Abstract
Objectives
The aim of this review was to systematically investigate long-term brain health in retired rugby players.
Methods
Six databases were systematically searched from inception to January 2018 using Medical Subject Headings and keywords. Two reviewers independently screened studies for inclusion. Cross-sectional studies of living retired male or female rugby players in which at least one cognitive test was used as an outcome measure were included. Data extraction was performed using Strengthening the Reporting of Observational Studies in Epidemiology guidelines. Methodological quality was assessed independently by two reviewers using the Downs and Black methodological quality tool.
Results
This review yielded six studies with an overall methodological quality of ‘moderate’. A total of 672 male retired rugby players (mean ages of 38–52 years) were included in this review. Three studies investigated neuropsychological functioning in retired rugby players in comparison with controls, with no significant evidence of decreased performance in the majority of tests when compared with controls. Five out of the six studies explored self-reported measures of cognition. Three studies compared retired rugby players to controls, one of which found significantly increased subjective cognitive complaints among retired rugby players. The other two studies found that persistent postconcussion symptoms were associated with a higher number of self-reported concussions. Two studies reported decreased fine motor control in retired rugby players in comparison with controls. Neurometabolites and electrophysiological changes were explored by two studies, with minimal and non-significant findings.
Conclusions
Overall findings are mixed. Methodological biases reduce the overall study quality and limited the conclusions that can be drawn. Findings of decreased fine motor control in retired athletes may be influenced by lack of controlling for evidence of upper limb musculoskeletal injuries. While some studies show evidence of reduced cognitive function among former athletes, the results are not significantly lower than population norms. Cognitive findings from this review are inconsistent within and across study cohorts and are biased towards positive findings when self-report methods were selected. Current evidence suggests that large gaps remain in the understanding of the cause-and-effect relationships between playing rugby and long-term brain health in retired players.
Keywords: retired, rugby player, aging, brain health, concussion, mild traumatic brain injury, neurocognitive function
What is already known?
Rugby is a popular sport worldwide.
Rugby has one of the highest incidence of concussion of all contact sports.
The long-term neurocognitive effects of a history concussion/repeated head impact exposure is a topic of increasing controversy and concern in rugby.
New findings?
There is modest objective evidence of decreased neuropsychological performance in retired rugby players in comparison with normative data/control cohorts.
Some retired rugby players appear to have increased self-report cognitive difficulties in retirement.
There is some evidence of decreased fine motor control in retired rugby players.
Neuroimaging and neurophysiological investigation is limited to date.
Introduction
Rugby is a popular full-contact sport played throughout the world at varying levels of competition, including professional level. There are many benefits of engaging in sport, with consistent evidence supporting associations with cognitive vitality, neural functioning and decreased risk of cognitive decline.1–6 However, the physically demanding nature of rugby exposes players to injury, particularly involving the tackle,7–9 a central tenant of the game.10 The frequent engagement in high-velocity collisions and impacts during contact activities such as the tackle and scrum, which varies depending on position, commonly result in musculoskeletal system injuries11 and place players at risk of head and neck injury.12 13 Given the physical nature of the sport, the incidence of concussion in rugby is high,14 15 estimated between 4 and 13.4 concussions per 1000 contact hours.13 16–18 This is recognised as one of the highest rates of concussion of all full-contact sports.13
Concussion in sport has been a topic of much attention and controversy. Postmortem investigations of former NFL American football players have raised concerns regarding neurodegenerative diseases, namely chronic traumatic encephalopathy (CTE), as potential adverse long-term outcomes of exposure to head impacts and concussions in sport.19–23 Given that rugby has a higher concussion incidence than American football, the issue of neurodegenerative decline is of particular importance.24 There is some evidence of worse neurocognitive performance associated with school-level participation in rugby. Adolescent male rugby players prospectively investigated have been found to have significantly lower neurocognitive performance over 3 years and poorer academic achievement over 6 years in comparison with non-contact sport controls.25 To date, there has been one case of CTE documented in a deceased former rugby player.26 However, information on long-term brain health among living retired rugby players is limited. Considering the high levels of participation of rugby worldwide,27 a formal objective investigation into long-term cognitive status of rugby players is needed. The aim of this review is to systematically investigate the literature examining long-term neurocognitive status of living retired rugby players.
Methods
This review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (www.prisma-statement.org) and was registered with PROSPERO, a registry of systematic reviews. Registration is available at https://www.crd.york.ac.uk/prospero/; registration number: CRD42017081586.
Eligibility criteria
Retired rugby players were included in this study. Studies were required to include retired male or female rugby players administered at least one form of cognitive testing as an outcome measure. Studies were excluded if they explored only active rugby players and/or case studies with five or fewer participants. The primary outcomes investigated were key domains of cognitive functioning including learning and memory, executive function and complex attention. Secondary outcomes included history of sports-related concussion (SRC)/mild traumatic brain injury (TBI).
Search strategy
A systematic literature search was undertaken using the electronic databases of MEDLINE/PubMed, Embase, Cochrane Central Register of Controlled Trials, PyscINFO, CINAHL and Web of Science from their inception to January 2018. The different search terms were adapted for use with each database (see online supplementary appendix 1). The search strategy keywords related to three components: (1) the participant (eg, retired rugby player), (2) the primary outcome measure (eg, cognitive functioning) and (3) secondary outcome (eg, history of sports concussion). No search restrictions were imposed. The electronic database searching was supplemented by searching abstracts of the international conference on sports concussion consensus meetings (2001–2018) along with conducting grey literature searching and a hand search of the reference lists of included studies. Abstracts (n=2) were found, which were not available in full text in the published literature. In this instance the authors were contacted via email/Research Gate seeking access to the full text of relevant studies. The titles and abstracts of the retrieved studies were independently screened to identify studies that met the eligibility criteria. Following this initial screening, the same two reviewers independently assessed the full texts of the selected studies. Any disagreements on inclusion of studies were resolved through discussion and consultation with a third reviewer to reach a consensus. Following this process of elimination, five studies were included for this review (figure 1).
bmjsem-2018-000356supp001.docx (15.5KB, docx)
Figure 1.
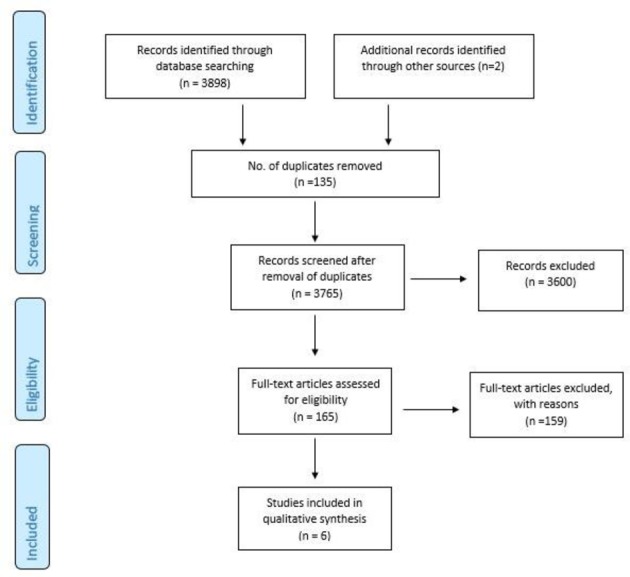
PRISMA flow diagram of study selection process.
Data extraction and analysis
A data extraction template was used as a checklist of items, which should be included in reports of cross-sectional studies, based on Strengthening the Reporting of Observational Studies in Epidemiology guidelines.28 Key details such as participant characteristics, details of concussion history, outcome measures used and relevant outcome data (group means and SD) were recorded and presented in table format (see table 1). A meta-analysis was deemed inappropriate due to the small number of studies, heterogeneity of study designs and varying cognitive assessments employed.
Table 1.
Data extraction
| Reference | Objective | Number of participants (n=); mean age (SD) | Number of concussions:mean (SD) | Definition of concussion | Cognitive outcome measures | Findings | Downs and Black Score; quality index |
| Gardner et al 32 | To examine brain neurometabolite concentrations in retired rugby league players | Retired rugby players n=1638.3±(3)Controls n=1637.9±(4.9) | Retired players reported an average of 33.44 (median=20; IQR=7–20; range 3–100) concussions, retired players reported an average of 5.9 concussions with LOC sustained during their careers (median=3.5; IQR=3.5–6; range 0–30). | Not Defined | ACS-TOPF, RAVLT, RCFT, TMT A and BCOWAT, WAIS-IV, RPQ | Retired players did not significantly differ in concentrations of 4 out of 5 neurometabolites tested. A significantly lower concentrations of grey matter glutathione (p=0.02) in retired players was detected. There were no significant differences between groups on measures of depression, anxiety or cognitive functioning. | 9; moderate |
| Lewis et al 34 | To assess measures of corticomotor excitability and inhibition in retired rugby players. | Elite rugby players n=2343±(7)Community-level rugby n=2845±(8)Retired non-contact sport controls n=2244±(9) | Elite rugby players: 0 concussions (n=0; 0%)1–2 concussions (n=3; 13%)≥3 concussions (n=20; 87%)Community-level rugby:0 concussions (n=1; 4%)1–2 concussions (n=3; 11%)≥3 concussions (n=23; 85%)Retired non-contact sport controls:0 concussions (n=16; 75%)1–2 concussions (n=5; 21%)≥3 concussions (n=1; 4%) | ‘A blow to the head followed by a variety of symptoms that may include any of the following: headache, dizziness, loss of balance, blurred vision, “seeing stars”, feeling in a fog or slowed down, memory problems, poor concentration, nausea or throwing-up. Getting “knocked out” or being unconscious does NOT always occur with a concussion’. | RPQ: predominantly early (RPQ-3) and late (RPQ-13) symptoms of brain injury. | RMT was significantly higher, and LICI was greater in the elite rugby group compared with the control group. | 8; limited |
| Hume et al 10 | To investigate cognitive function in former professional rugby players and assess the association between concussion history and cognitive function. | Retired elite rugby n=10341.3±(7.5)Retired community rugby n=19544.9±(8.4)Retired non-contact sport Group n=6542.1±(7.7) | Elite rugby=3.5±(2.0)Community rugby=2.9±(2.2)Non-contact=0.4±(0.8) | ‘A blow to the head followed by a variety of symptoms (LOC, headache, dizziness, loss of balance, blurred vision “seeing stars”, feeling in a fog or slowed down, memory problems, poor concentration, nausea or throwing up’. | Online CNS-Vital Signs Test | Elite rugby group performed worse compared with non-contact sports on tests of complex attention −0.67 (−0.07 to −0.26) processing speed −0.51 (−0.89 to −0.12) executive functioning −0.41 (−0.80 to −0.02) and cognitive flexibility −0.37 (−0.74 to 0.00). | 11; limited |
| McMillan et al 27 | To investigate symptoms and a range of cognitive and health outcomes in retired rugby players with history of repeated concussion. | Retired international rugby players n=5253.5±(13.0)Controls=2955.1±(9.0) | Retired international rugby players 13.9±(18.9)Controls 0.3±(0.5) | ‘Being a blow or injury to your head where you may or may not have lost consciousness and then had symptoms, such as dizziness, blurred vision, nausea, vomiting, headache, poor concentration’. | MOCA, SDT, TMT, RAVLT SART, JLO Test, Lafayette Grooved Pegboard, SF-36GOSE | RIRP performed poorer than controls on a test of verbal learning (p=0.022). No significant difference on the other cognitive tests were found (p>0.05). | 4; poor |
| Decq et al 33 | To assess the prevalence of major depressive disorder, mild cognitive disorders and headache in a population of retired rugby players. | Age (years) median (IQR)Retired rugby players (RRPs)=23952 (49–55.75)Other retired sportsmen (ORS)=13852 (49–55) | Retired rugby player: n=3.1±(5.01)Other sports: n=0.68±(1.83) | Not defined | Self-administered questionnaireF-TICS-m | A higher rate of major depressive disorder was observed among RRPs compared with ORS (p=0.04). The PHQ-9 score was increased with the number of reported concussions regardless of the type of sport (p=0.026). A higher rate of mild cognitive disorders was observed in RRPs compared with ORS (57% vs 40%) p=0.005. | 13; moderate |
| Thornton et al 35 | To examine the extent to which lifetime concussion exposure is associated with neurocognitive and symptomatic status in competitive versus recreational/retired players. | Male and female studyretired players (all male) n=1639.25±(10.99)Recreational players n=1550.53±(9.80)Older players n=3144.71±(11.75)Competitive players n=8026.43±(6.53) | Divided participants into no heavy concussion exposure groups (grade 2 or above) (n=37, 8:29), 1–2 heavy concussions (n=39, 4:35) and 3 or more (n=35, 1:34) | Criteria from the American Academy of Neurology. Grade 1 – transient confusion that resolves within 15 min with no LOC. Grade 2 – transient confusions that persist with for more than 15 min with no LOC. Grade 3 – any LOC. | ETS Kit, CCFT, WAIS-III, TMT-A and B, WMS-III, RAVLT, WCST, PCSC | Concussion exposure did not predict neurocognitive functioning but did predict PCS. Participants with no heavy concussions reported significantly fewer memory complaints (d=−0.68), less distress (d=−0.76) and less overall (total) PCS (d=−0.65) than did those with three or more heavy concussions. | 12; moderate |
ACS-TOPF, Advanced Clinical SolutionsTest of Premorbid Functioning; CCFT, Cattell’s Culture Fair Intelligence Test; COWAT, Controlled Oral Word Association Test; ETS Kit, Educational Testing Service; F-TICS-m, French version of the modified telephone interview for cognitive status; GOSE, Extended Glasgow Outcome Scale; JLO Test, Judgement of Line Orientation; LICI, long-interval intracortical inhibition; LOC, loss of consciousness; MOCA, Montreal Cognitive Assessment; PCS(C), Postconcussion Syndrome Checklist; PHQ-9, Patient Health Questionnaire; RAVLT, Rey Auditory Verbal Learning Test; RCFT, Rey Complex Figure Test; RPQ, Rivermead Post-concussion Symptoms Suestionnaire; RMT, resting motor threshold; SART, Sustained Attention to Response Task; SDT, Symbol Digit Test; SF-36, 36-Item Short Form Survey; TMT, Trail Making Test; WAIS, Wechsler Abbreviated Scale of Intelligence; WCST, Wisconsin Card Sorting Test; WMS, Wechsler Memory Scale.
Methodological assessment
An adapted Downs and Black checklist was used to evaluate the methodological quality of the studies.29 This was performed independently by two reviewers (JCC and FW). Disagreements between the reviewers were resolved through discussion to achieve consensus. Failing agreement, a third reviewer (SB) arbitrated. The checklist was modified to a maximum of 17 applicable questions, which addressed the following methodological components: reporting, external validity, internal validity (bias and confounding) and power. Seventeen items were rated either as yes (=1) or no/unable to determine (=0), and one item was rated on a three-point scale (yes=2, partial=1, and no=0). The maximum achievable score was 18, with higher scores indicating a better methodological quality of the study. Results were categorised according to the adapted Downs and Black checklist29 from Hartling et al 30 and Hignett.31 Interpretation of results was as follows: strong quality (≥14) represented the top 75%; moderate quality (9–13) represented 50%–74%; limited quality represented (5–8) represented 25%–49%; and poor quality (<5) represented <25%.
Results
The search strategy and selection process are summarised in figure 1, with 3765 records (after the removal of duplicates)initially identified. A total of six studies published between 2008 and 2017 met our criteria and were included in this review. Studies were cross-sectional observational studies that explored cognitive health measures in retired rugby players.
Types of outcome measures used
The studies included a wide variety of cognitive tests in order to assess the retired athlete’s cognitive capabilities. Some studies used a comprehensive battery of neuropsychological tests that subjectively or objectively explored different aspects of cognition, while others used only one cognitive test. To ease interpretability of tests used, individual neuropsychological tests were categorised according to the predominant cognitive domain they assessed. The eight domains explored were: attention, executive functioning, information processing, motor speed, verbal ability, verbal memory, visual memory and visuospatial ability. Neuropsychological tests and their corresponding cognitive domains are displayed in figure 2. In addition to the cognitive evaluation, subjective and self-administered questionnaires were used. One study used a self-reported measure of cognition: the Modified Telephone Interview for Cognitive Statu. Four other studies used a specific postconcussion questionnaires, for example, the Rivermead Post-Concussion Symptoms Questionnair and the Post-Concussion Symptom Checklist (PSCS), both of which assess for persistent symptoms associated with concussion, including cognitive complaints.
Figure 2.
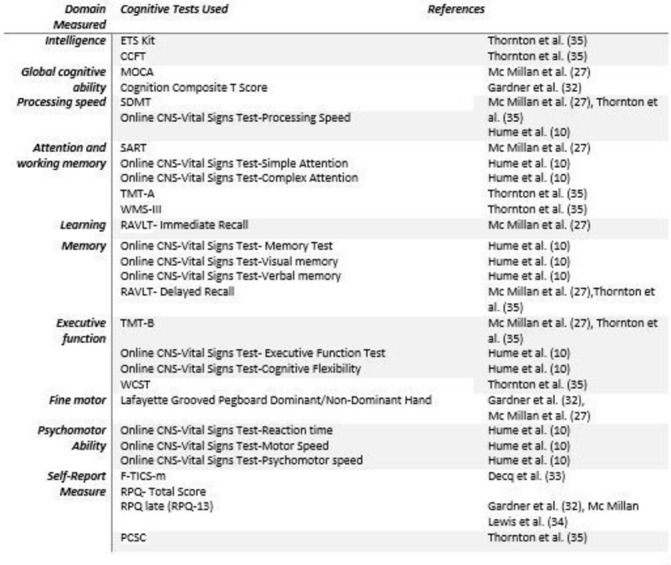
Cognitive tests and corresponding domains. CCFT, Cattell’s Culture Fair Intelligence Test; CNS, computerised neurocognitive assessment software; ETS Kit, Educational Testing Service; F-TICS-m, French version of the modified telephone interview for cognitive status; MOCA, Montreal Cognitive Assessment; PCSC, Post-Concussion Symptom Checklist; RAVLT, Rey Auditory Verbal Learning Test; RPQ, Rivermead Post-Concussion Symptoms Questionnaire; SART, Sustained Attention to Response Task; SDMT, Symbol Digit Modalities Test; TMT, Trail Making Test; WCST, Wisconsin Card Sorting Test; WMS, Wechsler Memory Scale.
Methodological assessment
The mean methodological quality score was 9.5 (SD 3.27) out of a total score of 18, giving an overall quality score of ‘moderate’ (percentage 50%–74%; 9–13). See table 1 for individual quality scores.
Study characteristics
Study characteristics and findings are summarised in table 1. A total of 672 male retired rugby players (range of mean ages 38–52 years) were included in this review. Participant characteristics varied in age, medical history, socioeconomic background, concussion exposure and number of concussions reported. Five out of the six studies included a control group.10 27 32–34 While the different cohorts in the study by Thornton et al 35 included rugby players across varying participation/competition levels.
Objective cognitive tests
Gardner et al 32 found no significant differences in cognitive functioning in retired rugby players in comparison with controls. Thornton et al 35 also found no between group differences in cognitive functioning in retired rugby players who were grouped based on self-reported concussion history in a non-controlled study. McMillan et al 27 found that retired rugby players performed significantly worse than controls on tests of verbal learning (p=0.022), but no significant differences were found in cognitive tests of global cognitive functioning, processing speed, attention, memory and executive function in comparison with controls (p>0.05). Hume et al10 found that a self-reported history of concussion was associated with small to moderate neurocognitive deficits in retired rugby players in areas such as cognitive flexibility, complex attention and executive function relative to the player group with no concussion history. The elite rugby group performed worse on tests of complex attention (effect size (EF) −0.67, 95% CI −1.07 to −0.26) and cognitive flexibility (EF −0.37, −0.74 to 0.00). The community rugby group performed worse than the non-contact group on executive functioning (EF −0.51, 95% CI −0.89 to −0.12).
Subjective cognitive tests
Gardner et al 32 and Lewis et al 34 found no significant differences in self-report (S-R) measures of postconcussion symptoms (PCS) in retired rugby players compared with controls. In contrast, Decq et al 33 found that retired rugby players had a higher rate of mild cognitive disorders (p=0.005), based on S-R of cognitive symptoms. McMillan et al 27 found that persistent symptoms attributed to concussion were more common in retired rugby players who self-reported more than nine concussion events (p=0.028). However, these symptoms were not perceived to affect social or work functioning. Thornton et al 35 separated the rugby players into groups based on self-reported concussion exposure. Players with higher past concussion exposure were found to have increased PCS. Participants with no ‘heavy’ concussions, which included grade 2 or 3 concussions as described by the American Academy of Neurology,36 reported significantly fewer memory complaints, less distress and less overall total PCS than did those with three or more heavy concussions.
Fine motor function
Gardner et al found that retired rugby players performed significantly worse in a test of manual dexterity with the non-dominant hand (p=0.03). Similarly, McMillan et al 27 found that retired rugby players had significantly lower scores on a test of fine coordination of the dominant hand (p=0.038). Both studies compared retired rugby players with healthy community control groups.
Neuroimaging
Gardner et al 32 were the only investigators to explore brain health in retired rugby players using a neuroimaging modality. Magnetic resonance spectroscopy was used to detect potential biochemical changes, showing that in comparison with non-sportsperson controls, retired rugby players had significantly lower concentrations of grey matter glutathione (p=0.02). No differences were found in grey matter (p=0.19) or white matter N-acetylaspartat (p=0.52) in retired athletes compared with controls.
Electrophysiology
Lewis et al 34 were the only authors to evaluate for electrophysiological changes in retired rugby players. Resting motor threshold was significantly higher (p=0.004), along with greater long-interval intracortical inhibition (p=0.005) in the elite rugby group compared with the control group. There was no evidence of altered corticomotor excitation and inhibition in the retired community rugby group who had experienced a similar number of concussions.
Discussion
This review shows modest objective evidence of decreased neuropsychological performance in retired rugby players. Some retired players appear to have persistent subjective postconcussion symptoms complaints, which were associated with the number of reported concussions in two studies. Fine motor control was found to be decreased in retired players compared with control groups in two studies. The evidence indicating declines in neurophysiological and neurochemical measures among retired rugby players were equivocal. Despite the research in this area being in its infancy, the long-term consequences of SRC remains a controversial topic of continuing interest. Some are now advocating for the banning of collision sports that expose athletes to head impacts, particularly at underage levels,37 but the current body of knowledge surrounding the long-term sequelae of playing rugby is not conclusive. As modern societies are largely inactive, banning a large number of sports is likely to counteract the goal of optimal brain health.38
Notably, investigations into the long-term effects of head impact exposure with/without concussion, associated with rugby participation on later-life brain health, are influenced by methodological biases. Primary among the studies evaluated here is the issue of self-selected participation on which results are based. It is therefore unclear whether the studies presented herein are representative of the entire retired rugby player the population. While five out of six studies provided a concussion definition,10 27 33–35 a wide variety of definitions used differed from international consensus recommended guidelines.39 40 The lack of standardised criteria may impact accurate injury surveillance.41 42 Gardner et al,32 Thornton et al 35 and McMillan et al 27 had the most robust exclusion criteria with participants excluded on the basis of a medical history of neurosurgery, diagnosis of chronic and debilitating neurological or psychiatric disturbance or other major medical conditions. These limitations make it difficult to draw conclusions regarding the long-term sequelae.
A control cohort was used in five out of six studies, with Thornton et al 35 being the only authors to exclude a control group. Instead, between-group differences among competitive, older/recreational and retired rugby players based on the number of previous concussions were analysed. While the lack of a non-contact control group is a potential bias, investigating groups of rugby players based on number of reported concussions offers an opportunity to evaluate the effect of concussion. However, given that the groups ranged from competitive to recreational to retired rugby players, different levels of head impact exposure without concussion would be expected. This is reflected in the different number of years played, which is highest in the older and recreational players. However, the influence of overall head impact exposure on cognitive tests was not explored. Additionally, the male-to-female ratio differed across the groups, with the ‘no concussions’ group having the highest percentage of females included. In predicting cognitive functioning based on neurocognitive test batteries, only demographic variables were found to be significant. Greater concussion exposure was found to be associated with total PCSC scores including the memory PCS scale. However, this relationship was only present in retired and recreational players and not competitive players. It is difficult to interpret the associations between self-report concussion history and subjective PCSC scores, given the potential for recall bias in self-report.
Three out of the five studies did not control for a history of non-sports-related TBI among participants,10 33 34 which may influence the results. Gardner et al 32 were the only investigators to control for a history of neurotrauma or participation in contact sports. Investigations in the majority of the studies have been on retired rugby players in comparison with controls who, in many cases, have a previous history of concussion/history of participation in sports that may expose to head impacts.10 27 34 For example, 34% of controls in the study by McMillan et al 27 had a concussion history and 63% had a history of playing rugby. A more robust comparison would include sport type, years played, position, along with number of concussions among all participants including controls. By failing to do so, there is a risk of biasing between group differences in cognitive tests. Ideally, former non-contact sport athletes, with and without concussion history, should be employed as a control group to allow for the evaluation of concussion alone, head impact exposure without concussion and head impact exposure with concussion. Length of time since most recent concussion and retirement (ie, time elapsed since potential head impact exposure) is also of significant importance when trying to differentiate between the potential long-term effects of a career in rugby as oppose to more short-term effects of a recent concussion. This was only assessed in two out of the five studies.32 34 Gardner et al 32 included two retired players who had sustained a concussion within the past year, 4 and 6 months prior. Among the retired players, four had retired sometime during the previous 12 months. Whereas, Lewis et al 34 required rugby players to be retired from competitive sport for at least 5 years.
The host of confounding factors that may influence brain functioning in the retired rugby players as they age were controlled to different extents in the studies. Gardner et al 32 was the only investigator to evaluate premorbid function. However, two participants who had a history of attendance in special education classes, reading problems and spelling problems were included in this study. Lewis et al 34 were the only investigators to control for drugs, physical activity and sleep quality. Importantly, participants were excluded if they were taking medication that are known to influence corticomotor excitability. Gardner et al 32 controlled for current prescription medication use among retired rugby players. It is important to view results within a wider context and to consider what implications the observed differences in test performance will have in terms of clinical performance and ongoing neurological function. Hume et al 10 found that rugby players performed worse than non-contact sport controls in certain areas of cognition. However, the elite rugby players still performed equally or better than US normative values on 6 out of 11 measures. In the other five measures (cognitive flexibility, processing speed, executive functioning, reaction time and verbal memory), they performed slightly lower (small to moderate EFs) than US normative values. The elite rugby players performed slightly better than the US norms on motor speed, while the community rugby players performed slightly better than US norms on a measure of complex attention. Collectively, the rugby cohort performed worse on 45% of the measures compared with the controls but were within one SD of normative values. For example, while there was a moderate difference in complex attention between the elite rugby group and the non-contact group, the mean score for the elite players on complex attention was 99, which was near the US standardised average of 100. The moderate between group differences is therefore due to the non-contact sport control group outperforming US standardised average, with a mean score of 106. McMillan et al 27 reported decreased performance in retired rugby players in two out of seven cognitive tests. However, the performance of the rugby player group still fell within the normal range for all tests. Where differences were found, they were not associated with a higher number of repeat concussions.
Similarly, results from the study by Gardner et al 32 are unclear. The study investigated neurometabolites, based on literature suggesting that they may be indicators of neuronal loss, neuroinflammation, axonal injury and possible neurodegenerative pathologies such as Alzheimer’s disease and mild cognitive Impairment (MCI).32 Biochemical alterations were evident for one out of five of these neurometabolites. The significance of this finding is unclear, particularly given the lack of clinical correlates. Lewis et al 34 found some evidence for altered corticomotor excitability and intracortical inhibition in retired elite rugby players in comparison with retired non-contact sport players. Importantly, both rugby groups reported a similar number of concussions and symptom severity compared with the control group, which did not influence the results. Given the absence of findings in the community rugby group, the association with previous concussion is unclear. However, one explanation may be the higher cumulative influence of repeated head impact exposure at the elite-level driving between group results. There is evidence to suggest that cumulative effect of multiple concussions or repeated head impact exposure may be more influential than the effect of a single concussion.43 An attempt to quantify overall head impact exposure was not undertaken, making it uncertain if long-term exposure to rugby without concussion is associated with alterations in corticomotor function.
Decq et al 33 did not find an association between S-R concussion history and cognitive outcome. However, smoking and higher education were factors independently associated with higher and lower S-R cognitive dysfunction, respectively. Among retired rugby players in the study by McMillan et al,27 persisting symptoms attributed to concussion were more common if reporting more than nine concussions (p=0.03), although these symptoms were not perceived to affect social or work functioning. McMillan and Gardner et al 32 both found significantly poorer performance in rugby players in comparison with controls on test of fine motor function. It is inconclusive whether this deficit can be attributed to a history of head impact exposure. There are also a number of other factors that may contribute to poorer fine motor control and dexterity in retired players, namely musculoskeletal injuries to the upper limb sustained throughout a career, which are extremely common in rugby.44 45 Four out of five studies included alcohol screening,10 32–34 Gardner et al 32 found that former rugby players reported significantly greater alcohol consumption in comparison with controls (0<0.01). This which may have impacted fine-motor performance.46
In addition to concussion, the brain itself is constantly changing primarily because of normal ageing. Two out of the five studies had an age cut-off as part of the exclusion criteria.33 34 Lewis et al 34 required former players to be aged 30–65 years. Similarly, Decq et al 33 excluded retired players over the age of 66 years. It is possible that the relatively young age of retired rugby players in the included studies and the age cut-off in two studies may have influenced results, given that younger retired rugby players would be expected to be more cognitively resilient. It may be more pertinent to follow players over the age of 65 years, due to the normal effects of the ageing process on cognitive functioning and the suggested role that head impact exposure may play in this process.47 Particularly, in light of the influence of cognitive reserve and neural compensatory mechanisms,48–50 which begin to diminish with age. Therefore, the exclusion of players in this age bracket in both studies may have limited the potential for greater observed between group differences. Follow-up studies are required on these rugby players as they enter older age. The neurocognitive findings in the study by Thornton et al 35 present an interesting finding, given there were minimal differences across the three concussion exposure groups in neuropsychological functioning. If there was a cumulative effect of head impact exposure on neuropsychological performance, a difference would be expected in the form of decreased performance in retired players in comparison with younger players.
The nature of rugby has changed over time and will continue to evolve. The modern game has larger, faster, stronger players, experiencing greater impact forces.10 Therefore, the nature of long-term health in former players may change over time. Equally, major strides in the awareness of SRC, availability of sideline medical assessment and treatment of players are evident across the board and specifically within the sport of rugby. The rigour with which concussion is identified and return to play managed has greatly improved.27 This may positively influence the long-term brain health of retired athletes and minimise any potential adverse long-term effects of rugby on brain functioning. Hence, findings from this review may not generalise to the modern rugby era but do not preclude additional investigation. Whether repeated concussions associated with playing rugby can be partly or completely responsible for longer term neurological disorders in older retired rugby players remains unanswered.
Conclusion
This review highlights the need for additional research to clarify the long-term influence of playing rugby on the brain health of retired rugby players. Despite the call for bans, the evidence is inconclusive and poorly developed. This review does offer positive findings in relation to objective neuropsychological performance in retired rugby players, the majority of which were normal, apart from fine motor control. Reliability of player self-reported concussion and the evidence of increased self-reported cognitive difficulties among some retired rugby players must be further investigated. In the absence of prospective epidemiological studies to establish a direct relationship between playing rugby and long-term brain health, the neurocognitive status of living retired rugby players remains unclear. Further research is required to investigate whether decreased cognitive functioning, both objectively and subjectively, may become more apparent in retired rugby players as they age. Equally, additional research is necessary to investigate evidence of neurochemical changes and fine motor functioning in retired rugby players and the aetiology and pathophysiology behind findings.
Recommendations
There is an urgent need for prospective longitudinal studies of brain health in rugby players, taking into account both history of concussion and overall head impact exposure. With regard participants in the current review, ongoing follow-up of rugby players is needed to clarify the potential long-term direct or indirect influence of head impact exposure on cognitive function in ageing rugby players.
Acknowledgement
We would like to thank David Mockler (medical librarian).
Footnotes
Contributors: JCC was first author. SB and FW were the two coauthors to this review. FW was involved with selection process of articles. She conducted methodology quality assessment for each study and edited the review. SB edited the review.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Ethics approval: This project was exempt from local Faculty of Health Sciences, St. James’s Hospital ethics approval as published data were pooled only.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res 2015;60:56–64. 10.1016/j.jpsychires.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roig M, Nordbrandt S, Geertsen SS, et al. . The effects of cardiovascular exercise on human memory: a review with meta-analysis. Neurosci Biobehav Rev 2013;37:1645–66. 10.1016/j.neubiorev.2013.06.012 [DOI] [PubMed] [Google Scholar]
- 3.Smith PJ, Blumenthal JA, Hoffman BM, et al. . Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med 2010;72:239–52. 10.1097/PSY.0b013e3181d14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bherer L, Erickson KI, Liu-Ambrose T. A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res 2013;2013:1–8. 10.1155/2013/657508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajewski PD, Falkenstein M. Physical activity and neurocognitive functioning in aging - a condensed updated review. Eur Rev Aging Phys Act 2016;13:1 10.1186/s11556-016-0161-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prakash RS, Voss MW, Erickson KI, et al. . Physical activity and cognitive vitality. Annu Rev Psychol 2015;66:769–97. 10.1146/annurev-psych-010814-015249 [DOI] [PubMed] [Google Scholar]
- 7.Fuller CW. Catastrophic injury in rugby union: is the level of risk acceptable? Sports Med 2008;38:975–86. [DOI] [PubMed] [Google Scholar]
- 8.Fuller CW, Ashton T, Brooks JH, et al. . Injury risks associated with tackling in rugby union. Br J Sports Med 2010;44:159–67. 10.1136/bjsm.2008.050864 [DOI] [PubMed] [Google Scholar]
- 9.Gardner AJ, Iverson GL, Quinn TN, et al. . A preliminary video analysis of concussion in the National Rugby League. Brain Inj 2015:1182–5. 10.3109/02699052.2015.1034179 [DOI] [PubMed] [Google Scholar]
- 10.Hume PA, Theadom A, Lewis GN, et al. . A comparison of cognitive function in former Rugby Union players compared with former non-contact-sport players and the impact of concussion history. Sports Med 2017;47:1209–20. 10.1007/s40279-016-0608-8 [DOI] [PubMed] [Google Scholar]
- 11.King DA, Hume PA, Milburn PD, et al. . Match and training injuries in rugby league: a review of published studies. Sports Med 2010;40:163–78. 10.2165/11319740-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 12.Brown JC, Lambert MI, Hendricks S, et al. . Are we currently underestimating the risk of scrum-related neck injuries in rugby union front-row players? Br J Sports Med 2014;48:1127–9. 10.1136/bjsports-2013-092869 [DOI] [PubMed] [Google Scholar]
- 13.Gardner AJ, Iverson GL, Williams WH, et al. . A systematic review and meta-analysis of concussion in rugby union. Sports Med 2014;44:1717–31. 10.1007/s40279-014-0233-3 [DOI] [PubMed] [Google Scholar]
- 14.King D, Gissane C, Brughelli M, et al. . Sport-related concussions in New Zealand: a review of 10 years of Accident Compensation Corporation moderate to severe claims and costs. J Sci Med Sport 2014;17:250–5. 10.1016/j.jsams.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 15.Shuttleworth-Edwards AB, Noakes TD, Radloff SE, et al. . The comparative incidence of reported concussions presenting for follow-up management in South African Rugby Union. Clin J Sport Med 2008;18:403–9. 10.1097/JSM.0b013e3181895910 [DOI] [PubMed] [Google Scholar]
- 16.Hollis SJ, Stevenson MR, McIntosh AS, et al. . Incidence, risk, and protective factors of mild traumatic brain injury in a cohort of Australian nonprofessional male rugby players. Am J Sports Med 2009;37:2328–33. 10.1177/0363546509341032 [DOI] [PubMed] [Google Scholar]
- 17.Kemp SP, Hudson Z, Brooks JH, et al. . The epidemiology of head injuries in English professional rugby union. Clin J Sport Med 2008;18:227–34. 10.1097/JSM.0b013e31816a1c9a [DOI] [PubMed] [Google Scholar]
- 18.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train 2007;42:311–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Hay J, Johnson VE, Smith DH, et al. . Chronic traumatic encephalopathy: the neuropathological legacy of traumatic brain injury. Annu Rev Pathol 2016;11:21–45. 10.1146/annurev-pathol-012615-044116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mez J, Daneshvar DH, Kiernan PT, et al. . Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 2017;318:360–70. 10.1001/jama.2017.8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKee AC, Cantu RC, Nowinski CJ, et al. . Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol 2009;68:709–35. 10.1097/NEN.0b013e3181a9d503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stern RA, Daneshvar DH, Baugh CM, et al. . Clinical presentation of chronic traumatic encephalopathy. Neurology 2013;81:1122–9. 10.1212/WNL.0b013e3182a55f7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKee AC, Stern RA, Nowinski CJ, et al. . The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136(Pt 1):43–64. 10.1093/brain/aws307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall SW, Spencer RJ. Concussion in Rugby: the hidden epidemic. J Athl Train 2001;36:334–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Alexander DG, Shuttleworth-Edwards AB, Kidd M, et al. . Mild traumatic brain injuries in early adolescent rugby players: Long-term neurocognitive and academic outcomes. Brain Inj 2015;29:1113–25. 10.3109/02699052.2015.1031699 [DOI] [PubMed] [Google Scholar]
- 26.Stewart W, McNamara PH, Lawlor B, et al. . Chronic traumatic encephalopathy: a potential late and under recognized consequence of rugby union? QJM 2016;109:11–15. 10.1093/qjmed/hcv070 [DOI] [PubMed] [Google Scholar]
- 27.McMillan TM, McSkimming P, Wainman-Lefley J, et al. . Long-term health outcomes after exposure to repeated concussion in elite level: rugby union players. J Neurol Neurosurg Psychiatry 2017;88:505–11. 10.1136/jnnp-2016-314279 [DOI] [PubMed] [Google Scholar]
- 28.von Elm E, Altman DG, Egger M, et al. . STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartling L, Brison RJ, Crumley ET, et al. . A systematic review of interventions to prevent childhood farm injuries. Pediatrics 2004;114:e483–96. 10.1542/peds.2003-1038-L [DOI] [PubMed] [Google Scholar]
- 31.Hignett S. Systematic review of patient handling activities starting in lying, sitting and standing positions. J Adv Nurs 2003;41:545–52. 10.1046/j.1365-2648.2003.02566.x [DOI] [PubMed] [Google Scholar]
- 32.Gardner AJ, Iverson GL, Wojtowicz M, et al. . MR spectroscopy findings in retired professional Rugby league players. Int J Sports Med 2017;38:241–52. 10.1055/s-0042-120843 [DOI] [PubMed] [Google Scholar]
- 33.Decq P, Gault N, Blandeau M, et al. . Long-term consequences of recurrent sports concussion. Acta Neurochir 2016;158:289–300. 10.1007/s00701-015-2681-4 [DOI] [PubMed] [Google Scholar]
- 34.Lewis GN, Hume PA, Stavric V, et al. . New Zealand rugby health study: motor cortex excitability in retired elite and community level rugby players. N Z Med J 2017;130:34–44. [PubMed] [Google Scholar]
- 35.Thornton AE, Cox DN, Whitfield K, et al. . Cumulative concussion exposure in rugby players: neurocognitive and symptomatic outcomes. J Clin Exp Neuropsychol 2008;30:398–409. 10.1080/13803390701443662 [DOI] [PubMed] [Google Scholar]
- 36.Practice parameter: the management of concussion in sports (summary statement). Report of the Quality Standards Subcommittee. Neurology 1997;48:581–5. [DOI] [PubMed] [Google Scholar]
- 37.Meehan WP, Taylor AM, Berkner P, et al. . Division III collision sports are not associated with neurobehavioral quality of life. J Neurotrauma 2016;33:254–9. 10.1089/neu.2015.3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giza CC, Prins ML, Hovda DA. It’s not all fun and games: sports, concussions, and neuroscience. Neuron 2017;94:1051–5. 10.1016/j.neuron.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 39.King DA, Gabbett TJ, Gissane C, et al. . Epidemiological studies of injuries in rugby league: suggestions for definitions, data collection and reporting methods. J Sci Med Sport 2009;12:12–19. 10.1016/j.jsams.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 40.McCrory P, Meeuwisse W, Dvořák J, et al. . Consensus statement on concussion in sport-the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med 2017;51:838–47. 10.1136/bjsports-2017-097699 [DOI] [PubMed] [Google Scholar]
- 41.Quarrie KL, Murphy IR. Towards an operational definition of sports concussion: identifying a limitation in the 2012 Zurich consensus statement and suggesting solutions. Br J Sports Med 2014;48:1589–91. 10.1136/bjsports-2014-094112 [DOI] [PubMed] [Google Scholar]
- 42.Carney N, Ghajar J, Jagoda A, et al. . Executive summary of Concussion guidelines step 1: systematic review of prevalent indicators. Neurosurgery 2014;75(Suppl 1):S1–2. 10.1227/NEU.0000000000000434 [DOI] [PubMed] [Google Scholar]
- 43.Manley G, Gardner AJ, Schneider KJ, et al. . A systematic review of potential long-term effects of sport-related concussion. Br J Sports Med 2017;51:969–977. 10.1136/bjsports-2017-097791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McIntosh AS. Rugby injuries. Med Sport Sci 2005;49:120–39. 10.1159/000085394 [DOI] [PubMed] [Google Scholar]
- 45.Usman J, McIntosh AS. Upper limb injury in rugby union football: results of a cohort study. Br J Sports Med 2013;47:374–9. 10.1136/bjsports-2012-091224 [DOI] [PubMed] [Google Scholar]
- 46.Brumback T, Cao D, King A. Effects of alcohol on psychomotor performance and perceived impairment in heavy binge social drinkers. Drug Alcohol Depend 2007;91:10–17. 10.1016/j.drugalcdep.2007.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moretti L, Cristofori I, Weaver SM, et al. . Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol 2012;11:1103–12. 10.1016/S1474-4422(12)70226-0 [DOI] [PubMed] [Google Scholar]
- 48.Bigler ED, Stern Y. Traumatic brain injury and reserve. Handb Clin Neurol 2015;128:691–710. 10.1016/B978-0-444-63521-1.00043-1 [DOI] [PubMed] [Google Scholar]
- 49.Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc 2002;8:448–60. 10.1017/S1355617702813248 [DOI] [PubMed] [Google Scholar]
- 50.Levi Y, Rassovsky Y, Agranov E, et al. . Cognitive reserve components as expressed in traumatic brain injury. J Int Neuropsychol Soc 2013;19:664–71. 10.1017/S1355617713000192 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjsem-2018-000356supp001.docx (15.5KB, docx)


