Abstract
Introduction
To assess if the apparent diffusion coefficient (ADC) value of magnetic resonance imaging (MRI) can discriminate between the cell type, histological grade and improve staging of urinary bladder cancer (BC).
Material and methods
102 patients with urinary bladder masses underwent MRI using a 1.5 T machine. T2 weighted and diffusion weighted imaging (DWI) using b values of 0, 150, 500 and 1000 s/mm2 were done. The ADC values of bladder masses were measured. These values were correlated with the histopathologic results. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of T2WI, DWI and T2WI plus DWI for detecting bladder lesions were evaluated.
RESULTS
The cut-off ADC value for diagnosing malignant bladder wall pathologies was ≤1 x 10-3 mm2/s with 94.5% sensitivity and 87.5% specificity. The mean ADC value of different malignant cell types was statistically insignificant. A significant difference in ADC values was found between G1 and G3 (P = 0.000), G2 and G3 (P = 0.045) but not between G1 and G2 (p = 0.066). Staging accuracy for differentiation between invasive and non-invasive lesions was nearly the same for all MRI data sets. For differentiation between organ confined (pT1–pT2) and non-organ confined lesions (pT3–pT4), staging accuracy was better in T2WI plus DWI (83%) as compared to DWI alone (77%) or T2WI alone (75%).
Conclusions
Adding DWI and the ADC value to T2WI improve the accuracy of MRI in BC detection and staging. However, at this time point, MRI cannot replace transurethral resection (TUR) biopsy or distinguish sharply between all different histologic grades and cell types.
Keywords: bladder cancer, neoplasm grading, neoplasm staging, diffusion magnetic resonance imaging
INTRODUCTION
Bladder cancer (BC) is one of the more common cancers in Egypt [1]. Differentiating the histopathological types, tumor grade and the depth of tumor invasion are critical for determining the therapeutic approach and are highly correlated with the progression, recurrence and patient's survival [2]. Neoadjuvant chemotherapy followed by radical cystectomy (RC) with lymphadenectomy is the standard treatment for muscle invasive tumors, whereas transurethral resection (TUR) ±chemo/immunotherapy is the treatment of choice for non-muscle invasive tumors [3].
Until now, the only sure method for assessing the stage and grade of BC is the TUR biopsy, which is invasive and not without hazards and may be risky in patients with hemorrhagic diathesis. Recent studies are searching for a less invasive alternative. T2-weighted and dynamic contrast enhanced MRI (DCE MRI) are used for staging of BC, which seemed to be superior to computed tomography urography (CTU)[4].
In a study evaluating the role of CTU in BC, Helenius et al. found that the detection rate of CTU was comparable to flexible cystoscopy [5]. In another study comparing CTU and MRU, Gandrup et al. found that the multiparametric MRI is superior to CTU in the diagnosis of BC where the sensitivity and specificity of CTU and MRU were 61.5% and 94.9% and 79.9% and 93.4% respectively [6].
However, overstaging remains a major drawback of MRI [4]. In addition, DCE MRI requires the use of gadolinium-based contrast agents, which should be avoided in patients with impaired renal function. To overcome these limitations, diffusion weighted imaging (DWI) has been investigated for diagnosis, staging, grading and assessment of treatment response in BC [7].
DW MRI tests the mobility of water molecules (Brownian motion) inside the tissues, by applying two equally sized but opposite diffusion sensitizing gradients (with 2 different b-values) to generate an image contrast [8, 9]. Water molecules' diffusion is inversely correlated to the tissue cellularity and the integrity of cell membranes. In malignant lesions with a high cellular density and intact cell membranes, diffusion is restricted. However, it is less restricted in areas of low cellular density [10, 11]. Herein, we evaluate the utility of the apparent diffusion coefficient (ADC) values of DW MRI in BC and its potential role in predicting clinical stage, grade and cell type.
MATERIAL AND METHODS
Between December 2013 and August 2015, 102 patients who attended our oncological urology outpatient clinic with bladder wall lesions diagnosed either clinically or radiologically (other than MRI) were included in this prospective study. They were subjected to MRI using a 1.5 T superconducting imager (Achieva, Philips health care, the Netherlands) using a 16 channel pelvic phased-array coil. Then, all patients underwent cystoscopy and biopsy. This study was approved by the local ethical committee. Exclusion criteria were contraindications for MRI (e.g. pacemaker, metallic prostheses or claustrophobia), contraindications for TUR biopsy (e.g. hemorrhagic diathesis) and patients with previous history of transurethral resection of bladder tumor (TURBT).
MRI examination
Patients were advised to drink plenty of fluids to distend the urinary bladder and to withhold micturition for at least 1 hour before MRI examination. In those with a fixed urethral catheter, clamping the catheter 2 hours before the examination or saline infusion into the bladder was done until the patients perceived the sensation of a full bladder. No bowel preparation was done.
Pulse sequences and scanning planes
High spatial resolution T2-weighted images (T2WI) of the bladder were obtained in both axial and sagittal planes using Repetition Time (TR) = 4154 ms, Echo Time (TE) = 120 ms, bandwidth = 1 kHz, 180 × 170 matrix, slice thickness of 6 mm, intersection gap of 0.5 mm and field of view (FOV) = 250 mm.
DWI was then obtained in the axial plane under free breathing using mono-directional gradients with a single shot spin-echo-planar sequence with the following parameters: TR = 1000 ms, TE = 67 ms, bandwidth = 142 kHz, 128 matrix, slice thickness of 6 mm, intersection gap of 1 mm and FOV = 240 mm, water excitation with b values of 0, 150, 500 and 1000 s/mm2.
Image analysis and interpretation: it was divided into
Qualitative analysis: The site, size, heterogeneity, tumor margins, and lymph nodes (LNs) signal intensity (SI) were assessed on T2WI, DWI, T2WI plus DWI and the ADC map (Figure 1 and Figure 2).
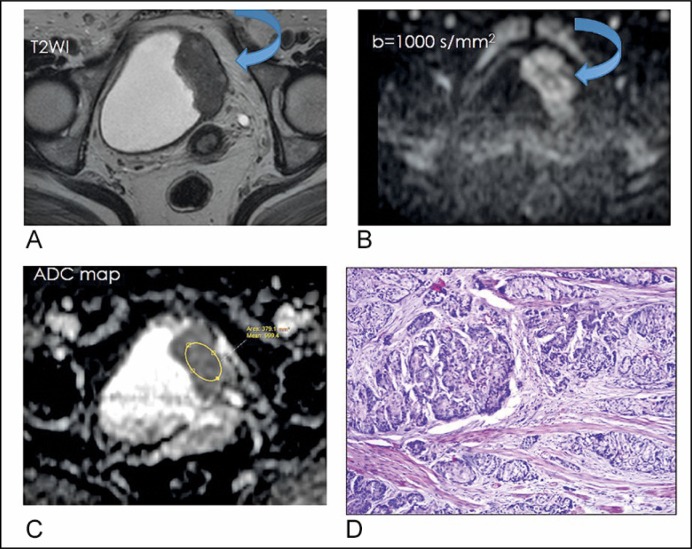
Figure 1.
A 50-years old woman with squamous cell carcinoma, GII, pT3. (A) T2-weighted axial imaging shows a tumor with intermediate signal intensity arising from the left lateral wall of the bladder (curved arrow). (B) Diffusion-weighted image (b = 1000 s/mm2) depicts the tumor as having hyperintense signal intensity with an irregular outline (curved arrow). (C) Apparent diffusion coefficient map: 0.9 x 10-3 mm2/s (D) Moderately differentiated Squamous cell carcinoma infiltrating the muscularis propria of the urinary bladder, H&E, x200.
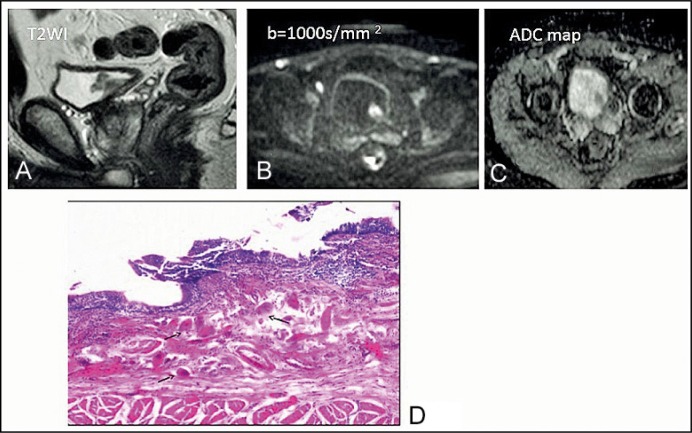
Figure 2.
A 43-year-old man with bilharzial cystitis. (A) T2-weighted imaging shows a tumor with intermediate signal intensity on the left posterolateral side of the bladder. (B) Diffusion-weighted image of the tumor shows restricted diffusion. (C) Apparent diffusion coefficient map: 1.4 x 10-3 mm2/s. (D) Bilharzial cystitis showing multiple bilharzial ova (arrows) in the lamina propria of the urinary bladder surrounded by chronic inflammatory cells, H&E, x200.
Quantitative analysis: The ADC was measured to estimate the degree of diffusion. It was measured by drawing a region of interest (ROI) over the most hypointense area within the tumor in the ADC map by only one examiner to avoid inter-observer variations. Malignancy is suggested at ADC value of 1 x 10-3 mm2/s at b value between 0 and 1000 s/mm2 [7–13]. The short axis of the lymph nodes was measured, and if it was >8 mm or if the lymph node had a low ADC value, it was considered malignant [14, 15]. All MR images sets were interpreted blinded to histopathologic information.
T staging on T2WI
Since normal bladder musculosa is seen as a low SI line, the tumors are considered stage T1 or lower if this line is preserved. Stage T2 or higher is suggested when this low SI line is disrupted focally in the region underlying the tumor. Lesions extending beyond this line into the perivesical fat are considered T3 and extending to the adjacent organs as T4 [10–16].
T staging on DWI
Cancer is reported as a high SI lesion. Hence, T1 or lower stages are considered if: a thin, flat, high SI area or high SI mass with a low SI submucosal stalk (stalk sign) or a thickened submucosa was found. A high SI tumor without a submucosal stalk and with a smooth margin indicates stage T2; extension into the perivesical fat with an irregular margin indicates T3; and extension into adjacent organs indicates T4 [13].
Histopathologic analysis
All specimens were fixed in 10% formalin, embedded in paraffin, cut and stained with a hematoxylin and eosin stain. Specimens were examined histopathologically to assess the type of lesion, type of malignant tumor, its grade and stage. Malignant tumors were classified and graded according to the World Health Organization classification [17]. Tumor staging was identified according to the American Joint Committee on Cancer/Union for International Cancer Control TNM system [18].
Statistical analysis
T-test was used to compare between benign and malignant bladder lesions. ANOVA with post Hoc test was used to compare the mean ADCs and the histologic grades. ROC curve was used to determine the cutoff value of ADC. P value <0.05 is considered statistically significant. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of T2WI, DWI and T2WI plus DWI were evaluated. Diagnostic accuracy of MRI staging as compared to pathologic staging was assessed on a stage-by-stage basis. To assess the three MRI data sets for diagnosing muscle and perivesical invasion, ROC curve analyses was used. The area under the curve (AUC) was then compared for the three data sets.
RESULTS
Malignant lesions were encountered in 94/102 patients (92.2%) and benign lesions in 8/102 (7.8%). The latter were excluded from the staging process. The presenting symptoms, tumour characteristics and histopathologic analysis are shown in Table 1.
Table 1.
Patients’ characteristics
| Number | 102 |
| Age range, years (mean ±SD) | 13–80 (56.5 ±11.1) |
| Sex, No. (%) | 93 males (91.2%), 9 females (8.8%) |
| Smoking history | 38 (37.2%) |
| Presenting symptoms • Hematuria • Dysuria • Burning micturition • Others |
80% 11% 8% 1% |
| Operative technique • Transuretheral resection biopsy • Transuretheral resectionof tumor • Radical/Partial cystectomy |
40 18 44 |
| Nature of the lesion • Malignant • Benign |
94 (92.2%) 8 (7.8%) |
| Mean size of bladder tumours | 4.7 ±1.8 cm (range: 1–10 cm) |
| The histopathologic types • malignant lesions • Benign lesions |
Transitional cell carcinoma (No = 67) Squamous cell carcinoma (No = 22) Adenocarcinoma (No = 4) Sarcoma (No = 1) Billharzial cystitis (4) Non-specific cystitis (4) |
ADC and histological cell types
All malignant lesions (No = 94) and 5/8 of the benign lesions showed restriction in DWI. There was a statistically significant difference between benign and malignant bladder wall pathologies (P <0.001). The cut-off value by the ROC curve for diagnosing malignant bladder wall pathologies according to ADC values was ≤1 x 10-3 mm2/s with 94.5% sensitivity and 87.5% specificity, P <0.0001, AUC = 0.934, and 95% confidence interval of 0.867 to 0.974. Table 2 shows the mean ADC of different cell types.
Table 2.
The mean apparent diffusion coefficient of different cell types
| Meanapparent diffusion coefficient | P values | |
|---|---|---|
| Benign lesions Malignant tumours |
1.2 ±0.15 x 10-3 mm2/s 0.78 ±0.19 x 10-3 mm2/s |
P <0.001 |
| Malignant cell types Transitional cell carcinoma Squamous cell carcinoma Adenocarcinoma Sarcoma |
0.76 ±0.21 x 10-3 mm2/s 0.83 ±0.14 x 10-3 mm2/s 0.9 ±0.12 x 10-3 mm2/s 0.6 ±0 x 10-3 mm2/s |
P = 0.156 |
ADC and histological grade
There was an inverse relationship between the tumor grade and the ADC value. According to the WHO grading system (1998), the majority of the tumors were G3 [No = 65 (69.2%)]. G2 was found in 19 patients (20.2%) and G1 in 10 patients (10.6%). A significant difference in ADC values was found between G1 and G3 (P <0.001), G2 and G3 (P = 0.045) but not between G1 and G2 (p = 0.066). According to the WHO grading system (2004), high-grade tumors [No = 84 (89.4%)] showed significantly lower ADC than low-grade tumors [No = 10 (10.6%)] (P = 0.0460, AUC: 0.74, 95% Confidence interval = 0.641–0.825). The ADC cutoff <0.9 x 10-3 mm2/s identified high-grade from low grade tumors with 91.7% sensitivity and 60% specificity.
Histopathologic staging
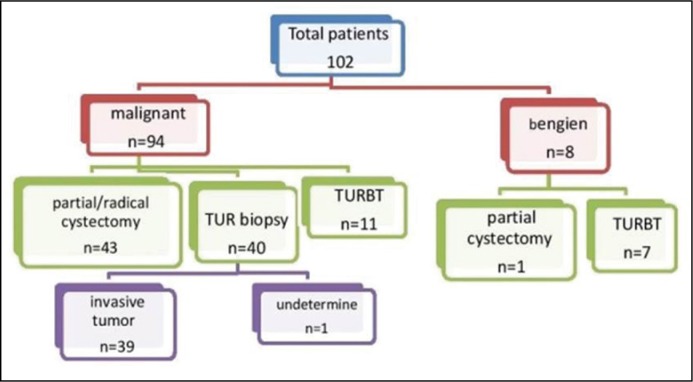
T stage was pathologically (p) confirmed in 54 patients by TURBT and partial or radical cystectomy, while 39 patients underwent TUR biopsy only which did not allow the differentiation between T2 and higher lesions (these were excluded from the analysis of organ confined vs. non-organ confined tumors). One patient had undetermined T stage (Tx) and was excluded from T stage analysis. The 54 pathologically proven stages were as follows: T1 [No =10 (9.8%)], T2 [No = 20 (19.6%)], T3 [No =16 (15.7%)] and T4 [No = 8 (7.8%)] (Figure 3).
Figure 3.
A flow chart showing the procedures done to the patients.
Tumor detection
All lesions identified by DWI were clearly shown on images obtained at b = 1000 S/mm2 as high SI in relation to the bladder wall and the surrounding urine. The sensitivity and PPV for T2WI in identifying bladder tumors were 100% and 91.5% respectively, and 98% and 96.6% respectively for DWI alone or plus T2WI. One false negative case in DWI – which was proven to be pT1 – decreased the sensitivity of both DWI and DWI plus T2WI.
Accuracy for differentiation between non-muscle-invasive bladder cancer (NMIBC) (pTis–pT1) and muscle-invasive bladder cancer MIBC (pT2–pT4), was nearly the same for all MRI data sets (96.7% for DWI alone or T2WI plus DWI and 95.7% for T2WI). When the stages were grouped as organ confined (pT1–pT2) and non-organ confined (pT3–pT4), staging accuracy was better in T2WI plus DWI (83%) as compared to DWI alone (77.4%) or T2WI alone (75.9%).
There was a statistically significant difference in diagnosis of invasive tumor depending on ADC value (P <0.001) with a cutoff ≤0.8 x 10-3 mm2/s with 72.3% sensitivity and 81.8% specificity.
Stage prediction using stalk sign or submucosal component
Nineteen tumors with a positive stalk sign were detected by DWI and/or T2WI. Seventeen tumors (in 10 patients) proved to be pT1, while 2 (in 2 patients) proved to be pT2.
In DWI: stalk sign was detected in 14 (82.4%) and was absent in 3 (17.7%) of the pT1 tumors. Also, it was detected in the 2 pT2 tumors.
In T2WI: stalk sign was detected in 4 (23.5%) and was absent in 13 (76.5 %) of the pT1 tumors. However, it was detected in the 2 pT2 tumors.
Prediction of metastatic lymph nodes
T2WI identified suspicious lymph nodes in 17/44 patients (39.5%) who underwent cystectomy due to a short axis diameter of 8 mm. DWI identified 18 (41.9%) patients based upon the restriction of a high b value and low ADC value. Only 2 patients had pathologically proven lymph-node metastases (ADC of 0.8 x 10-3 mm2/s), while other LNs proved to be reactive hyperplasia (mean ADC = 0.4 x 10-3 mm2/s).
DISCUSSION
Proper staging and grading of BC is important for choosing a suitable treatment option [18, 19]. Also, differentiation between organ-confined and non-organ-confined BC is essential, as patients with extravesical tumors show higher recurrence rates and worse survival than those with organ-confined ones [4, 19].
Several reports investigated the ability of DW MRI to distinguish the cell type, stage and grade of BC as a non-invasive method [4, 13]. In our study group, complications of TUR occurred in 11 (10.7%) patients. Bleeding occurred in 5 (4.9%) – two required hemostatic dose of radiotherapy after failure of conservative measures – postoperative high grade fever in 3 (2.9%), ureteric obstruction in 2 (1.9%) and bladder perforation which required immediate drainage occurred in one patient (0.9%). The resultant increased hospital stay and the additional cost of the TUR biopsy are less cost-effective in comparison to the less invasive and somewhat expensive MRI.
In the current study, all bladder tumors were clearly visible in DWI at a high b value (1000 s/mm2) as a bright SI relative to the bladder wall and surrounding tissue with 98% sensitivity as previously reported by other researchers [12, 20, 21]. Also, and in accordance with others [22, 23], there was a statistically significant difference between malignant and benign bladder wall pathologies.
Although ADC cut off values differ between centers and there is no standard for how to perform DWI, different scanners may give different results. However, every center performing and reporting on the technique rely, to a considerable extent, on its validity to differentiate between benign and malignant lesions.
In the current study, the mean ADC of malignant tumors was 0.78 x 10-3 mm2/s which was lower than other studies, reporting a mean ADC of malignant tumors of 1 x 10-3 mm2/s [12, 13, 22, 23]. The cut-off value in the ROC curve for differentiating malignant and benign tumors was ≤1 x 10-3 mm2/s with 94.5% sensitivity and 87.5% specificity. However, Avcu et al. reported a higher cut-off ADC value of 1.5 x 10-3 mm2/s with nearly the same sensitivity (94.1%) and a higher specificity (95.7%) [22]. In their report, they evaluated 17 non-malignant pathologies including 4 with restricted diffusion (2 polypoidal masses and 2 eosinophilic cystitis). The other 13 cases with facilitated diffusion included 12 with bladder wall thickening due to BPH and 1 eosinophilic cystitis. This difference in ADC cut-off value may be explained by the fact that only bladder masses were evaluated in our study and most of benign masses (5/8) showed restriction in DWI, hence, the lower ADC cut-off value. Other studies also reported mild restriction in benign lesions if compared to the normal bladder wall [11–24].
Proper T staging of BC in the current study was improved by adding DWI to T2WI. Both together showed higher accuracy (68.5%) than T2WI alone (63%). This was in accordance with other researchers. [13, 21, 25, 26].
In our study, the cut-off ADC value that differentiated high grade from low grade tumors was 0.9 x 10-3 mm2/s. Other studies reported cut-off values as 0.82 x 10-3 mm2/s [19] and 1.135 x 10-3 mm2/s [22]. In these studies, a smaller sample size may explain the difference between our cut-off values and theirs. The inverse relationship between ADC values and tumor grade was previously reported by others [13, 27, 28].
Regarding the histological subtypes of BC, we didn't encounter a statistically significant difference between mean ADC values of different cell types. However, a larger case study may find a significant difference between different cell types. Other investigators had similar results but with a very small number of urothelial squamous cell carcinoma (SCC) [23, 28]. Dağgülli et al., reported on 22 patients with transitional cell carcinoma (TCC) and 13 with urothelial SCC. They found a significantly lower mean ADC for SCC at b 100 and b 600 [24]. This difference may be related to the small number of patients and lower b value used in his study in comparison to ours.
MIBC patients showed significantly lower ADC values than those with NMIBC, with a cut-off ADC value of 0.80 x 10-3 mm2/s. This was similar to other studies. [10, 26, 27] DWI alone and DWI plus T2WI had a higher accuracy (96.7% and 96% respectively) than T2WI in differentiating between NMIBC and MIBC. These results are in accordance with other investigators [13, 25, 26, 29]. However, El-Assmy's et al., [30] and Abdel-Rahman et al. [20] reported accuracy of DWI alone of 63.6% and 75% respectively.
Abdel-Rahman et al., reported accurate staging in 30 patients (75%), under-staging in 8 (20%) and over-staging in 2 patients (5%) [20]. This was comparable to our study where DWI staging was accurate in 37 (68.5%) patients.
Regarding differentiation of organ confined from non-organ confined BC, staging accuracy of DWI alone was 77.4% which was higher than El-Assmy's et al. (69.6%) [30] and lower than Abdel-Rahman et al. [20] and Wu et al. [29] who reported accuracy between 90–95%. DWI plus T2WI had a higher accuracy (83%) in differentiating organ confined from non-organ confined tumors as compared to T2WI alone (75.9%) or DWI alone (77.4%). Also, other studies reported a higher accuracy for combined modalities ranging between 87–99%. [11–26, 29].
Most of the tumors with a stalk in T2WI and DWI were pT1. DWI alone had 82.4% accuracy while T2WI alone had 23.5% accuracy in the diagnosis of pT1 using the stalk sign. This corresponded with other studies [11, 13]. Although the stalk sign is seen more in pT1, it was also present in pT2 [11].
In the current study, only 2/18 LNs proved to be malignant; the other 16 had reactive hyperplasia. The marked overlap of ADC values in our study between benign and malignant lymph nodes can be explained by the increased restriction of the water molecules' movement in the reactive hyperplastic nodes. Papalia et al., 2012, reported a mean ADC of metastatic LNs of 0.85 x 10-3 mm2/s and 1 x 10-3 mm2/s for benign LNs, with a cutoff point of 0.86 x 10-3 mm2/s. However, the small number in their study (36 patients) and the nature of reactive hyperplasia which restricts the water molecules' movement undermines these results [31].
The inherent limitations to MRI are its availability as a basic diagnostic tool and the artifacts that might occur due to the mobility of the examined organs with breathing, a corrugated mucosa in a poorly distended bladder, a very thinned out bladder wall due to overfilling or susceptibility artifacts that can occur next to gas-filled bowel or post-cystoscopy gas bubbles within the bladder [32]. To minimize these artifacts, suitable bladder filling and performing cystoscopy after MRI examination were adopted. The artifacts due to mobility are probably minimal in the bladder in relation to other organs due to its pelvic location. The susceptibility artifact usually gives a false positive test or may lead to upstaging. While there was no false positive diagnosis of a bladder mass, some of our patients didn't perform cystectomy, leaving upstaging to non-organ confined disease a possibility.
CONCLUSIONS
Adding DWI and ADC value to T2WI improves the accuracy of MRI in BC detection and staging. However, at this point in time, MRI cannot replace TUR biopsy or distinguish sharply between all different histologic grades and cell types.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Ibrahim AS, Khaled HM, Mikhail NN, Baraka H, Kamel H. Cancer incidence in Egypt: results of the national population-based cancer registry program. J Cancer Epidemiol. 2014;2014:437971.. doi: 10.1155/2014/437971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green DA, Rink M, Hansen J, et al. Accurate preoperative prediction of non-organ-confined bladder urothelial carcinoma at cystectomy. BJU Int. 2013;111:404–411. doi: 10.1111/j.1464-410X.2012.11370.x. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 4.Lee M, Shin SJ, Oh YT, et al. Non-contrast magnetic resonance imaging for bladder cancer: fused high b value diffusion-weighted imaging and T2-weighted imaging helps evaluate depth of invasion. Eur Radiol. 2017;27:3752–3758. doi: 10.1007/s00330-017-4759-2. [DOI] [PubMed] [Google Scholar]
- 5.Helenius M, Brekkan E, Dahlman P, Lönnemark M, Magnusson A. Bladder cancer detection in patients with gross haematuria: Computed tomography urography with enhancement-triggered scan versus flexible cystoscopy. Scand J Urol. 2015;49:377–381. doi: 10.3109/21681805.2015.1026937. [DOI] [PubMed] [Google Scholar]
- 6.Gandrup KL, Løgager VB, Bretlau T, Nordling J, Thomsen HS. Diagnosis of bladder tumours in patients with macroscopic haematuria: a prospective comparison of split-bolus computed tomography urography, magnetic resonance urography and flexible cystoscopy. Scand J Urol. 2015;49:224–229. doi: 10.3109/21681805.2014.981203. [DOI] [PubMed] [Google Scholar]
- 7.Thoeny HC, Forstner R, De Keyzer F. Genitourinary applications of diffusion- weighted MR imaging in the pelvis. Radiology. 2012;263:326–342. doi: 10.1148/radiol.12110446. [DOI] [PubMed] [Google Scholar]
- 8.Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622–1635. doi: 10.2214/AJR.06.1403. [DOI] [PubMed] [Google Scholar]
- 9.Afaq A, Koh DM, Padhani A, van As N, Sohaib SA. Clinical utility of diffusion-eighted magnetic resonance imaging in prostate cancer. BJU Int. 2011;108:1716–1722. doi: 10.1111/j.1464-410X.2011.10256.x. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi S, Koga F, Yoshida S. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol. 2011;21:2178–2186. doi: 10.1007/s00330-011-2174-7. [DOI] [PubMed] [Google Scholar]
- 11.Abou-El-Ghar ME, El-Assmy A, Refaie HF, El-Diasty T. Bladder cancer: diagnosis with diffusionweighted MR imaging in patients with gross hematuria. Radiology. 2009;251:415–421. doi: 10.1148/radiol.2503080723. [DOI] [PubMed] [Google Scholar]
- 12.Matsuki M, Inada Y, Tatsugami F, Tanikake M, Narabayashi I, Katsuoka Y. Diffusion weighted MR imaging for urinary bladder carcinoma: initial results. Eur Radiol. 2007;17:201–204. doi: 10.1007/s00330-006-0281-7. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi M, Sasaki S, Ito M, et al. Urinary Bladder cancer: diffusion weighted MR imaging-accuracy for diagnosing T stage and estimating histologic grade. Radiology. 2009;251:112–121. doi: 10.1148/radiol.2511080873. [DOI] [PubMed] [Google Scholar]
- 14.Barentsz JO, Engelbrecht MR, Witjes JA, de la Rosette JJ, van der Graaf M. MR imaging of the male pelvis. Eur Radiol. 1999;9:1722–1736. doi: 10.1007/s003300050916. [DOI] [PubMed] [Google Scholar]
- 15.Dorfman RE, Alpern MB, Gross BH, Sandler MA. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180:319–322.. doi: 10.1148/radiology.180.2.2068292. [DOI] [PubMed] [Google Scholar]
- 16.Narumi Y, Kadota T, Inoue E, et al. Bladder wall morphology: in vitro MR imaging-histopathologic correlation. Radiology. 1993;187:151–155. doi: 10.1148/radiology.187.1.8451403. [DOI] [PubMed] [Google Scholar]
- 17.Eble JN, Sauter G, Epstein JL, Sesterhenn IA, editors. Pathology and genetics: tumours of the urinary system and male genital organs. Lyon: IARC Press, International Agency for Research on Cancer; World Health Organization classification of tumours; 2004. v 6.s. [Google Scholar]
- 18.Sobin LH, Gospodariwicz MK, Wittekind C. International Union against Cancer. TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell; 2009. [Google Scholar]
- 19.Sherif A, Jonsson MN, Wiklund NP. Treatment of muscle-invasive bladder cancer. Expert Rev Anticancer Ther. 2007;7:1279–1283. doi: 10.1586/14737140.7.9.1279. [DOI] [PubMed] [Google Scholar]
- 20.Abdel-Rahman HM, El Fiki IM, Desoky EAE, Elsayed ER, Abd Samad KM. The role of diffusion-weighted magnetic resonance imaging in T staging and grading of urinary bladder cancer. Egypt J Radiol Nucl Med. 2015;46:741–747. [Google Scholar]
- 21.Watanabe H, Kanematsu M, Kondo H, et al. Preoperative T staging of urinary bladder cancer: does diffusion-weighted MRI have supplementary value? AJR. 2009; 192:1361–1366. doi: 10.2214/AJR.08.1430. [DOI] [PubMed] [Google Scholar]
- 22.Avcu S, Koseoglu MN, Ceylan K, Bulut MD, Unal O. The value of diffusion- weighted MRI in the diagnosis of malignant and benign urinary bladder lesions. Br J Radiol. 2011;84:875–882. doi: 10.1259/bjr/30591350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ceylan K, Taken K, Gecit T, et al. Comparison of cystoscopy with diffusion- weighted magnetic resonance images used in the diagnosis and follow-up of patients with bladder tumors. Asian Pac J Cancer Prev. 2010;11:1001–1004. [PubMed] [Google Scholar]
- 24.Dağgülli M, Onur MR, Fırdolaş F, Onur R, Kocakoç E, Orhan İ. Role of diffusion MRI and apparent diffusion coefficient measurement in the diagnosis, staging and pathological classification of bladder tumors. Urol Int. 2001;87:346–352. doi: 10.1159/000330925. [DOI] [PubMed] [Google Scholar]
- 25.Yamada Y, Kobayashi S, Isoshima S, Arima K, Sakuma H, Sugimura Y. The usefulness of diffusion weighted magnetic resonance imaging in bladder cancer staging and functional analysis. J Cancer Res Ther. 2014;10:878–882. doi: 10.4103/0973-1482.138225. [DOI] [PubMed] [Google Scholar]
- 26.Gupta N, Sureka B, Kumar MM, Malik A, Bhushan TB, Mohanty NK. Comparison of dynamic contrast-enhanced and diffusion weighted magnetic resonance image in staging and grading of carcinoma bladder with histopathological correlation. Urol Ann. 2015;7:199–204. doi: 10.4103/0974-7796.150480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Guan J, Guo Y, Guang Z. Estimation of bladder carcinoma histologic grade with diffusion weighted MR imaging. Eur Soc Radiol. 2014;138:409–414. [Google Scholar]
- 28.Sherif MF. The value of diffusion weighted MR imaging in T staging and correlation with histologic grading in urinary bladder cancer. Egypt J Radiol Nucl Med. 2015;46:189–194. [Google Scholar]
- 29.Wu LM, Chen XX, Xu JR, Zhang XF, et al. Clinical value of T2-weighted imaging combined with diffusion-weighted imaging in preoperative T staging of urinary bladder cancer: a large-scale, multiobserver prospective study on 3.0-T MRI. Acad Radiol. 2013;20:939–946. doi: 10.1016/j.acra.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 30.El-Assmy A, Abou-El-Ghar ME, et al. Bladder tumour staging: comparison of diffusion- and T2-weighted MR imaging. Eur Radiol. 2009;19:1575–1581. doi: 10.1007/s00330-009-1340-7. [DOI] [PubMed] [Google Scholar]
- 31.Papalia R, Simone G, Grasso R, Augelli R, et al. Diffusion-weighted magnetic resonance imaging in patients selected for radical cystectomy: detection rate of pelvic lymph node metastases. BJU Int. 2012;109:1031–1036. doi: 10.1111/j.1464-410X.2011.10446.x. [DOI] [PubMed] [Google Scholar]
- 32.Lin WC, Chen J-H. Pitfalls and limitations of diffusion-weighted magnetic resonance imaging in the diagnosis of urinary bladder cancer. Transl Oncol. 2015;8:217–230. doi: 10.1016/j.tranon.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]