. Pyogenic liver abscess is most often due to spread of an infection from an abdominal focus either by portal vein pyaemia or a direct spread from biliary infection. When these are ruled out, haematogenous seeding from the systemic circulation has been well-documented, as in our patient.
The other considerations of this CT appearance of the liver are hepatic hydatid (echinococcal) cyst which are multiple in 60% and hypodense but well defined, often with internal ‘daughter’ cysts and rim calcifications—unlike here, and biliary cystadenocarcinoma which is rare and occurs mostly in women.
Treatment
Despite sensitivity to penicillin (minimum inhibitory concentration <0.06), ceftriaxone 2 g/day and metronidazole were continued over 6 weeks counted from the first negative blood culture.
Outcome and follow-up
The patient showed significant improvement with time and repeat ultrasound showed complete resolution of the liver lesions.
Discussion
S. anginosus is a very unusual aetiological agent of IE, and patients with endocarditis whose presentation includes extensive liver abscesses are exceedingly rare. S. anginosus group includes three species: Streptococcus constellatus, Streptococcus intermedius and S. anginosus. These bacteria can be alpha, beta or non-haemolytic and can possess Lancefield group antigens A, C, G and F.1 Formerly called ‘S. milleri group’, these streptococci are commensals of the oropharynx and gastrointestinal tract, but are also associated with pyogenic invasive infections and predilection for abscess formation. For example, abscesses in varied organs have been reported, including periodontal abscess, cerebral abscess, lung abscess and pleural empyema; as well as deep abdominal infections such as liver abscess, peritonitis, cholangitis and appendicitis. The different species appear to be associated with varying clinical infections: S. constellatus is more likely than S. anginosus to cause deep abscesses, S. intermedius has an apparent predilection for liver and brain abscesses formation,2 and S. anginosus is more likely to cause bacteraemia, often part of a polymicrobial infection.3
Overall, the S. anginosus group are very rarely associated with IE. However, of all the members of its group, S. anginosus appears to have the highest predilection for infection of the heart valves.4 Woo et al reviewed 377 cases of IE and found only six caused by S. milleri group, all identified as S. anginosus using 16S rRNA gene sequencing.5 This association is also supported by an experimental model of endocarditis showing significantly higher microbial loads in vegetations of S. anginosus-infected rats.4 Junckerstorff et al did not find a statistically significant correlation between the species and infection site, but all three cases of IE in their study were caused by S. anginosus.6 Previous studies of S. milleri bacteraemia also had very low rates of IE, but bacteria were not identified to the species level.7
The taxonomy of S. anginosus group has caused confusion for many years, and the distinction of this group from other Viridans group streptococci (VGS) may still be problematic. Optimal subspecies identification should probably rely on molecular methods. VITEK 2 identification system can commonly misidentify S. anginosus as other types of the VGS,8 as occurred in our case. In the study of Woo et al, none of the six S. anginosus isolates were confidently identified by this or the Analytical Profile Index 20E (bioMérieux) systems. Matrix-assisted laser desorption/ionisation (MALDI) mass spectrometry may provide adequate identification of S. anginosus and S. constellatus subspecies, but does not reliably identify S. intermedius.9 The clinical implications are significant because the S. anginosus group have varying degrees of sensitivity to antibiotics, and different subtypes of VGS can be associated with varying clinical characteristics as mentioned previously. Studies have shown better accuracy in the identification of VGS when using alternative techniques with better sensitivity such as Phoenix10 and 16S sequencing. However, due to cost constraints, 16S sequencing is unavailable in most facilities. VITEK or MALDI provide rapid results and are more affordable; therefore, they are more commonly used on a daily basis in many facilities.
To the best of our knowledge, this is the first well-described case of a documented S. anginosus patient with IE presenting with multiple liver abscesses. Our patient also had RLL pneumonia, and although we have no proof of the aetiological agent, it is reasonable to assume that it was also due to S. anginosus.11 He had no risk factors for aspiration; however, poor oral hygiene and tooth brushing may be associated with a significant incidence of bacteraemia with IE-related species which is similar to tooth extraction.12 13 This may be a potential portal of entry of S. anginosus, causing IE and continuous bacteraemia with secondary pneumonitis and liver abscesses in a splenectomised patient. IE diagnosis in our patient satisfies the modified Duke’s criteria (one major, three minor). Rashid et al reported a case of S. milleri IE associated with liver abscess, but the abscess was polymicrobial, diagnostic criteria of IE were not specified, and the bacterium was not identified to the species level.14 One of the virulent mechanisms of the S. milleri group is its polysaccharide capsule, enabling its evasion from phagocytosis.15 Encapsulated bacteria are notorious causes of severe disease in splenectomised patients, and this may explain the unusual formation of multiple abscess in our patient.
In summary, we still have a lot to learn about the virulence mechanisms of the S. anginosus group, its clinical presentations and best diagnostic practices. This case serves as a reminder that S. anginosus is a bacterium that continues to surprise us and should be kept in mind when treating splenectomised patients with abscesses, endocarditis or bacteraemia due to any VGS. As result of this case, we have changed our approach to VGS isolates and all clinically significant VGS isolates will be subsequently sent for 16S sequencing for identification confirmation.
Patient’s perspective.
At first, I thought I had nothing but the influenza. Then, every day they discovered another problem…Now I have to endure endless antibiotic treatment by infusions every day, but I am really much better, thank G-d.
Learning points.
Streptococcus anginosus can cause liver abscesses, but it rarely causes infective endocarditis and the occurrence of both of these infections together is an even rarer entity.
Compared with the other bacteria in the S. anginosus group, S. anginosus is more likely to cause endocarditis.
S. anginosus are encapsulated bacteria which may account for their ability to cause abscesses at different sites, in our case—the liver. Splenectomised patients, such as our patient, may possibly be more vulnerable, but it has been reported in non-splenectomised patients as well.
Misidentification within the S. anginosus group is common using regular microbiological techniques and reliance on molecular methods (16S gene sequencing) is advisable.
Footnotes
Contributors: The patient was treated by all the authors and follow-up was done by TF and RC. TF, AS and RC planned the investigations and interpreted the results. The manuscript was primarily written by AS with significant input by TF, ID and RC.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Doern CD, Burnham CA. It’s not easy being green: the viridans group streptococci, with a focus on pediatric clinical manifestations. J Clin Microbiol 2010;48:3829–35. 10.1128/JCM.01563-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tran MP, Caldwell-McMillan M, Khalife W, et al. Streptococcus intermedius causing infective endocarditis and abscesses: a report of three cases and review of the literature. BMC Infect Dis 2008;8:154 10.1186/1471-2334-8-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claridge JE, Attorri S, Musher DM, et al. Streptococcus intermedius, Streptococcus constellatus, and Streptococcus anginosus ("Streptococcus milleri group") are of different clinical importance and are not equally associated with abscess. Clin Infect Dis 2001;32:1511–5. 10.1086/320163 [DOI] [PubMed] [Google Scholar]
- 4.Kitada K, Inoue M, Kitano M. Experimental endocarditis induction and platelet aggregation by Streptococcus anginosus, Streptococcus constellatus and Streptococcus intermedius. FEMS Immunol Med Microbiol 1997;19:25–32. 10.1111/j.1574-695X.1997.tb01069.x [DOI] [PubMed] [Google Scholar]
- 5.Woo PC, Tse H, Chan KM, et al. "Streptococcus milleri" endocarditis caused by Streptococcus anginosus. Diagn Microbiol Infect Dis 2004;48:81–8. 10.1016/j.diagmicrobio.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 6.Junckerstorff RK, Robinson JO, Murray RJ. Invasive Streptococcus anginosus group infection-does the species predict the outcome? Int J Infect Dis 2014;18:38–40. 10.1016/j.ijid.2013.09.003 [DOI] [PubMed] [Google Scholar]
- 7.Siegman-Igra Y, Azmon Y, Schwartz D. Milleri group streptococcus – a stepchild in the viridans family. Eur J Clin Microbiol Infect Dis 2012;31:2453–9. 10.1007/s10096-012-1589-7 [DOI] [PubMed] [Google Scholar]
- 8.Gavin PJ, Warren JR, Obias AA, et al. Evaluation of the Vitek 2 system for rapid identification of clinical isolates of gram-negative bacilli and members of the family Streptococcaceae. Eur J Clin Microbiol Infect Dis 2002;21:869–74. 10.1007/s10096-002-0826-x [DOI] [PubMed] [Google Scholar]
- 9.Woods K, Beighton D, Klein JL. Identification of the ’Streptococcus anginosus group' by matrix-assisted laser desorption ionization--time-of-flight mass spectrometry. J Med Microbiol 2014;63(Pt 9):1143–7. 10.1099/jmm.0.076653-0 [DOI] [PubMed] [Google Scholar]
- 10.Brigante G, Luzzaro F, Bettaccini A, et al. Use of the Phoenix automated system for identification of Streptococcus and Enterococcus spp. J Clin Microbiol 2006;44:3263–7. 10.1128/JCM.00299-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinzato T, Saito A. The Streptococcus milleri group as a cause of pulmonary infections. Clin Infect Dis 1995;21(Suppl 3):S238–S243. 10.1093/clind/21.Supplement_3.S238 [DOI] [PubMed] [Google Scholar]
- 12.Lockhart PB, Brennan MT, Sasser HC, et al. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008;117:3118–25. 10.1161/CIRCULATIONAHA.107.758524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mougeot FK, Saunders SE, Brennan MT, et al. Associations between bacteremia from oral sources and distant-site infections: tooth brushing versus single tooth extraction. Oral Surg Oral Med Oral Pathol Oral Radiol 2015;119:430–5. 10.1016/j.oooo.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 14.Rashid RM, Salah W, Parada JP. ’Streptococcus milleri' aortic valve endocarditis and hepatic abscess. J Med Microbiol 2007;56(Pt 2):280–2. 10.1099/jmm.0.46781-0 [DOI] [PubMed] [Google Scholar]
- 15.Whitworth JM. Lancefield group F and related streptococci. J Med Microbiol 1990;33:135–51. 10.1099/00222615-33-3-135 [DOI] [PubMed] [Google Scholar]


