Abstract
Introduction
Urethral pain syndrome is a subgroup of chronic pelvic pain syndromes and comprises a relatively challenging patient group in urological practice. Several different treatments have been used for the management of the condition from a mostly empirical basis. In this article, we present the results of a small cohort of young male patients treated with sertraline and gabapentin therapy.
Material and methods
The data of 52 patients was retrospectively evaluated and 31 patients' data was included in this study. Clinical symptom scores, including International Prostate Symptoms Score, Hamilton Anxiety Rating Scale, Visual Analog Scale for Pain, Quality of Life due to Lower Urinary Tract Symptoms, and Urinary, Psychosocial, Organ Specific, Infection, Neurologic/Systemic, Tenderness of Skeletal Muscles (UPOINT) classifications were retrospectively analyzed from the patient records and charts.
Results
We observed improvement in clinical scores involving anxiety, lower urinary tract symptoms, quality of life, and pain. Statistical analysis revealed significant amelioration of the symptoms with gaba- pentin and sertraline treatment in our cohort.
Conclusions
Gabapentin and sertraline treatment may be considered in the second step management of urethral pain syndrome. To draw an evidence-based recommendation, prospective and comparative studies should be conducted in the future.
Keywords: chronic pain ‹› urethral pain ‹› pelvic pain ‹› gabapentin ‹› sertraline
INTRODUCTION
Chronic pelvic pain holds a significant case load in urological practice with an important economic burden for health care systems [1]. The exact etiology of the condition is still uncertain. Several different treatment methods from pelvic muscle exercises and neuromodulation to antibiotics, antidepressants and neuromuscular agents have been used, and different success rates have been reported in previous reports [2]. Spontaneous evanesce of the syndrome is also possible.
Urethral pain can also be a part of the somatic symptom disorder and the improvement of somatic symptoms is possible after the appropriate psychiatric treatment [3, 4]. In this study, we evaluated the clinical success of combined sertraline and gabapentin therapy in a selected group of male patients who had not responded to close observation and common treatment options, and report the six-month follow-up results of 31 patients with urethral pain syndrome who were treated with sertraline and gabapentin therapy.
MATERIAL AND METHODS
Between 2010 and 2016, data of adult male patients who applied to our outpatient clinic with occurrence of chronic or recurrent urethral pain for at least six months was recorded. All patients were evaluated with urine analysis, the International Prostate Symptom Score (I-PSS), micturition diary, urinary system ultrasonography, uroflowmetry, and cystoscopy during the first admission to exclude other associative diseases i.e. benign prostatic hyperplasia, urinary tract infection, interstitial cystitis, and neurogenic bladder dysfunction. Physical examination of the abdomen, pelvis, scrotum and perineum with digital rectal examination was performed in all patients. For definition of chronic pelvic pain syndrome, we excluded patients who had urinary tract infection, pelvic malignancy, radiotherapy history, neurological disease, cognitive dysfunction and neurogenic bladder dysfunction history. Neither of the patients had previous urologic surgery. One patient had an open appendectomy when he was 8 years old, and one patient had a hemorrhoidectomy two years before admission.
Empirical non-steroidal anti-inflammatory drugs and alpha blockers were given to all patients as first line treatment with conservative therapies (pain education and dietary treatment) to all patients (52 patients total). Three months after the first line treatment, all patients were evaluated again. The complaints of twenty-one patients had improved.
The remaining 31 patients were included to this retrospective analysis. Uroflowmetry and urine analysis tests were repeated. Additionally, a filling cystometry, and pressure-flow study were performed in these patients. In all patients, urodynamic studies revealed physiologic filling and micturition phases. The urine analysis and uroflowmetry results for all patients were normal. Twenty-nine patients' six-domain Urinary, Psychosocial, Organ-Specific, Infection, Neurologic/Systemic and Tenderness (UPOINT) classifications were available. Sertraline 50 mg and gabapentin 300 mg were administered once daily to these patients as second-line therapy. All patients who were included in this study were evaluated for anxiety [with the Hamilton-Anxiety Rating Scale (HAM-A)], pain [Visual Analogue Scale (VAS)] and lower urinary tract symptoms (with International Prostate Symptom Score (I-PSS) and Quality of Life Due to Urinary Symptoms Score (QoL) before the initiation of the second-line treatment. All scores of all patients were also recorded at the end of the first and sixth months.
Statistical analysis
For statistical analysis, SPSS 16.0 (IBM Company, Chicago, Illinois, USA) was used. The mean clinical scores between treatment timelines were compared with Wilcoxon and Friedman tests. For statistical significance, a p-value of <0.05 was accepted.
RESULTS
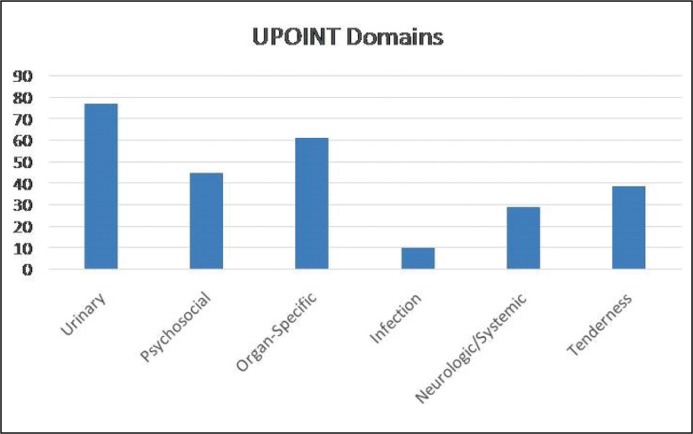
The mean age, mean maximum bladder capacity during urodynamic study and mean maximum flow rate during uroflowmetry were 22.4 (range: 18–28 years), 281 (range: 204–387) ml, 22.2 (range: 16–32) ml/sec, respectively. The longest interval between two micturitions was one and a half hours in the cohort. Seven patients reported more than ten micturitions during work hours. There were no married patients in our cohort. Six patients had history of paid sexual intercourse which occurred just before the onset of their symptoms. The mean duration of the symptoms on admission was 9.2 (range: 6–14) months. Distribution of the patients according to the UPOINT classification in the cohort can be seen in Figure 1. The Urinary, Organ-Specific, and Psychosocial domains were the most common positive sub-domains with percentages of 82.7%, 65.5%, and 48.2%, respectively.
Figure 1.
Distribution of the sub-kohorty to the domen UPOINT.
UPOINT – Psychosocial, Organ Specific, Infection, Neurologic/Systemic, Tenderness of Skeletal Muscles
Before sertraline and gabapentin treatment, the mean HAM-A, VAS, I-PSS, and QoL scores were 17, 4.83, 20.8, and 3.7, respectively. After the sixth month of the second line treatment, the mean HAM-A, VAS, IPSS, and QoL scores were 8.67, 1.61, 11.96, and 1.45, respectively. All scores are detailed in Table 1. Patient reported side effects were common, albeit mild to moderate. There was no drop-out in our cohort during the six-month follow-up period. We noted that 3 patients complained of dizziness and were re-admitted to the outpatient clinic in the first week of the treatment. However, the situations could have been managed with gradual dose titration.
Table 1.
Mean Hamilton Anxiety Rating Scale (HAM-A), Visual Analog Scale (VAS), International Prostate Symptom Score (I-PSS) and Quality of Life (QoL) scores of the patients
| Before second-line treatment, mean (range) | At the first month of second-line treatment, mean (range) | At the sixth month of second-line treatment, mean (range) | p-value for comparisons | |
|---|---|---|---|---|
| VAS: | 4.86 (3–8) | 2.9 (1–5) | 1.61 (1–3) | <0.01a,b,c |
| IPSS: | 21.05 (15–31) | 15.58 (4–25) | 11.96 (4–20) | <0.01a,b,c |
| QoL: | 3.7 (2–6) | 3.38 (1–5) | 1.45 (0–4) | <0.01a,b,c |
| HAM-A: | 17.11 (12–24) | 13.16 (4–21) | 8.67 (4–9) | <0.01a,b,c |
Comparison of the scores after the treatment at the first month of treatment vs. baseline
Comparison of the scores after the treatment at the sixth month of treatment vs. baseline
Comparison of the scores after the treatment at the sixth month vs. first month of treatment
We observed significantly lower clinical scores after the treatment at the first month of the treatment vs. baseline, at the sixth month of the treatment vs. baseline, and at the sixth month vs. first month of the treatment (p <0.01 for all comparisons). All comparisons are detailed in Table 1.
DISCUSSION
Chronic pelvic pain syndrome (CPSS) is an important clinical entity that holds a significant case load in urological practice. Even though its common place in daily practice, the exact etiology and pathogenesis are still not completely understood [5, 6, 7]. In previous reports, CPSS was associated with diet, shift-based working, lifestyle, history of childhood abuse, sexual dysfunction, depression, substance abuse, somatization, and anxiety [8–11]. In its common clinical course, the symptom intensity is variable [12]. Urethral pain syndrome is classified as a subgroup of urological pelvic pain syndromes, and as is the characteristic of pelvic pain syndrome subtypes, urethral pain syndrome also has an uncertain etiology [2]. The diagnosis is based on patients' symptoms. In most circumstances, laboratory tests, cystoscopy, transrectal ultrasound examination of the prostate and urodynamic tests are undertaken. However, these tests are performed mainly to exclude other conditions.
A current report by Chelimsky et al. showed an autonomic dysfunction in patients with CPPS [13]. On the other hand, CPPS was also associated with previous abuse, adult sexual dysfunction, anxiety, major depression and even as a compulsive guilt expression. In our patient cohort, six out of 31 patients had history of paid sexual intercourse just before the initiation of symptoms. Therefore, we can speculate about the effect of feelings of guilt in the psychological basis of urethral pain syndrome, which was previously descripted as “spousal revenge syndrome” by Makovey et al. [14]. According to the Diagnostic and Statistical Manual of Mental Disorders V (DSM-V) classification, urethral pain syndrome that has persisted for at least six months may also be classified as a somatic symptom disorder [3, 15].
Treatment of the condition is challenging and may require a multidisciplinary approach with high endurance on both patients' and physicians' sides. In contemporary practice, treatment with paraceta- mol, non-steroidal anti-inflammatory drugs (NSAIDs), antidepressants, opioids, anticonvulsants, gabapentin, α-blockers, anticholinergic drugs, anxiolytics, quercetin, antibiotics, as well as physiotherapy, neuromodulation, acupuncture, and pelvic floor exercises are recommended, and concurrent sexual dysfunction and mental disorders should be evaluated and treated accordingly [2, 3]. The contemporary clinical guidelines written by the European Association of Urology (EAU) recommend using a phenotypically directed classification in a multi-disciplinary management strategy. Unfortunately, even the cumulative evidence that shaped the current guidelines is far from drawing a common treatment pathway. Therefore, the EAU guidelines on chronic pelvic pain recommend initiating the therapy with general options and planning a multi-disciplinary treatment for urethral pain syndrome [16]. In our selected cohort, all patients had a treatment history with an α-blocker, an anticholinergic drug, and a NSAID which were unsuccessful. It is also known that a trial and error approach is not uncommon in urethral pain syndrome, and the symptoms may even resolve without any treatment [17]. In our institution, the UPOINT classification, which has been successfully used in clinical practice, is also widely used in the initial evaluation of the patients. Currently, Infection, Neurologic/Systemic, Psychosocial, Ulcers and Tenderness of Muscles (INPUT) classification, that incorporates Hunner's ulcers into the clinical domains, is proposed and has been validated in a wide range of patients [18]. The INPUT classification system is a promising tool which will find more use in clinical practice in the future.
In our cohort, no patients had physiotherapy, pelvic muscle exercise, neuromodulation or psychiatric medication usage history. In their retrospective analysis of 80 patients whose median age was 45.1 years with chronic prostatitis (CP)/CPSS, Shoskes et al. reported a stepwise treatment approach that involves two to four weeks of antibiotic treatment with weekly prostate massages in patients with detected pathogens in prostatic secretions. For patients with urinary symptoms or significant post-voiding residual urine, tamsulosin therapy was initiated. They used anti-inflammatory drugs as a second step, and if both steps were unsuccessful, they proceeded to finasteride, pentosane polysulfate, amitriptyline, and gabapentin therapy. In their report, they concluded the high clinical benefit of the stepwise approach along with a one year follow-up period in their patient group [19].
Successful management of chronic pelvic pain in women with gabapentin and amitriptyline has also been reported. In their open-label study, Sator-Katzenschlager et al. increased the daily doses to the maximum levels, which was 3600 mg/day for gabapentin. Gabapentin was synthesized as a structural gamma-aminobutyric acid (GABA) analogue and is currently used for the treatment of epilepsy, restless leg syndrome, and neuropathic pain [20]. Apart from its primary indication for use as adjunctive treatment for partial seizures, it has also been used in the treatment of neuropathic pain disorders and movement disorders [21, 22]. Gabapentin has been reported to be effective as an adjunctive therapy for the treatment of anxiety disorders with somatic symptoms [23]. Sertraline is a specific serotonin reuptake inhibitor (SSRI) that is used in daily practice for treating posttraumatic stress disorder, generalized anxiety disorder, obsessive compulsive disorder, major depressive disorder, and somatoform disorder with proven safety in both adults and adolescents [24–28]. Sertraline treatment has also been shown to be successful in the treatment of chronic pelvic pain in both men and women [29, 30]. On the other hand, the clinical failure of pregabalin, which is a powerful neuropathic medication, therapy was also reported [31]. Despite the controversy on the current management options and the lack of a specific treatment of the different sub-types, clinicians should be aware that the chronic pelvic pain syndromes are manageable and a subjective cure is possible, at least in patients with moderate pain scores [32].
Due to the relatively young age group, completely normal urine analysis, diagnostic cystourethroscopy and urodynamic results, and previous failure of empirical treatments, we preferred to manage these patients with neuropathic medicine. In our patient group, we started with 300 mg/day gabapentin dosage, titrated to 600 mg/day. For sertraline, we initiated the treatment with a 50 mg/day dosage, titrated to 100 mg/day in four patients and increased the dose to 200 mg/day in three patients. We observed a significant improvement in symptoms, and symptom-related anxiety. Side effects were manageable with careful titration. However, considering the refractory and severe nature of our cohort, we can speculate that the successful continuation of the therapy might be a result of the patients' situation. In the first step therapy drop-out rates may be higher because of side effects of gabapentin and SSRIs, if used. Sertraline may also have had a positive impact by decreasing pain-related anxiety with its anxiolytic effect. Therefore, our results may have been affected by this bias in the HAM-A scores. The HAM-A score is developed and validated for scoring in general anxiety states, and it is also used in our routine practice in evaluation of pelvic pain syndromes. At the same time, this scale evaluates the treatment outcome in anxiety disorders [33]. Therefore, its role in evaluating urethral pain-related anxiety in our cohort is arguable. We also observed individual differences in the reflection of urinary symptoms as anxiety. Some patients had lower HAM-A scores with high IPSS scores; however, some were the opposite. An exact differentiation of whether urethral pain causes anxiety or vice versa would be possible with a comprehensive multi-disciplinary approach. Anyway, we have noticed a significant decrease in HAM-A scores with concurrent improvement in I-PSS and QoL due to urinary symptoms scores. Our patient group comprises 31 young patients, which was a specific cohort with a moderate size. Despite this moderate patient cohort with retrospective data analysis, our results are encouraging to strengthen the place of sertraline and gabapentin in the treatment of urethral pain syndrome. Our current follow-up on the cohort has just reached 12 months. We usually attempt a trial of gradual dose reduction after three months of symptomatic remission. We may further publish our long term clinical results and patient compliance rates in the future.
The main shortcoming of our study was its retrospective nature, lack of any comparison and randomization, and moderate patient number. Therefore, we cannot conclude any superiority of the management we have used, nor can we suggest the usage of the combined sertraline-gabapentin therapy in the routine practice by the current level of clinical evidence. However, we can suggest our approach as a promising management strategy in multi-disciplinary fashion that should undergo validation and prospective-comparative clinical trials.
CONCLUSIONS
We observed significant symptom and anxiety amelioration with sertraline and gabapentin treatment in patients with urethral pain syndrome. Thus, combination of sertraline and gabapentin can be used in the secondary treatment of the syndrome. Further prospective, case-matched, and comparative studies should be undertaken to evaluate the superiority of the management options as well as the place of neuropathic medicine in the primary management of the urethral syndrome.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Schaeffer AJ. Chronic prostatitis and the chronic pelvic pain syndrome. New Engl J Med. 2006;355:1690–1698. doi: 10.1056/NEJMcp060423. [DOI] [PubMed] [Google Scholar]
- 2.Magistro G, Wagenlehner FM, Grabe M, Weidner W, Stief CG, Nickel JC. Contemporary management of chronic prostatitis/chronic pelvic pain syndrome. Eur Urol. 2016;69:286–297. doi: 10.1016/j.eururo.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 3.American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM-V®. 2013. ISBN 978-0-89042-554-1. [Google Scholar]
- 4.Egan KJ, Krieger JN. Psychological problems in chronic prostatitis patients with pain. Clinical J Pain. 1994;10:218–226. doi: 10.1097/00002508-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Schaeffer AJ. Etiology and management of chronic pelvic pain syndrome in men. Urology. 2004;3(Suppl 1):75–84. doi: 10.1016/j.urology.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Bartoletti R, Cai T, Mondaini N, et al. Prevalence, incidence estimation, risk factors and characterization of chronic prostatitis/chronic pelvic pain syndrome in urological hospital outpatients in Italy: results of a multicenter case-control observational study. J Urol. 2007;178:2411–2415. doi: 10.1016/j.juro.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 7.Pontari MA, Ruggieri MR. Mechanisms in prostatitis/chronic pelvic pain syndrome. J Urol. 2004;172:839–845. doi: 10.1097/01.ju.0000136002.76898.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Hu C, Peng Y, Lu J, et al. Association of diet and lifestyle with chronic prostatitis/chronic pelvic pain syndrome and pain severity: a case-control study. Prostate Cancer Prostatic Dis. 2016;19:92–99. doi: 10.1038/pcan.2015.57. [DOI] [PubMed] [Google Scholar]
- 9.Walker E, Katon W, Harrop-Griffiths J, Holm L, Russo J, Hickok LR. Relationship of chronic pelvic pain to psychiatric diagnoses and childhood sexual abuse. Am J Psychiatry. 1988;145:75–80. doi: 10.1176/ajp.145.1.75. [DOI] [PubMed] [Google Scholar]
- 10.Brünahl C, Dybowski C, Albrecht R, Gregorzik S, Löwe B. Psychiatric comorbidity in patients with chronic pelvic pain syndrome (CPPS. J Psychosom Res. 2016;76:127–133. [Google Scholar]
- 11.Kroenke K. Patients presenting with somatic complaints: epidemiology, psychiatric co-morbidity and management. Int J Methods Psychiatr Res. 2003;12:34–43. doi: 10.1002/mpr.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens-Shields AJ, Clemens JQ, Jemielita T, et al. Symptom variability and early symptom regression in the mapp study, a prospective study of urologic chronic pelvic pain syndrome. J Urol. 2016;196:1450–1455. doi: 10.1016/j.juro.2016.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chelimsky G, Simpson P, McCabe N, et al. Autonomic testing in women with chronic pelvic pain. J Urol. 2016;196:429–434. doi: 10.1016/j.juro.2016.03.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Makovey I, Dolinga R, Shoskes DA. 'Spousal Revenge Syndrome' - description of a new chronic pelvic pain syndrome patient cohort. Can J Urol. 2016;23:8176–8178. [PubMed] [Google Scholar]
- 15.Dimsdale JE, Creed F, Escobar J, et al. Somatic symptom disorder: an important change in DSM. J Psychosom Res. 2013;75:223–228. doi: 10.1016/j.jpsychores.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 16.Engeler D, Baranowski AP, Borovicka J, et al. EAU Guidelines on Chronic Pelvic Pain. EAU Guidelines. The Netherlands: EAU Guidelines Office, Arnhem; ISBN 978-90-79754-91-5. [Google Scholar]
- 17.Kaur H, Arunkalaivanan AS. Urethral pain syndrome and its management. Obstet Gynecol Surv. 2007;62:348–351. doi: 10.1097/01.ogx.0000261645.12099.2a. [DOI] [PubMed] [Google Scholar]
- 18.Crane A, Lloyd J, Shoskes DA. Improving the utility of clinical phenotyping in interstitial cystitis/painful bladder syndrome: from upoint to input. J Urol. 2017;(Suppl):e386–387. [PubMed] [Google Scholar]
- 19.Shoskes DA, Hakim L, Ghoniem G, Jackson CL. Long-term results of multimodal therapy for chronic prostatitis chronic pelvic pain syndrome. J Urol. 2003;169:1406–1410. doi: 10.1097/01.ju.0000055549.95490.3c. [DOI] [PubMed] [Google Scholar]
- 20.Sator-Katzenschlager SM, Scharbert G, Kress HG, et al. Chronic pelvic pain treated with gabapentin and amitriptyline: a randomized controlled pilot study. Wien Klin Wochensch. 2005;117:761–768. doi: 10.1007/s00508-005-0464-2. [DOI] [PubMed] [Google Scholar]
- 21.Backonja M, Glanzman RL. Gabapentin dosing for neuropathic paIn: evidence from randomized, placebo-controlled clinical trials. Clin Ther. 2003;25:81–104. doi: 10.1016/s0149-2918(03)90011-7. [DOI] [PubMed] [Google Scholar]
- 22.Sills GJ. The mechanisms of action of gabapentin and pregabalin. Curr Opin Pharmacol. 2006;6:108–113. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Karatas G, Tamam L, Ozpoyraz N. Anksiyete bozukluklarında gabapentin tedavisi. Klinik Psikofarmakoloji Bulteni-Bulletin of Clinical Psychopharmacology. Bull Clin Psychopharmacol. 2003;13:37–42. [Google Scholar]
- 24.Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283:1837–1844. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- 25.Rynn MA, Siqueland L, Rickels K. Placebo-controlled trial of sertraline in the treatment of children with generalized anxiety disorder. Am J Psychiatry. 2001;158:2008–2014. doi: 10.1176/appi.ajp.158.12.2008. [DOI] [PubMed] [Google Scholar]
- 26.Wagner KD, Ambrosini P, Rynn M, et al. Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials. JAMA. 2003;290:1033–1041. doi: 10.1001/jama.290.8.1033. [DOI] [PubMed] [Google Scholar]
- 27.March JS, Biederman J, Wolkow R, et al. Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA. 1998;280:1752–1756. doi: 10.1001/jama.280.20.1752. [DOI] [PubMed] [Google Scholar]
- 28.Han C, Pae C-U, Lee BH, et al. Fluoxetine versus sertraline in the treatment of patients with undifferentiated somatoform disorder: a randomized, open-label, 12-week, parallel-group trial. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:437–444. doi: 10.1016/j.pnpbp.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Engel CC, Walker EA, Engel AL, Bullis J, Armstrong A. A randomized, double-blind crossover trial of sertraline in women with chronic pelvic pain. J Psychosom Res. 1998;44:203–207. doi: 10.1016/s0022-3999(97)00215-8. [DOI] [PubMed] [Google Scholar]
- 30.Lee R, West R, Wilson J. The response to sertraline in men with chronic pelvic pain syndrome. Sex Transm Infect. 2005;81:147–149. doi: 10.1136/sti.2004.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pontari MA, Krieger JN, Litwin MS, et al. Pregabalin for the treatment of men with chronic prostatitis chronic pelvic pain syndrome a randomized controlled trial. Arch Intern Med. 2010;170:1586–1593. doi: 10.1001/archinternmed.2010.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichard CA, Makovey I, Shoskes DA. Phenotype, symptom severity and treatment in a 'cured' cohort of chronic pelvic pain syndrome patients. Can J Urol. 2015;22:7623–7626. [PubMed] [Google Scholar]
- 33.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]