Abstract
The aims of the current statement are to refine the definition of quality in cardiovascular imaging and to propose novel methodological approaches to inform the demonstration of quality in imaging in future clinical trials and registries. We propose defining quality in cardiovascular imaging using an analytical framework put forth by the Institute of Medicine whereby quality was defined as testing being safe, effective, patient-centered, timely, equitable, and efficient. The implications of each of these components of quality health care are as essential for cardiovascular imaging as they are for other areas within health care. Our proposed statement may serve as the foundation for integrating these quality indicators into establishing designations of quality laboratory practices and developing standards for value-based payment reform for imaging services. We also include recommendations for future clinical research to fulfill quality aims within cardiovascular imaging, including clinical hypotheses of improving patient outcomes, the importance of health status as an end point, and deferred testing options. Future research should evolve to define novel methods optimized for the role of cardiovascular imaging for detecting disease and guiding treatment and to demonstrate the role of cardiovascular imaging in facilitating healthcare quality.
Keywords: AHA Scientific Statements, cardiac imaging techniques
Quality in health care has been challenging to define but has evolved over the past several decades to represent adherence to sets of performance measures associated with outcomes for specific treatments. Quality for cardiovascular imaging, however, is currently not well defined. Historically, the term quality in cardiovascular imaging has had many meanings, including excellence in technical standards and interpretive acumen. The underlying premise in evaluating quality is that the procedure would lead to improved patient outcomes, including both a reduction in adverse health outcomes and an improvement in the patient’s experience. However, cardiovascular imaging has often lacked the depth and strength of quality evidence typically produced for cardiovascular therapeutic interventions. Moreover, there is a perception within the medical community that quality has 1 standard to be uniformly applied to both therapeutic interventions and imaging. Previous efforts to evaluate the quality of cardiovascular imaging have focused on structural and process measures of quality, as assessed by accreditation standards and patient selection through the development of appropriateness criteria. Although these represent important initial forays into improving quality, there is a need for a broader conceptual framework to ensure that the maximal value of cardiovascular imaging is being routinely provided and may be easily measured. Although therapeutic agents may have a direct impact on patient outcomes, the mechanism by which imaging tests improve outcomes is indirect and subject to multiple confounders affecting patient outcomes, including how physicians decide on which imaging test to order and how to use the imaging results in patient management. Defining how to measure quality in cardiovascular imaging is essential because imaging remains a widely used and potentially costly resource affecting all aspects of cardiovascular patient care.
For the imager, past standards of imaging quality focused on producing a timely and accurate report.1 In 2006, an intersociety conference of 53 professional societies met to discuss quality in imaging and produced a statement expanding on our previous limited view to define diverse metrics related to access, appropriateness, and patient safety.2 In the present statement from the American Heart Association (AHA) Clinical Cardiology Council’s Cardiovascular Imaging and Intervention Subcommittee in collaboration with the Council on Quality of Care and Outcomes Research, we propose to further refine the definition of quality in cardiovascular imaging and suggest novel methodological approaches to inform the demonstration of quality within future clinical trials and registries. It is our goal that indicators used to define quality in cardiovascular imaging will be used for improvement in patient care interventions and reporting, in research aimed at comparing the effectiveness of various imaging approaches, and in future policy broadly designed to enhance healthcare value with an understanding of outcomes relative to cost.
Current Policies Related to Quality in Cardiovascular Imaging
Curbing high rates of growth in medical imaging has been the focus of policy initiatives over the past decade, with aims to control overuse and inappropriate procedural use patterns. The strain on the healthcare system resulting from increasingly high costs related to cardiovascular imaging volume was unsustainable without concurrent programs focused on constraint or reductions in imaging growth.3 The consequential policies for controlling cardiovascular imaging included initiatives for decreasing procedural reimbursement, use management (such as previous notification and authorization) programs, and the development of appropriate use criteria to identify optimal candidates for testing within all of the conventional imaging modalities.4,5
Evidence across health plans reveals substantial variability in the type of modalities used and differing frequencies of follow-up or serial testing patterns.6–9 The wide variability in use, particularly for expensive imaging modalities, has contributed to sociodemographic disparities for regions of low-compared with high-use patterns.6 Some of the patterns in regional variation may be a result of a variable workforce of imagers, expertise, and imaging equipment.10 In 1 report using Centers for Medicare & Medicaid Services Physician Supplier Procedure Summary Master Files (1998–2007), cardiovascular imaging had the greatest variability in use: as high as a 70.2-fold difference between high- and low- use regions of the country.11 In a recent statement from the American College of Cardiology and AHA on use and costs associated with cardiovascular imaging, high-use rates were not driven by any identifiable clinical need or increasing risk within a given population.12 Rather, it has been stated that use patterns for imaging have been influenced by the economic potential of a test for market penetration.13 The variability in use of noninvasive cardiovascular imaging was evaluated by Safavi et al14 in the Premier registry of 224 hospitals. They observed that among patients with suspected ischemia, the rate of imaging ranged from 0.2% to 55.7% and that hospitals with higher rates of ischemia testing also more often used invasive coronary angiography. Although some of the observed variability can be attributed to differences in patient case mix, these results highlight a critical need to clarify which patients referred for testing are most (and least) likely to experience a benefit from such testing.
Defining Quality in Cardiovascular Imaging
For a cardiovascular imaging procedure, defining quality is complex because it relates to multiple dynamic variables, including the appropriateness of the initial referral decision, the equipment used or available, the use of standardized imaging protocols and reporting, the accuracy of image interpretation, and the likelihood that imaging results will be directly tied to subsequent patient management (Figure 1). These are additive and essential components for achieving high-quality imaging. Along the diagnostic evaluation pathway, any one of these components can be variably performed and result in missed opportunities for achieving optimal patient outcomes or reducing costs. Although imaging findings often play a prominent role in the patient’s course of care, quality parameters are not routinely tracked, and to date, knowledge is lacking about the clinical sequelae in populations receiving poor- and high-quality imaging-guided care. Although we currently have widespread implementation of electronic health records, there is a lack of interoperability across imaging and patient records that contributes to silos of evidence that are rarely combined to measure imaging quality and to prevent overuse and duplication of services.
Figure 1.
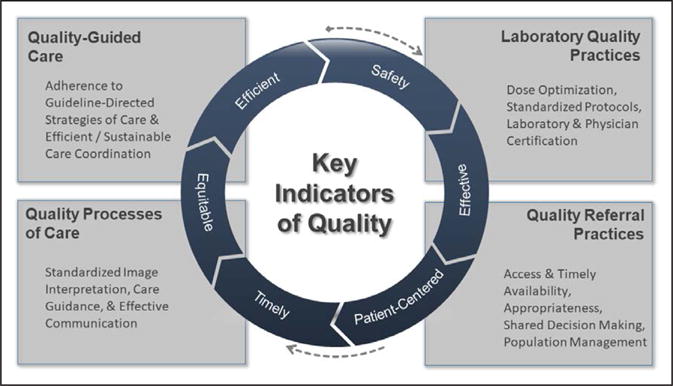
Key indicators of quality in cardiovascular imaging.
No single definition can comprehensively define quality in imaging; however, there have been several efforts to devise an analytical framework that distills core components of a working definition of quality in other aspects of health care. Several earlier statements on quality of cardiovascular imaging have been published, and the present document aims to expand on these reports.15–18 One of the most prominent analytical frameworks put forth is that from the Institute of Medicine whereby quality was defined with 6 key domains. These key dimensions of quality healthcare delivery include testing that is safe, effective, patient-centered, timely, equitable, and efficient.19 The implications of each of these components of quality health care are as essential for cardiovascular imaging as for other areas within health care and are highlighted in the following sections.
Safety
For each of the commonly used cardiovascular imaging modalities, safety issues exist (eg, a black box warning for intravenous contrast use for echocardiography). Even for exercise treadmill testing (with or without imaging), there is a very small but definable safety risk.20 From the 2001 AHA statement on exercise testing, incident myocardial infarction or sudden cardiac death occurs in 0.005% of tested patients.21
The AHA has focused several statements on radiation safety.22,23 Guiding principles for the use of imaging procedures exposing patients to ionizing radiation include justification, dose optimization, and dose limitation.24 Justification for procedural use is based on guideline-derived clinical indications and appropriateness criteria from the American College of Radiology or American College of Cardiology.4,25,26 The Society of Cardiovascular Computed Tomography and American Society of Nuclear Cardiology have guidance statements on optimization of radiation dose reduction techniques that should be used uniformly for all patients.27–29 Across cardiovascular computed tomography (CT) and nuclear imaging procedures, there have been marked reductions in radiation dose over the past decade.28,29 The application of high effective doses of ≥20 mSv is strongly discouraged and should be tracked.30 In a recent report from the Intersocietal Accreditation Commission Data Repository, 9.6% of 1074 nuclear cardiology laboratories had a median dose ≥20 mSv.31 The identification of laboratories with high median dose patterns should prompt quality assurance programs and initiation of laboratory-wide dose reduction strategies. A goal for nuclear cardiology laboratories set by the American Society of Nuclear Cardiology was a median dose of ≤9 mSv, achieved largely through greater use of stress-only imaging and elimination of dual-isotope myocardial perfusion single-photon emission CT (rest thallium-201 and technetium-99m for stress at 24 mSv).32 Recent guidance documents also support imaging with the lower exposure, rest/stress positron emission tomography with rubidium-82 or N-13 ammonia (≈2–3 mSv).30,33 However, in this recent report from the Intersocietal Accreditation Commission Data Repository, only 1.5% of nuclear cardiology laboratories met the goal of a median dose of ≤9 mSv.31 Many single-photon emission CT laboratories have aging equipment that is not suitable for reducing patient doses without software or hardware upgrades.34
Accreditation of imaging laboratories such as through the Intersocietal Accreditation Commission, American College of Radiology, or The Joint Commission helps to ensure that appropriate quality and dosing standards are met. Laboratory dose patterns should be tracked with registries such as the American College of Radiology’s Dose Index Registries to ensure adherence to recognized radiation dose limitations and to allow national benchmarking (stratified by body part, examination type, geographic region, and facility type [eg, academic versus community hospital]).35 Via these benchmarking capabilities, diagnostic reference level values (defined as the 75th percentile of the histogram of aggregated dose data) should be derived from the distributions of dosimetric quantities observed.36,37 To date, the Dose Index Registries contain >20 million scans from >1000 radiological facilities,36 are certified as a Qualified Clinical Data Registry for the Centers for Medicare & Medicaid Services’ Physician Quality Reporting System, and meet The Joint Commission’s dose incident identification review and external benchmarking requirements.38
Other safety measures should also be included when imaging is applied by nonimagers (eg, bedside echocardiography).
Appropriateness
Appropriate imaging involves the use of testing within a given indication with supportive high-quality evidence. For cardiovascular imaging, appropriate testing requires a link to our body of evidence to comprehend the test-guided strategy of care as it is relevant within a given indication for the procedure. There are appropriate use criteria from the American College of Cardiology and appropriateness criteria from the American College of Radiology with a primary aim of limiting the use of rarely appropriate or inappropriate indications.4 Reports vary, but ranges of rarely appropriate indications from 16% to 45% have been reported (ie, for single-photon emission CT imaging),39–41 and elimination of such inappropriate testing could provide tremendous cost savings to the healthcare system.42–46 Alterations to the appropriate imaging definitions have implications to the rate of inappropriate or rarely appropriate indications and are important considerations for tracking purposes with electronic health records. Moreover, the relations between appropriate use criteria and clinical outcomes are not well defined. From a recent series, appropriate indications identified a higher-risk patient subgroup (hazard ratio for cardiac death or myocardial infarction, 3.7; P=0.006) and that documentation of stress-induced ischemia led to higher use of invasive coronary angiography and revascularization.41 Conversely, inappropriate testing in lower-risk cohorts harmed by such testing has not been documented. How appropriate indications could improve processes of care and ultimately outcome should be the focus of future research endeavors.
Consensus across imaging societies reveals certain indications when resource use should be minimized such as with the American Board of Internal Medicine’s Choose Wisely Program.
Patient-Centeredness
Patient-centeredness entails a mutual engagement and partnership among patients, their families, and healthcare providers (Figure 2). Cardiovascular medicine strategies are often algorithmic and aim for adherence to guideline-directed care. The tracking of variable outcomes, appropriate use criteria, and performance measure patterns exemplify recent efforts to understand population management. These remain important, but a primary focus of testing is the care of a given patient. It remains vital not only that patient care reflect state-of-the-art decision making but also that the patient is fully engaged in the strategic planning of his or her care. Patient engagement requires collaboration and shared decision making but is challenging within an imaging environment in which only the primary care physician has the patient’s complete medical history and in which imaging laboratories may be intimidating to a patient. Moreover, health literacy, notably numeric literacy, is vital to understanding the value of a test on the basis of the patient’s clinical condition and how test results affect clinical decision making.22 Laboratories that tailor educational materials, including educational videos, to diverse patients with various educational backgrounds and languages improve patient comprehension. Standardized discussions led by laboratory staff should facilitate patient understanding of the reason for the test, the process of the imaging procedure, and how it may affect the patient’s care. Emotional support for the patient during the procedure should be provided by all staff and imagers. Although who is primarily responsible for discussing the imaging findings with the patient remains uncertain, a follow-up visit with the patient’s primary care provider or specialist should be prompt and remains important to provide communication about test findings and for the development of a future pathway of care.30 A major focus of patient-centeredness is for the imager to fully engage in care coordination. In addition, continued efforts must be made to ensure that referring physicians understand how to integrate the results of any test appropriately into downstream therapeutic decision making.
Figure 2.
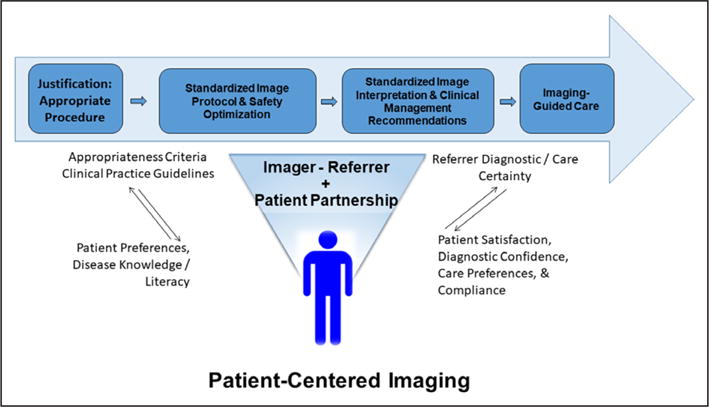
Imager care coordination.
From the stress imaging literature, a small proportion of patients with stress ischemia have follow-up changes in medical management or downstream referral to invasive angiography.47–49 Similarly, fewer than half of patients with moderate to severe ischemia on myocardial perfusion imaging or obstructive coronary artery disease (CAD) on coronary CT angiography (CTA) are referred to invasive angiography.48 Although there may be legitimate reasons for a lack of change in patient management, these data suggest a disconnect between an imaging procedure and follow-up visits and therapeutic intervention. Further data on the timing and effectiveness of follow-up visits and patient satisfaction with care and testing during his or her episodic evaluation are necessary components of future research.
Timeliness
Delays in implementing changes in management after testing decrease the likelihood that the test will affect patient outcomes, and timeliness varies widely across healthcare systems and practices. As an example, the 1-month delay in treatment change observed in the SCOT-HEART trial (Scottish Computed Tomography of the Heart) may have contributed to the failure of the trial to show a reduction of cardiac events in the CTA arm of the trial.50 This is typical of care within the UK National Health Service and may be less relevant in the United States but serves as an example of the issue of timeliness. Another example is delays in referral to invasive angiography from 6 to 24 months.47 The documentation of clinically worsening states, including visits to the emergency department, in the interim between testing and follow-up evaluation is an important component of documentation of delays within the diagnostic evaluation. Assurance of a timely referral or an office visit is an important indicator of quality health care. Direct communication from the imager to the referring physician and, in turn, to the patient effectively explaining the findings and allowing a discussion of a future planned strategy of care based on imaging findings is vital to timely care. Ultimately, timely communication should immediately prompt communication at the time of the index evaluation between the imager and patient.
Effectiveness
Effectiveness of cardiovascular imaging may be defined by examining the extent to which test findings promptly lead to guideline-directed care, thus defining postimaging strategies of care providing the link between a test and directed therapy that may improve patient outcomes. Using the SCOT-HEART50 trial (n=4146 patients randomized after an index chest pain evaluation [including exercise electrocardiography in 85% of enrollees] to standard care versus CTA-guided care), a post-hoc analysis revealed that CTA led to a 3- to 12-fold higher initiation of new preventive therapies and was associated with a 50% relative risk reduction for myocardial infarction compared with standard care (P=0.02). The National Institutes of Health–National Heart, Lung, and Blood Institute–sponsored PROMISE trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) similarly reported more initiation of aspirin, statins, and β-blockers among patients randomized to CTA with obstructive CAD compared with those with an abnormal functional study.51 Although hypothesis generating, the SCOT-HEART results, subsequently validated in a national Danish cohort,52 suggest that linked therapeutic intervention after an imaging procedure may positively affect patient outcomes.
There are numerous examples of potential effectiveness measures, including the extent to which a given procedure reduces clinical worsening (eg, emergency department visit for symptom instability) or improves patient symptoms. Some have proposed that the rate of normal invasive coronary angiography may also be used as a measure of test effectiveness because 59% of patients undergoing elective coronary angiography have previous noninvasive testing but no obstructive CAD.53,54 The extent to which a newly introduced imaging procedure may improve the identification of obstructive CAD or a reduced fractional flow reserve remains important to guide appropriate referrals to invasive coronary angiography. A pooled analysis from 3 recent randomized trials reveals that the diagnostic yield for detection of angiographic obstructive CAD (ie, positive predictive value) after abnormal test findings is higher with CTA (71% of 1047) than with stress testing (52.5% of 819).55
Equitability
Decades of evidence supports sex, racial, and ethnic differences in the quality of care received, including reduced stress testing and angiography rates, which contribute to worsening clinical outcomes commonly observed among diverse population subgroups.56–60 An all-encompassing term for this is social determinants of health, which include important population subgroups and the influence of access to care, limited financial means, and other factors affecting clinical resource use and outcomes. Standardized care remains vital to providing equitable care across our diverse patient populations. Improvements in cardiovascular mortality, for example, among women have been attributed to the implementation of guideline-directed care and the tracking of performance measures.61 Reasons for ineffective care for women and men from diverse races and ethnicities are multidimensional and include sociocultural preferences, educational background, financial resources and preferences, distance to healthcare facilities, having a usual source of health care, and many others that will affect the use and follow-up of imaging services. Interoperability of imaging databases with follow-up documentation of clinical management within the electronic health record is an important means to document and track health equity in the use of cardiovascular imaging procedures across a given clinical indication for testing.
Efficiency
The definition of efficiency varies throughout the published literature but often includes the fewest resources (or costs) associated with a given diagnosis or time period. Cost savings, in particular as they relate to comparing a higher-with a lower-cost procedure, have been reported.47,62,63 There have been examples of efficiency with randomized trials comparing CTA with usual testing strategies in the evaluation of acute, low-risk chest pain in the emergency department.64–68 Moreover, cost savings have been reported when CTA is used in the emergency department in large part because of a prompt discharge and accurate exclusion of CAD.68 A synthesis of this evidence reported that a strategy of CTA compared with usual testing resulted in reduced time to diagnosis of ≈8 hours.66 When the emergency department evaluation leads to hospitalization, CTA often is followed by invasive coronary angiography and revascularization, which further add costs to the evaluation strategy.67 Thus, the early cost savings may be eliminated when ensuing hospital costs are considered. In general, the early discharge after CTA does not result in increased near-term readmissions for an acute coronary syndrome (odds ratio, 1.2; 95% confidence interval, 0.7–2.2).66
Moreover, serial or routine testing without clinical indications is an important example of inefficiency within cardiovascular imaging. Deferred testing options are increasingly discussed as a potential alternative to our current approaches of prompt imaging.55 The imaging community has already focused on deferred testing through the elimination of routine or periodic evaluations (ie, yearly stress testing) for the stable patient. In lieu of serial testing, symptom-guided re-evaluation is considered an accepted indication for retesting.4
Efficiency may also be defined as cost minimization or savings while achieving equivalent outcomes to ensure preservation of quality of care. Near-term reductions in cost should not be observed as advantageous when higher long-term costs or resource patterns also occur. The shifting of costs to a later time period should be carefully differentiated on the basis of indications for testing related to repeat, but potentially unnecessary, testing to procedural use associated with clinical worsening states. Examples of cost savings include using stress imaging as an index diagnostic procedure compared with direct coronary angiography or applying CTA in the immediate evaluation of low-risk chest pain.64,68–70 An important consideration for calculation of cost savings is that tracking should include the cost consequences of induced care and testing patterns after the initial imaging procedure.71
In summary, each of the above indicators provides a framework for defining quality of imaging. This set of quality indicators is multidimensional and distills portions of care with minimal overlap because each contributes uniquely to optimal patient care. Each of these indicators can be applied not only to the care of the individual patient but also for population management. With regard to the latter, there are few performance metrics or quality reporting measures in cardiovascular imaging, but they may be helpful for quality assurance purposes.72 The extent to which laboratory practices with imager leadership adhere to each of these indicators is of primary concern. Later in this statement, we review each of these quality indicators and suggest metrics to be applied in clinical practice and in research. In the remaining sections, we propose how each of these components should form the basis for future clinical research and clinical registries for demonstration of high-quality cardiovascular imaging practices.
Quality Framework for Cardiovascular Imaging Research
Figure 3 details the hierarchy of evidence for cardiovascular imaging. Ranking the quality of cardiovascular imaging evidence is based largely on the selectivity or bias related to the enrolled patient series and the face validity of the primary aim results. For single-site or smaller patient series, selection bias is operational, and the generalizability of the findings is limited to patients similar to those included in the reported series. As the range of centers and the diversity of patient enrollment increase across modalities and multiple enrolling centers, generalizability of the study findings is enhanced.
Figure 3.
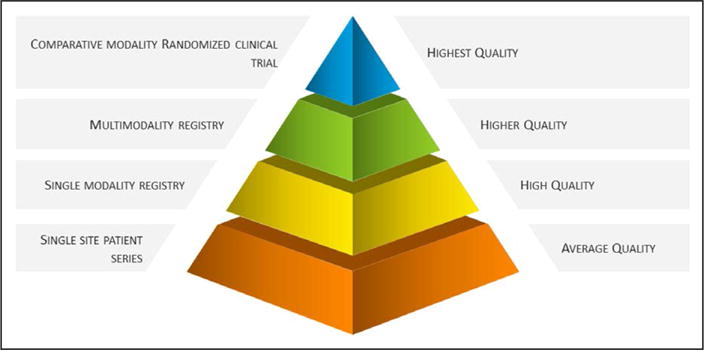
Hierarchy of cardiovascular imaging evidence.
For the purposes of Figure 3, a randomized trial is at the top of the hierarchy with the potential for the highest quality of evidence; however, selectivity in enrollment is problematic for this type of study design. A major benefit of a randomized trial is the potential to answer the clinical hypotheses without the need (generally) for multivariable risk adjustment or covariate consideration, as is the case for observational series. The traditional end points such as death or myocardial infarction occur less often in stable patient cohorts and affect much of imaging populations. Randomized trials in lower-risk, stable populations require substantially larger sample sizes. Moreover, cost analysis often lacks a depth of detail or the collection of actual healthcare system billing data and may rely on decision analytic modeling (ie, simulations). Additional considerations for a randomized trial are the much higher expense associated with a trial and the historic lack of funding in imaging trials.
Although multimodality registries are considered second-tier evidence, in many scenarios, the findings from such data can provide tremendous insight into real-world cardiovascular imaging practices, with the result being more generalizable to patient subgroups who are less likely to be enrolled in clinical trials. The depth of high-quality evidence for any cardiovascular imaging procedure can foster a greater knowledge base to inform imaging-guided strategies of care.
Pragmatism in Cardiovascular Imaging Research
The ideal framework for the evaluation of quality in cardiovascular imaging is one that emulates the real-world environment such that the findings can be easily assimilated into daily clinical practice. This focus on deriving real-world evidence has been a motivating force for the emergence of pragmatic trials or registries. A pragmatic trial may be defined as that which includes broad eligibility criteria and minimal guidance for postrandomization clinical care such that the findings emulate everyday clinical practice.73 A pragmatic trial is one that emulates real-world practice and relies on local image interpretation and local physician-guided care. The benefits of and challenges with pragmatic trials are highlighted in this section. The rationale for pragmatism in a trial is based on the well-documented selectivity in enrollment of previous randomized trials, reflecting a limited proportion of the denominator of eligible patients. The ratio of enrolled to eligible patients may be as little as 10%.74 When trial enrollment is overly selective, the trial may be viewed as examining efficacy (ie, in a laboratory or restricted research setting where testing and treatment may be outside the standard of care [experimental]) or findings emulating an ideal environment and not effectiveness that reflects the usual care setting.75
Pragmatism in clinical research must foster or evaluate adherence to each of our key quality indicators for cardiovascular imaging. The suboptimal but real-world rates of appropriate testing and guideline-directed care hinder strategies that improve patient outcomes. Maintenance of quality cardiovascular imaging practices within the clinical research environment remains a vital aim of current clinical trial designs. The pragmatic framework may not be optimal for the applications of all clinical hypotheses, as recently reviewed by Ford and Norrie.73 Specialty-based imaging requires a complex knowledge base, the interaction of multiple stakeholders, and variable practice patterns that may be less suited to pragmatic approaches within clinical research. Moreover, the efficacy of imaging-guided management in many cases is not well established, and embarking on more traditional approaches, including selective enrollment, the use of core laboratories to document imaging findings, and a more structured approach to posttest care, may provide important stepping stones to develop effectiveness research within cardiovascular imaging.
Embedded Randomized Trials
It has been suggested that trials embedded within disease-specific registries with proven enrollment from seasoned investigators may serve as an optimal environment for answering specific imaging-related hypotheses.73 This would include enrollment of patients from within the clinical workflow of an imaging laboratory and with randomization within the electronic health record. Thus, a patient may present to an imaging laboratory, and his or her testing and care pattern may be guided in the patient’s electronic record to 1 arm of the trial, while another patient may be allocated to a varied testing and treatment approach. All of this would be completed electronically. Using this approach of an embedded trial, the investigators may have a run-in period for observing enrollment patterns and posttest management approaches to ascertain the feasibility of the trial and the potential for true equipoise for the proposed clinical trial aims.
Optimal Follow-Up and the Episode of Care
Additional considerations for cardiovascular imaging research include a targeted follow-up that resembles the episode of care and provides a sufficient observation time period to answer the primary hypothesis and to report any adverse consequences after randomization. Recent randomized trials in the emergency department for the evaluation of low-risk chest pain have been criticized for a limited duration of follow-up from only 28 days to 6 months.64–68 The enrolled population must reflect those typically referred to the imaging procedure. Careful review of clinical characteristics of enrolled patients (eg, age, risk factor burden) and observed clinical outcome rates can provide insight into whether the trial included a lower- or higher-risk patient subset, an important consideration for trial generalizability. There will be selectivity or bias in the enrollment of lower-risk patients, which may lead to underestimation of the effect of a strategy on patient outcomes. Enrollment should emulate population diversity to allow validation of findings across targeted priority populations of racially and ethnically diverse subgroups of women and men.
Figure 4 details critical components of high-quality cardiovascular imaging research. The clinical hypothesis includes an emphasis on evidentiary gaps and unmet clinical needs that may broadly affect a large population subgroup. The clinical end points include a broad range of acceptable outcomes, including clinical worsening and disease progression. Moreover, the diverse patient-centered outcomes used in clinical research projects are highlighted in Figure 4. The importance of targeted patient enrollment that fulfills the primary and secondary hypotheses of the research endeavor is also emphasized, including patients of diverse race and ethnicity.
Figure 4.
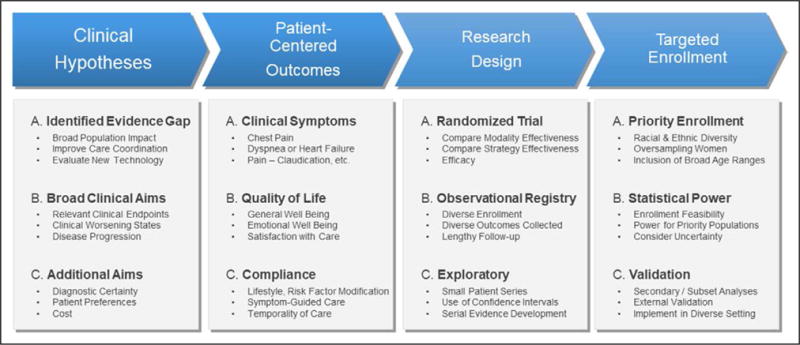
Critical components of high-quality cardiovascular imaging evidence.
Patient-Centered Outcomes and Optimal Cardiovascular Imaging End Points
Increasingly, there is growing consideration for the measurement of diverse outcomes in cardiovascular imaging research, including sentinel events that are most relevant to patients. Our previous discussion on effectiveness prioritizes the use of clinical end points that may be quantified such as mortality or myocardial infarction. This has been the historic approach for outcomes assessment, and the use of other important outcomes such as patient-reported symptoms was less favorable because of their subjective nature. There is emerging evidence for a focus on patient-reported outcomes (PROs) as part of a comprehensive strategy of care.76,77 In cardiovascular medicine, patient symptoms and other quality-of-life measures have been studied, in particular their importance for therapeutic symptom reduction (eg, a major goal of percutaneous coronary intervention in stable CAD is improving patients’ symptoms, physical functioning, and quality of life).74,78 A focus on a broader range of self-reported outcomes may capture the clinical reasons why cardiovascular imaging is being conducted and improve patient-centeredness in clinically effective care. This section focuses on the emerging tools for PROs as measures of quality as supported by a recent report by a National Quality Forum staff member76 and a statement from the National Quality Forum.77
Although we define this section as patient-centered outcomes, this is also called patient-reported outcomes, which reflect the self-narration of health status measures without interpretation by a clinical researcher. Examples include symptoms, functioning, and overall well-being. Questionnaires collecting PROs can be generic or disease specific. Commonly used disease-specific measures in cardiovascular medicine include the Seattle Angina Questionnaire and the Kansas City Cardiomyopathy Questionnaire to capture the impact of coronary disease and heart failure on a patient’s health status.79,80
Given the large number of patients undergoing cardiovascular imaging, the use of end points characterizing progressively worsening conditions or clinical worsening has advantages as a near-term outcome compared with mortality, which may be realized only with lengthy observational follow-up and can be influenced by factors other than those warranting the original imaging study. Recent efforts to categorize standard PRO measurement sets have been undertaken, such as the International Consortium of Health Outcomes Measurement.81 Standard sets for cardiovascular PROs may more clearly define the role and value of imaging in patient care and should ideally be routinely collected.
This recent focus on health status as an important consideration for cardiovascular imaging research is consistent with patient-centered outcomes research or, for this statement, patient-centered imaging. Patient-centered outcomes were first advocated by Donabedian,82 who defined the quality of health care as more than technical excellence but also interpersonal care, including meeting the emotional needs of patients and keeping within their preferences. More recently, patient-centered outcomes research has focused on patients as active stakeholders throughout the process of evidence development and technology evaluation. For cardiovascular imaging, this would include prioritizing of research that evaluates clinical management and testing options that are important to patients. It would also include an adaptation of data to address patient-centered effective implementation of scientific findings into real-world clinical environments. Important considerations for patient-centered outcomes research as applied to cardiovascular imaging are detailed in the Table.83 A novel part of patient-centered outcomes research is the burgeoning concept of the patient-scientist collaboration for priority setting, proposal development, and valuation of the research findings as they relate to improved patient-centered imaging. Moreover, the use of a patient-specific informed consent for cardiovascular imaging may further support the concept of patient-centered imaging.
Table.
Key Components of Patient-Centered Outcomes Research in Cardiovascular Imaging
| Methodological considerations for randomized trials and observational research |
| PROs (eg, angina, functional status) |
| Assessment of benefits and risks (ie, safety, radiation exposure) |
| Maintenance of representative sampling for targeted priority population subgroups |
| Really informed consent, individualized |
| Effectiveness |
| Clinically representative indications for cardiovascular imaging |
| Meaningful effect size |
| Heterogeneity of effectiveness |
| “Given my characteristics, conditions, and preferences…”* |
| Patient-scientist collaborations |
| Transparent involvement for setting priorities, proposal development, and valuation of the findings |
PRO indicates patient-reported outcome.
Adapted from the Patient-Centered Outcomes Research Institute.83
Improving Patient Symptoms
Consistent with patient-centered outcomes, primary goals of improved symptoms or quality of life are valuable goals for cardiovascular imaging research. There are numerous examples of recent clinical trials using health status measures. For example, the CAPP trial (Cardiac CT for Assessment of Pain and Plaque) used improvements in angina as its primary end point,84 and the CECaT trial (Cost-Effectiveness of Noninvasive Cardiac Testing) had functional capacity as its primary end point.85 Insofar as patients seek care for the evaluation of symptoms, health status measurements are highly relevant clinical outcomes. From the perspective of patient-centered outcomes, the patient is often the best evaluator of changes in clinical status and can observe early signs or transitions within symptom characteristics and patterns. Figure 5 details the value of early capturing of patient-specific symptom transitioning states that may signal clinical worsening or precede an acute event. However, the possibilities of symptoms waxing and waning and random variation also intertwine with cyclic variation in patient symptoms. More information should be devised for the relationship between imaging findings and patient-reported symptoms and quality of life.
Figure 5.
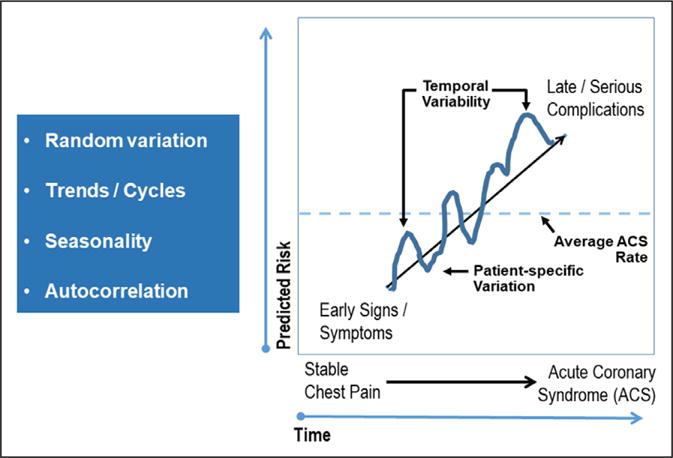
Refining appropriate end points for cardiovascular imaging research, including health status and symptoms, and associated sequelae, including variation in symptoms and risk of major coronary artery disease events. ACS indicates acute coronary syndrome.
Imaging-Guided Therapeutic Initiation and Intervention?
Several approaches to clinical hypotheses involve therapeutic or interventional efficacy. For these trials, imaging is used as a guide to the intervention but may not be how outcome is improved. One example was published from the Veterans Affairs–sponsored COURAGE trial (Clinical Outcomes Using Revascularization and Aggressive Drug Evaluation) in which serial myocardial perfusion single-photon emission CT before treatment and again at ≈1 year demonstrated a reduction in ischemic myocardium after randomized treatment.86 Additional examples include the use of cardiovascular imaging as an aid in assessing procedural success or complications and for documentation of disease progression or clinical worsening.
With the rapid development of new transcatheter approaches to valvular and structural heart disease, 3-dimensional imaging, including CT and echocardiography, have become central to determine device selection and sizing to optimize procedural outcomes. In this setting, imaging of the noncircular aortic annulus allows improved sizing and thus reduces the occurrence of paravalvular regurgitation and mitigates complications such as annular rupture and coronary occlusion. In the definition of quality in imaging in interventional procedures, imaging is used as a tool to assist procedural performance and to guide therapeutic intervention. Isolating the impact of imaging on patient outcomes of these complex procedures is difficult because of the multiplicity of factors, but whenever possible, studies should be designed to attempt to validate the incremental value of new imaging techniques.
Improving Outcomes and Test Effectiveness After Cardiovascular Imaging
The performance of a cardiovascular imaging procedure provides largely an indirect link to improve clinical outcomes. Improvements in patient outcomes may be realized when postimaging care is prompt and consistent with guideline-directed care. Thus, the intervening treatment initiation or modification is the rate-limiting link to improving the lives of patients after an imaging procedure. This link between imager and referring physician is critical to achieve optimal patient outcomes and communication about the recommended treatment.
Linking imaging with outcomes forms the basis for clinical effectiveness research. This framework of linking imaging to outcomes forms the basis for understanding clinical research designs aimed at improved patient outcomes and the effectiveness of cardiovascular imaging. Negative trials or registries can be expected when minimal change in patient management is observed after randomization regardless of the treatment strategy. The PET and Recovery Following Revascularization trial is an example in which a secondary analysis revealed a clinical benefit of treatment only for the subset adhering to 18F-fluorodeoxyglucose positron emission tomography–assisted treatment recommendations (P=0.019).87 To date, comparative-effectiveness trials in imaging have not included secondary hypotheses on adherence to guideline-directed care.55 Evolutionary trial or registry designs should be expanded to consider clinical outcome differences by varied therapeutic strategies of care, which may include imaging-directed treatment decisions. Noninferiority designs may prove useful by comparing the intensity of treatment strategies guided by cardiovascular imaging findings and usual care approaches. In some cases, documentation of physician certainty in the diagnosis may further contribute to adherence to and consistency in implementing guideline-directed care.50
Real-world use patterns for cardiovascular imaging reveal inappropriate referral patterns for cardiovascular imaging, suboptimal treatment guidance, and variability in use patterns. Innovative study designs are needed to embed links from cardiovascular imaging findings to guided treatment that is timely and appropriate. Because cardiovascular imaging use patterns support that treatment guidance is often lacking, trial designs cannot assume that appropriate care will ensue after the index cardiovascular imaging procedure.
Importance of Diverse Recruitment and Secondary Analysis Informing Equity in Cardiovascular Imaging
Imaging laboratories serve a diverse patient population, and enrollment in clinical trials and registries should be proportionally representative of racial and ethnic subgroups of women and men. If proportional enrollment goals are reached and the subgroups are sufficiently powered, then analysis should inform secondary analysis of research findings to unique patient subsets. Beyond race and ethnicity, the degree of comorbidity and pretest risk in a cohort should emulate typical referral patterns. Personalization of the research findings to targeted priority populations should be a critical aim and include both imaging and therapeutic strategies of care. The admixture of comorbidity, symptom characteristics, and risk factors affects the incidence of imaging abnormalities and clinical outcomes. Careful planning should be undertaken to achieve a balance between high rates of enrollment and achieving representative sampling of diverse patient groups. The ultimate goal of patient enrollment in research is to be equitable, inclusive, and representative of the patient population undergoing cardiovascular imaging.
Defining Value Within the Quality Imaging Framework
As our healthcare system enters an era of constrained resource use, cardiovascular imaging must be used only while focusing on quality of care resulting in improved outcomes and value.88,89 This approach of considering quality and then value may be defined as intelligent cost-effectiveness. Valuation of costs must be made by consumers and physicians within the framework of definable quality. The defining of value as it pertains to a fiscal choice includes a tradeoff in options that have yet to be evaluated by all stakeholders, including patients, physicians, governments, and private payers.
The goal of value-based analyses is to define opportunities for cost savings or improved deployment or allocation of efficient cardiovascular imaging procedures. Understanding the costs or resources allocated to a given patient population provides the primary framework for identifying economic savings. In some cases, the allocation of resources will vary on the basis of clinical risk and imaging findings, and costs would be expected to be higher for higher-risk patients (and lower for lower-risk patients).
The most common aim of economic analysis is to calculate cost savings, efficiency, or minimization within a comparative analysis. Given the transition of US health care toward value-based reimbursement with bundled payments, the focus is on cost minimization without worsening patient outcomes. Cardiovascular imaging modalities are added to a given diagnostic evaluation, and as reimbursement shifts toward minimizing cost, selective imaging will be of primary importance. When the goal is to define minimal resources expended, variable approaches should be used, including no or deferred testing options. The defining of cost savings should be framed within standard resources applied on the basis of guideline-directed care. For example, invasive coronary angiography is indicated after moderate to high levels of stress ischemia detected on imaging. Thus, given this expected pattern of care, cumulative costs associated with guideline-directed care should be differentiated by a lack of follow-up testing, as indicated by clinical practice guidelines or appropriate use criteria. Moreover, standard costs associated with a given evaluation metric have yet to be defined but are important for identifying any pattern of excess cost and evaluation metrics that are cost appropriate but underused.
Beyond defining the costs of care, deterioration of a patient’s clinical status may confound calculation of the cumulative costs of strategies. Temporal patterns may exist as a result of near-term testing when the patient is stable or deferred testing when the patient develops clinical worsening or clinical instability requiring an immediate evaluation (eg, in the emergency department). Cumulative costs should differentiate deferred costs associated with clinical worsening from redundant or unnecessary repeat testing patterns occurring in the near term.90
Cost-effectiveness is commonly calculated as the cost per life-year saved with or without a quality adjustment. Recently, the American College of Cardiology/AHA task forces on performance measures and practice guidelines defined cost-effectiveness in terms of low (incremental cost-effectiveness ratio >$150 000 per quality-adjusted life-year saved) to high (<$50 000 per quality-adjusted life-year saved or better outcomes at lower costs of care) value.88 When cost-effectiveness modeling is based on expert opinion and simulated findings, the result may be divergent from real-world data and bias that reflects the data inputs. More recent clinical trials have used patient-specific resource use or mixed-model cost analysis whereby resource use data are collected and then nationwide or payer-specific costs are applied to the patterns of testing, treatment, and hospitalization.91 There are clinical scenarios in which the cost-effectiveness of imaging may be difficult to evaluate. First, there are fundamental differences between cost-effectiveness evaluations of imaging technologies and therapeutic interventions. In patients with disease, it may be easy to demonstrate the impact of an imaging test that guides the use of an effective therapy (eg, mitral valve replacement after echocardiographic diagnosis of severe mitral regurgitation). However, in patients without a disease, it could be difficult to quantify the benefit from the reassurance gained from a negative imaging test compared with no testing at all. Imaging may also reveal incidental findings of an unknown subclinical disease. In addition, regional practices of imaging with variable reimbursement and use patterns influence overall cost inputs.
Knowledge Translation
Implementation science defines the degree to which clinical trial or guideline-directed evidence is assimilated into real-world practice and includes the development of novel approaches to improve knowledge translation into various clinical care settings. Such implementation programs may focus on behavior interventions or clinical decision support mechanisms for appropriate use of cardiovascular imaging or for imaging-directed clinical management strategies. The result is that documentation of poor adherence to high-quality evidence can identify gaps in optimal imaging use that provide opportunities for tracking quality. Consideration should be given to the adaptation of research findings to clinical testing strategies on the basis of not only positive trial or registry findings but also negative information. For example, the recently published Prospective Observational Longitudinal Registry of Patients With Stable Coronary Artery Disease Registry of 32 105 stable outpatients with CAD failed to report any association between ischemia and major cardiovascular outcomes, including death, myocardial infarction, or stroke.92 Findings from this registry contrast with expert imaging centers but may reflect real-world practice. These findings may reflect ischemia-guided treatment that alters its natural history or may reflect imprecision in interpretive acumen, which would then require a greater focus on the quality of image interpretation. Negative and positive research findings should prompt revisions to quality-based imaging practices. Future trials that may affect cardiovascular imaging practice include the National Institutes of Health–National Heart, Lung, and Blood Institute–sponsored ongoing International Study of Comparative Health Effectiveness With Medical and Invasive Approaches trial. Eligibility criteria limit enrollment to patients with moderate to severe ischemia and CTA-defined obstructive CAD. Eligible patients are then randomized to a strategy of optimal medical therapy compared with cardiac catheterization with optimal medical therapy.93
Additional Considerations for Enhancing Cardiovascular Imaging– Guided Quality of Care
Effective Educational Programs
Educational programs aimed at informing patients and referring physicians about the imaging procedure and its clinical benefits and risks, along with how the results would be expected to alter treatment, should become routinely incorporated into clinical care. The goal is to improve appropriate referral patterns over the long term and patient and physician adherence to postimaging guideline-directed care, including empowering patients to be more educated participants in shared decision making for subsequent treatments.
Personalized Image Reporting
An additional approach to guiding care coordination is to optimize a set of parsimonious standardized reporting algorithms tailored to individual physicians and informed by patients’ needs within their evaluation algorithm. Such reports could include the execution of risk models with patient-specific data to better define prognosis and the relative benefits of alternative treatment strategies. Although efforts have focused on a comprehensive standardized report, adherence is low, and parsimony and targeted responses to the clinical referral indication may streamline and improve effective patient clinical management.
Quality Metrics for Continuous Quality Initiatives
The key indicators of quality have the means to be tracked within interoperable electronic health records and to be incorporated into continuous quality initiatives for optimal image-guided population management. For example, within safety, a proposed radiation exposure metric would be >90% of patients below the dose reference level for a given procedure. Measurement of adherence to each of the quality indicators for cardiovascular imaging within the electronic health record–embedded registries is an optimal means to define low- and high-performing laboratories.
The Quality Payment Program is part of the 2015 Medicare Access and CHIP Reauthorization Act, which repealed the Sustainable Growth Rate formula, a method that served as the underpinning of Medicare physician payment, established to control spending by Medicare on physician services. The Quality Payment Program sets reform for Medicare Part B payments with a primary aim of providing high-value, patient-centered care within the Medicare system.94,95 Within the Quality Payment Program, 2 tracks are available for physician participation (Advanced Alternative Payment Models and Merit-Based Incentive Payment System). For the majority of the estimated 600 000 participating physicians, the Merit-Based Incentive Payment System will be the standard to achieve compliance with the Quality Payment Program. Within this program, a payment adjustment is based on evidence-based and practice-specific quality data. The Medicare Access and CHIP Reauthorization Act defines 4 performance categories: quality, cost, improvement activities, and advancing care information. Our discussion of the Medicare Access and CHIP Reauthorization Act is brief with regard to this ongoing program; additional guidance documents are available from the Centers for Medicare & Medicaid Services website. For our discussion, we now see operational opportunities for incentivizing high-value, patient-centered cardiovascular imaging and orchestrating high-quality imaging practices as a means to improve comprehensive strategies of care for patients.
Conclusions
Periodic evaluation of gaps in cardiovascular imaging evidence and targeted priorities of research should be undertaken within the imaging community, both within specific imaging societies and also broadly within the radiology and cardiology communities. In many cases, large trials and registries of real-world evidence are often considered complementary and definitive, even if the results are negative. This statement from the AHA’s Cardiovascular Imaging and Intervention Subcommittee in collaboration with the Council on Quality of Care and Outcomes Research provides a broad-based discussion of quality in cardiovascular imaging. The definition for quality in cardiovascular imaging was based on the Institute of Medicine’s key indicators: safe, effective, patient-centered, timely, equitable, and efficient. Moreover, we discuss the importance of patient-centered outcomes and introduce novel methodological approaches to devising high-quality evidence in cardiovascular imaging. Our proposed statement may serve as the foundation for integrating each of the quality indicators for establishing designations of quality laboratory practices and as standards for value-based payment reform for imaging procedures.96
With regard to orienting future clinical research to quality in cardiovascular imaging, overarching clinical hypotheses of improving patient outcomes, the importance of health status as an end point, and deferred testing options in clinical research are important components of this statement. Future research should evolve to clarify appropriate methods optimized for the role of cardiovascular imaging for detection and for guiding treatment and to demonstrate the role of cardiovascular imaging in facilitating healthcare quality. Alternative research options should be explored, which may require approaches different from those for traditional effectiveness trials for therapies. Strategic priorities from patient stakeholders collaborating with the imaging community remain a vital means to define high-quality evidence for cardiovascular imaging. The life cycle of evidence development for quality cardiovascular imaging practices is shown in Figure 6. As we learn from current imaging evidence, evolution in methodological approaches should prompt more refined approaches to defining the quality of cardiovascular imaging.
Figure 6.
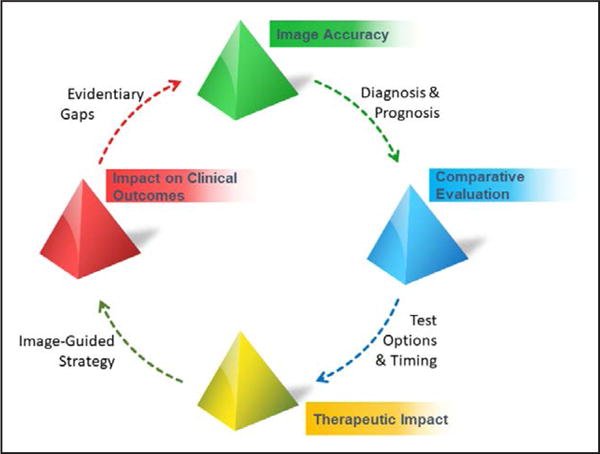
Life cycle of evidence development for quality standards for cardiovascular imaging.
Disclosures
Writing Group Disclosures.
| Writing Group Member |
Employment | Research Grant | Other Research Support |
Speakers’ Bureau/ Honoraria |
Expert Witness |
Ownership Interest |
Consultant/ Advisory Board |
Other |
|---|---|---|---|---|---|---|---|---|
| Leslee J. Shaw | Emory University | None | None | None | None | None | None | None |
| Marcelo F. Di Carli | Brigham & Women’s Hospital | Spectrum Dynamics* | None | None | None | None | None | None |
| Rob S.B. Beanlands | University of Ottawa Heart Institute Cardiology | JubilantDraxImage†; Lantheus Medical Imaging† | None | None | None | None | JubilantDraxImage*; Lantheus Medical Imaging*; KAI Research* | None |
| Ron Blankstein | Brigham & Women’s Hospital | Amgen* | None | None | None | None | EKOS, Inc*; Amgen, Inc* | None |
| Scott D. Flamm | Cleveland Clinic Imaging Institute | None | None | None | None | Precision Image Analysis*; Arterys* | Precision Image Analysis*; Arterys* | None |
| Thomas C. Gerber | Mayo Clinic | None | None | None | None | None | None | None |
| Jill E. Jacobs | NYU Langone Medical Center | None | None | None | None | None | None | None |
| Raymond Y. Kwong | Brigham & Women’s Hospital | None | None | None | None | None | None | None |
| Jonathon A. Leipsic | University of British Columbia | None | Medtronic†; Neovasc†; Tendyne† | GE Healthcare*; Samsung* | None | PI Cardia*; eartflow† | Circle†; Edwards*; Heartflow† | None |
| Jennifer H. Mieres | Northwell Health Cardiology | None | None | None | None | None | None | None |
| John Spertus | Saint Luke’s Mid America Heart Institute | None | None | None | None | None | None | None |
| Viviany R. Taqueti | Brigham and Women’s Hospital Heart and Vascular Center, Harvard Medical School | None | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Reviewer Disclosures.
| Reviewer | Employment | Research Grant |
Other Research Support |
Speakers’ Bureau/ Honoraria |
Expert Witness |
Ownership Interest |
Consultant/Advisory Board | Other |
|---|---|---|---|---|---|---|---|---|
| Daniel S. Berman | Cedars-Sinai Medical Center | None | None | None | None | None | None | None |
| Raymond J. Gibbons | Mayo Clinic Rochester | None | None | None | None | None | Astellas Pharmaceutical*; Lantheus Medical Imaging*; Peer View Institute* | None |
| David N. Levin | University of Toronto (Canada) | None | None | None | None | None | None | None |
This table represents the relationships of reviewers that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all reviewers are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Footnotes
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This statement was approved by the American Heart Association Science Advisory and Coordinating Committee on August 7, 2017, and the American Heart Association Executive Committee on December 11, 2017. A copy of the document is available at http://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 843-216-2533 or e-mail kelle.ramsay@wolterskluwer.com.
Expert peer review of AHA Scientific Statements is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit http://professional.heart.org/statements. Select the “Guidelines & Statements” drop-down menu, then click “Publication Development.”
References
- 1.Blackmore CC. Defining quality in radiology. J Am Coll Radiol. 2007;4:217–223. doi: 10.1016/j.jacr.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Dunnick NR, Applegate KE, Arenson RL. Quality–a radiology imperative: report of the 2006 Intersociety Conference. J Am Coll Radiol. 2007;4:156–161. doi: 10.1016/j.jacr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Leppo JA. Is more imaging better than less? J Nucl Cardiol. 2009;16:849–850. doi: 10.1007/s12350-009-9122-9. [DOI] [PubMed] [Google Scholar]
- 4.Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, Min JK, Patel MR, Rosenbaum L, Shaw LJ, Stainback RF, Allen JM, American College of Cardiology Foundation Appropriate Use Criteria Task Force ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Shaw LJ, Marwick TH, Zoghbi WA, Hundley WG, Kramer CM, Achenbach S, Dilsizian V, Kern MJ, Chandrashekhar Y, Narula J. Why all the focus on cardiac imaging? JACC Cardiovasc Imaging. 2010;3:789–794. doi: 10.1016/j.jcmg.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Onega T, Tosteson TD, Wang Q, Hillner BE, Song Y, Siegel BA, Tosteson AN. Geographic and sociodemographic disparities in PET use by Medicare beneficiaries with cancer. J Am Coll Radiol. 2012;9:635–642. doi: 10.1016/j.jacr.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao VM, Parker L, Levin DC, Sunshine J, Bushee G. Use trends and geographic variation in neuroimaging: nationwide Medicare data for 1993 and 1998. AJNR Am J Neuroradiol. 2001;22:1643–1649. [PMC free article] [PubMed] [Google Scholar]
- 8.Levin DC, Parker L, Rao VM. Recent trends in imaging use in hospital diabesettings: implications for future planning. J Am Coll Radiol. 2017;14:331–336. doi: 10.1016/j.jacr.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 9.Levin DC, Parker L, Halpern EJ, Rao VM. Recent trends in imaging for suspected coronary artery disease: what is the best approach? J Am Coll Radiol. 2016;13:381–386. doi: 10.1016/j.jacr.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Rosenkrantz AB, Hughes DR, Duszak R., Jr The U.S. radiologist workforce: an analysis of temporal and geographic variation by using large national datasets. Radiology. 2016;279:175–184. doi: 10.1148/radiol.2015150921. [DOI] [PubMed] [Google Scholar]
- 11.Parker L, Levin DC, Frangos A, Rao VM. Geographic variation in the utilization of noninvasive diagnostic imaging: national Medicare data, 1998-2007. AJR Am J Roentgenol. 2010;194:1034–1039. doi: 10.2214/AJR.09.3528. [DOI] [PubMed] [Google Scholar]
- 12.Mark DB, Anderson JL, Brinker JA, Brophy JA, Casey DE, Jr, Cross RR, Edmundowicz D, Hachamovitch R, Hlatky MA, Jacobs JE, Jaskie S, Kett KG, Malhotra V, Masoudi FA, McConnell MV, Rubin GD, Shaw LJ, Sherman ME, Stanko S, Ward RP. ACC/AHA/ASE/ASNC/HRS/IAC/Mended Hearts/NASCI/RSNA/SAIP/SCAI/SCCT/SCMR/SNMMI 2014 health policy statement on use of noninvasive cardiovascular imaging: a report of the American College of Cardiology Clinical Quality Committee. J Am Coll Cardiol. 2014;63:698–721. doi: 10.1016/j.jacc.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Vitola JV, Shaw LJ, Allam AH, Orellana P, Peix A, Ellmann A, Allman KC, Lee BN, Siritara C, Keng FY, Sambuceti G, Kiess MC, Giubbini R, Bouyoucef SE, He ZX, Thomas GS, Mut F, Dondi M. Assessing the need for nuclear cardiology and other advanced cardiac imaging modalities in the developing world. J Nucl Cardiol. 2009;16:956–961. doi: 10.1007/s12350-009-9104-y. [DOI] [PubMed] [Google Scholar]
- 14.Safavi KC, Li SX, Dharmarajan K, Venkatesh AK, Strait KM, Lin H, Lowe TJ, Fazel R, Nallamothu BK, Krumholz HM. Hospital variation in the use of noninvasive cardiac imaging and its association with downstream testing, interventions, and outcomes. JAMA Intern Med. 2014;174:546–553. doi: 10.1001/jamainternmed.2013.14407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douglas PS, ACC Think Tank on the Future of Cardiac Imaging Steering Committee The author replies. JACC Cardiovasc Imaging. 2017;10:98–99. doi: 10.1016/j.jcmg.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Douglas PS, Chen J, Gillam L, Hendel R, Hundley WG, Masoudi F, Patel MR, Peterson E. Achieving Quality in Cardiovascular Imaging II: proceedings from the Second American College of Cardiology–Duke University Medical Center Think Tank on Quality in Cardiovascular Imaging. JACC Cardiovasc Imaging. 2009;2:231–240. doi: 10.1016/j.jcmg.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Douglas PS, Cerqueira MD, Berman DS, Chinnaiyan K, Cohen MS, Lundbye JB, Patel RA, Sengupta PP, Soman P, Weissman NJ, Wong TC, ACC Cardiovascular Imaging Council The future of cardiac imaging: report of a think tank convened by the American College of Cardiology. JACC Cardiovasc Imaging. 2016;9:1211–1223. doi: 10.1016/j.jcmg.2016.02.027. [DOI] [PubMed] [Google Scholar]
- 18.Douglas PS, Taylor A, Bild D, Bonow R, Greenland P, Lauer M, Peacock F, Udelson J. Outcomes research in cardiovascular imaging: report of a workshop sponsored by the National Heart, Lung, and Blood Institute. JACC Cardiovasc Imaging. 2009;2:897–907. doi: 10.1016/j.jcmg.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. 2001 Mar; http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Accessed June 27, 2017. [PubMed]
- 20.Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, Froelicher VF, Leon AS, Piña IL, Rodney R, Simons-Morton DA, Williams MA, Bazzarre T. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104:1694–1740. doi: 10.1161/hc3901.095960. [DOI] [PubMed] [Google Scholar]
- 21.National Heart, Lung, and Blood Institute. What are the risks of stress testing? https://www.nhlbi.nih.gov/health/health-topics/topics/stress/risks. Accessed June 27, 2017.
- 22.Fazel R, Gerber TC, Balter S, Brenner DJ, Carr JJ, Cerqueira MD, Chen J, Einstein AJ, Krumholz HM, Mahesh M, McCollough CH, Min JK, Morin RL, Nallamothu BK, Nasir K, Redberg RF, Shaw LJ, on behalf of the American Heart Association Council on Quality of Care and Outcomes Research, Council on Clinical Cardiology, and Council on Cardiovascular Radiology and Intervention Approaches to enhancing radiation safety in cardiovascular imaging: a scientific statement from the American Heart Association [published correction appears in Circulation. 2014;130:e172] Circulation. 2014;130:1730–1748. doi: 10.1161/CIR.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 23.Gerber TC, Carr JJ, Arai AE, Dixon RL, Ferrari VA, Gomes AS, Heller GV, McCollough CH, McNitt-Gray MF, Mettler FA, Mieres JH, Morin RL, Yester MV. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation. 2009;119:1056–1065. doi: 10.1161/CIRCULATIONAHA.108.191650. [DOI] [PubMed] [Google Scholar]
- 24.Kase KR. Radiation protection principles of NCRP. Health Phys. 2004;87:251–257. doi: 10.1097/00004032-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 25.White RD, Patel MR, Abbara S, Bluemke DA, Herfkens RJ, Picard M, Shaw LJ, Silver M, Stillman AE, Udelson J, American College of Radiology; American College of Cardiology Foundation 2013 ACCF/ACR/ASE/ASNC/SCCT/SCMR appropriate utilization of cardiovascular imaging in heart failure: an executive summary: a joint report of the ACR Appropriateness Criteria® Committee and the ACCF Appropriate Use Criteria Task Force. J Am Coll Radiol. 2013;10:493–500. doi: 10.1016/j.jacr.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Carr JJ, Hendel RC, White RD, Patel MR, Wolk MJ, Bettmann MA, Douglas P, Rybicki FJ, Kramer CM, Woodard PK, Shaw LJ, Yucel EK, American College of Cardiology Foundation; American College of Cardiology Foundation 2013 Appropriate utilization of cardiovascular imaging: a methodology for the development of joint criteria for the appropriate utilization of cardiovascular imaging by the American College of Cardiology Foundation and American College of Radiology. J Am Coll Radiol. 2013;10:456–463. doi: 10.1016/j.jacr.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 27.Depuey EG, Mahmarian JJ, Miller TD, Einstein AJ, Hansen CL, Holly TA, Miller EJ, Polk DM, Samuel Wann L. Patient-centered imaging. J Nucl Cardiol. 2012;19:185–215. doi: 10.1007/s12350-012-9523-z. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RC, Burgett EV. Patient centered imaging and the dose of radiopharmaceuticals. J Nucl Cardiol. 2016;23:143–144. doi: 10.1007/s12350-015-0263-8. [DOI] [PubMed] [Google Scholar]
- 29.Halliburton SS, Abbara S, Chen MY, Gentry R, Mahesh M, Raff GL, Shaw LJ, Hausleiter J, Society of Cardiovascular Computed Tomography SCCT guidelines on radiation dose and dose-optimization strategies in cardiovascular CT. J Cardiovasc Comput Tomogr. 2011;5:198–224. doi: 10.1016/j.jcct.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Einstein AJ, Berman DS, Min JK, Hendel RC, Gerber TC, Carr JJ, Cerqueira MD, Cullom SJ, DeKemp R, Dickert NW, Dorbala S, Fazel R, Garcia EV, Gibbons RJ, Halliburton SS, Hausleiter J, Heller GV, Jerome S, Lesser JR, Raff GL, Tilkemeier P, Williams KA, Shaw LJ. Patient-centered imaging: shared decision making for cardiac imaging procedures with exposure to ionizing radiation. J Am Coll Cardiol. 2014;63:1480–1489. doi: 10.1016/j.jacc.2013.10.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jerome SD, Tilkemeier PL, Farrell MB, Shaw LJ. Nationwide laboratory adherence to myocardial perfusion imaging radiation dose reduction practices: a report from the Intersocietal Accreditation Commission Data Repository. JACC Cardiovasc Imaging. 2015;8:1170–1176. doi: 10.1016/j.jcmg.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Cerqueira MD, Allman KC, Ficaro EP, Hansen CL, Nichols KJ, Thompson RC, Van Decker WA, Yakovlevitch M. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol. 2010;17:709–718. doi: 10.1007/s12350-010-9244-0. [DOI] [PubMed] [Google Scholar]
- 33.Einstein AJ. Effects of radiation exposure from cardiac imaging: how good are the data? J Am Coll Cardiol. 2012;59:553–565. doi: 10.1016/j.jacc.2011.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tilkemeier P, Green J, Einstein AJ, Fazel R, Reames P, Shaw LJ. The evolving practice of nuclear cardiology: results from the 2011 ASNC member survey. J Nucl Cardiol. 2012;19:1170–1175. doi: 10.1007/s12350-012-9624-8. [DOI] [PubMed] [Google Scholar]
- 35.American College of Radiology. National Radiology Data Registry. http://www.acr.org/Quality-Safety/National-Radiology-Data-Registry. Accessed June 27, 2017.
- 36.Morin RL, Seibert JA, Boone JM. Radiation dose and safety: informatics standards and tools. J Am Coll Radiol. 2014;11(pt B):1286–1297. doi: 10.1016/j.jacr.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Brink JA, Miller DL. U.S. national diagnostic reference levels: closing the gap. Radiology. 2015;277:3–6. doi: 10.1148/radiol.2015150971. [DOI] [PubMed] [Google Scholar]
- 38.Bohl MA, Goswami R, Strassner B, Stanger P. Meeting The Joint Commission’s dose incident identification and external benchmarking requirements using the ACR’s Dose Index Registry. J Am Coll Radiol. 2016;13:936–942. doi: 10.1016/j.jacr.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 39.Nelson KH, Willens HJ, Hendel RC. Utilization of radionuclide myocardial perfusion imaging in two health care systems: assessment with the 2009 ACCF/ASNC/AHA appropriateness use criteria. J Nucl Cardiol. 2012;19:37–42. doi: 10.1007/s12350-011-9467-8. [DOI] [PubMed] [Google Scholar]
- 40.Carryer DJ, Hodge DO, Miller TD, Askew JW, Gibbons RJ. Application of appropriateness criteria to stress single photon emission computed tomography sestamibi studies: a comparison of the 2009 revised appropriateness criteria to the 2005 original criteria. Am Heart J. 2010;160:244–249. doi: 10.1016/j.ahj.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Doukky R, Hayes K, Frogge N, Balakrishnan G, Dontaraju VS, Rangel MO, Golzar Y, Garcia-Sayan E, Hendel RC. Impact of appropriate use on the prognostic value of single-photon emission computed tomography myocardial perfusion imaging. Circulation. 2013;128:1634–1643. doi: 10.1161/CIRCULATIONAHA.113.002744. [DOI] [PubMed] [Google Scholar]
- 42.Matulevicius SA, Rohatgi A, Das SR, Price AL, DeLuna A, Reimold SC. Appropriate use and clinical impact of transthoracic echocardiography. JAMA Intern Med. 2013;173:1600–1607. doi: 10.1001/jamainternmed.2013.8972. [DOI] [PubMed] [Google Scholar]
- 43.Johnson TV, Rose GA, Fenner DJ, Rozario NL. Improving appropriate use of echocardiography and single-photon emission computed tomographic myocardial perfusion imaging: a continuous quality improvement initiative. J Am Soc Echocardiogr. 2014;27:749–757. doi: 10.1016/j.echo.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Gurzun MM, Ionescu A. Appropriateness of use criteria for transthoracic echocardiography: are they relevant outside the USA? Eur Heart J Cardiovasc Imaging. 2014;15:450–455. doi: 10.1093/ehjci/jet186. [DOI] [PubMed] [Google Scholar]
- 45.Gibbons RJ, Miller TD, Hodge D, Urban L, Araoz PA, Pellikka P, McCully RB. Application of appropriateness criteria to stress single-photon emission computed tomography sestamibi studies and stress echocardiograms in an academic medical center. J Am Coll Cardiol. 2008;51:1283–1289. doi: 10.1016/j.jacc.2007.10.064. [DOI] [PubMed] [Google Scholar]
- 46.Lin FY, Dunning AM, Narula J, Shaw LJ, Gransar H, Berman DS, Min JK. Impact of an automated multimodality point-of-order decision support tool on rates of appropriate testing and clinical decision making for individuals with suspected coronary artery disease: a prospective multicenter study. J Am Coll Cardiol. 2013;62:308–316. doi: 10.1016/j.jacc.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 47.Shaw LJ, Mieres JH, Hendel RH, Boden WE, Gulati M, Veledar E, Hachamovitch R, Arrighi JA, Merz CN, Gibbons RJ, Wenger NK, Heller GV, for the WOMEN Trial Investigators Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) trial. Circulation. 2011;124:1239–1249. doi: 10.1161/CIRCULATIONAHA.111.029660. [DOI] [PubMed] [Google Scholar]
- 48.Hachamovitch R, Nutter B, Hlatky MA, Shaw LJ, Ridner ML, Dorbala S, Beanlands RS, Chow BJ, Branscomb E, Chareonthaitawee P, Weigold WG, Voros S, Abbara S, Yasuda T, Jacobs JE, Lesser J, Berman DS, Thomson LE, Raman S, Heller GV, Schussheim A, Brunken R, Williams KA, Farkas S, Delbeke D, Schoepf UJ, Reichek N, Rabinowitz S, Sigman SR, Patterson R, Corn CR, White R, Kazerooni E, Corbett J, Bokhari S, Machac J, Guarneri E, Borges-Neto S, Millstine JW, Caldwell J, Arrighi J, Hoffmann U, Budoff M, Lima J, Johnson JR, Johnson B, Gaber M, Williams JA, Foster C, Hainer J, Di Carli MF, SPARC Investigators Patient management after noninvasive cardiac imaging results from SPARC (Study of Myocardial Perfusion and Coronary Anatomy Imaging Roles in Coronary Artery Disease) J Am Coll Cardiol. 2012;59:462–474. doi: 10.1016/j.jacc.2011.09.066. [DOI] [PubMed] [Google Scholar]
- 49.Shaw LJ, Berman DS, Picard MH, Friedrich MG, Kwong RY, Stone GW, Senior R, Min JK, Hachamovitch R, Scherrer-Crosbie M, Mieres JH, Marwick TH, Phillips LM, Chaudhry FA, Pellikka PA, Slomka P, Arai AE, Iskandrian AE, Bateman TM, Heller GV, Miller TD, Nagel E, Goyal A, Borges-Neto S, Boden WE, Reynolds HR, Hochman JS, Maron DJ, Douglas PS, National Institutes of Health/National Heart, Lung, and Blood Institute-Sponsored ISCHEMIA Trial Investigators Comparative definitions for moderate-severe ischemia in stress nuclear, echocardiography, and magnetic resonance imaging. JACC Cardiovasc Imaging. 2014;7:593–604. doi: 10.1016/j.jcmg.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.SCOT-HEART Investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet. 2015;385:2383–2391. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 51.Mark DB, Anstrom KJ, Sheng S, Baloch KN, Daniels MR, Hoffmann U, Patel MR, Cooper LS, Lee KL, Douglas PS, on behalf of the PROMISE Investigators Quality-of-life outcomes with anatomic versus functional diagnostic testing strategies in symptomatic patients with suspected coronary artery disease: results from the PROMISE randomized trial. Circulation. 2016;133:1995–2007. doi: 10.1161/CIRCULATIONAHA.115.020259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jørgensen ME, Andersson C, Nørgaard BL, Abdulla J, Shreibati JB, Torp-Pedersen C, Gislason GH, Shaw RE, Hlatky MA. Functional testing or coronary computed tomography angiography in patients with stable coronary artery disease. J Am Coll Cardiol. 2017;69:1761–1770. doi: 10.1016/j.jacc.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 53.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel MR, Dai D, Hernandez AF, Douglas PS, Messenger J, Garratt KN, Maddox TM, Peterson ED, Roe MT. Prevalence and predictors of nonobstructive coronary artery disease identified with coronary angiography in contemporary clinical practice. Am Heart J. 2014;167:846–852.e2. doi: 10.1016/j.ahj.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Shaw LJ, Phillips LM, Nagel E, Newby DE, Narula J, Douglas PS. Comparative effectiveness trials of imaging-guided strategies in stable ischemic heart disease. JACC Cardiovasc Imaging. 2017;10:321–334. doi: 10.1016/j.jcmg.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 56.Shaw LJ, Butler J. Targeting priority populations to reduce disparities in cardiovascular care: health equity for all. J Am Coll Cardiol. 2014;64:346–348. doi: 10.1016/j.jacc.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 57.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132:997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, D’Onofrio G, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–345. doi: 10.1016/j.jacc.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Caillier JG. Race, gender, and cardiovascular disease: do disparities exist at hospitals that serve majority black populations when patients present with ischemic heart disease and myocardial infarction? J Cult Divers. 2006;13:202–207. [PubMed] [Google Scholar]
- 60.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD, Stamler J. Race/ethnicity, income, major risk factors, and cardiovascular disease mortality. Am J Public Health. 2005;95:1417–1423. doi: 10.2105/AJPH.2004.048165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK, on behalf of the American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 62.Lee SP, Jang EJ, Kim YJ, Cha MJ, Park SY, Song HJ, Choi JE, Shim JI, Ahn J, Lee HJ. Cost-effectiveness of coronary CT angiography in patients with chest pain: comparison with myocardial single photon emission tomography. J Cardiovasc Comput Tomogr. 2015;9:428–437. doi: 10.1016/j.jcct.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, Voigt JU, Zamorano JL, European Association of Echocardiography Stress echocardiography expert consensus statement– executive summary: European Association of Echocardiography (EAE) (a registered branch of the ESC) Eur Heart J. 2009;30:278–289. doi: 10.1093/eurheartj/ehn492. [DOI] [PubMed] [Google Scholar]
- 64.Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB, Hollander JE. CT angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med. 2012;366:1393–1403. doi: 10.1056/NEJMoa1201163. [DOI] [PubMed] [Google Scholar]
- 65.Hulten E, Pickett C, Bittencourt MS, Villines TC, Petrillo S, Di Carli MF, Blankstein R. Outcomes after coronary computed tomography angiography in the emergency department: a systematic review and meta-analysis of randomized, controlled trials. J Am Coll Cardiol. 2013;61:880–892. doi: 10.1016/j.jacc.2012.11.061. [DOI] [PubMed] [Google Scholar]
- 66.Goodacre S, Thokala P, Carroll C, Stevens JW, Leaviss J, Al Khalaf M, Collinson P, Morris F, Evans P, Wang J. Systematic review, meta-analysis and economic modelling of diagnostic strategies for suspected acute coronary syndrome. Health Technol Assess. 2013;17:v–vi. 1–188. doi: 10.3310/hta17010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoffmann U, Truong QA, Schoenfeld DA, Chou ET, Woodard PK, Nagurney JT, Pope JH, Hauser TH, White CS, Weiner SG, Kalanjian S, Mullins ME, Mikati I, Peacock WF, Zakroysky P, Hayden D, Goehler A, Lee H, Gazelle GS, Wiviott SD, Fleg JL, Udelson JE, ROMICAT-II Investigators Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med. 2012;367:299–308. doi: 10.1056/NEJMoa1201161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goldstein JA, Chinnaiyan KM, Abidov A, Achenbach S, Berman DS, Hayes SW, Hoffmann U, Lesser JR, Mikati IA, O’Neil BJ, Shaw LJ, Shen MY, Valeti US, Raff GL, CT-STAT Investigators The CT-STAT (Coronary Computed Tomographic Angiography for Systematic Triage of Acute Chest Pain Patients to Treatment) trial. J Am Coll Cardiol. 2011;58:1414–1422. doi: 10.1016/j.jacc.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 69.Marwick TH, Shaw L, Case C, Vasey C, Thomas JD. Clinical and economic impact of exercise electrocardiography and exercise echocardiography in clinical practice. Eur Heart J. 2003;24:1153–1163. doi: 10.1016/s0195-668x(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 70.Underwood SR, Godman B, Salyani S, Ogle JR, Ell PJ. Economics of myocardial perfusion imaging in Europe: the EMPIRE study. Eur Heart J. 1999;20:157–166. doi: 10.1053/euhj.1998.1196. [DOI] [PubMed] [Google Scholar]
- 71.Xie JX, Shaw LJ. Measuring diagnostic health care costs in stable coronary artery disease: should we follow the money? Ann Intern Med. 2016;165:147–148. doi: 10.7326/M16-1048. [DOI] [PubMed] [Google Scholar]
- 72.QualityNet. Imaging efficacy measures. https://www.qualitynet.org/dcs/ContentServer?c=Page&pagenamec=QnetPublicc%2FPage%2FQnetTier2&cid=1228695266120. Accessed June 27, 2017.
- 73.Ford I, Norrie J. Pragmatic trials. N Engl J Med. 2016;375:454–463. doi: 10.1056/NEJMra1510059. [DOI] [PubMed] [Google Scholar]
- 74.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS, COURAGE Trial Research Group Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 75.Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chronic Dis. 1967;20:637–648. doi: 10.1016/0021-9681(67)90041-0. [DOI] [PubMed] [Google Scholar]
- 76.Basch E, Torda P, Adams K. Standards for patient-reported outcome-based performance measures. JAMA. 2013;310:139–140. doi: 10.1001/jama.2013.6855. [DOI] [PubMed] [Google Scholar]
- 77.National Quality Forum website. http://www.qualityforum.org/Publications/2012/12/Patient-Reported_Outcomes_in_Performance_Measurement.aspx. Accessed June 27, 2017.
- 78.Weintraub WS, Spertus JA, Kolm P, Maron DJ, Zhang Z, Jurkovitz C, Zhang W, Hartigan PM, Lewis C, Veledar E, Bowen J, Dunbar SB, Deaton C, Kaufman S, O’Rourke RA, Goeree R, Barnett PG, Teo KK, Boden WE, Mancini GB, COURAGE Trial Research Group Effect of PCI on quality of life in patients with stable coronary disease. N Engl J Med. 2008;359:677–687. doi: 10.1056/NEJMoa072771. [DOI] [PubMed] [Google Scholar]
- 79.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–49. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 80.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 81.International Consortium for Health Outcomes Measurement. ICHOM website. http://www.ichom.org/. Accessed September 21, 2017.
- 82.Donabedian A. The quality of care in a health maintenance organization: a personal view. Inquiry. 1983;20:218–222. [PubMed] [Google Scholar]
- 83.PCORI: Patient-Centered Outcomes Research Institute. Patient-centered outcomes research. http://www.pcori.org/research-results/patient-centered-outcomes-research. Accessed June 27, 2017.
- 84.McKavanagh P, Lusk L, Ball PA, Verghis RM, Agus AM, Trinick TR, Duly E, Walls GM, Stevenson M, James B, Hamilton A, Harbinson MT, Donnelly PM. A comparison of cardiac computerized tomography and exercise stress electrocardiogram test for the investigation of stable chest pain: the clinical results of the CAPP randomized prospective trial. Eur Heart J Cardiovasc Imaging. 2015;16:441–448. doi: 10.1093/ehjci/jeu284. [DOI] [PubMed] [Google Scholar]
- 85.Sharples L, Hughes V, Crean A, Dyer M, Buxton M, Goldsmith K, Stone D. Cost-effectiveness of functional cardiac testing in the diagnosis and management of coronary artery disease: a randomised controlled trial: the CECaT trial. Health Technol Assess. 2007;11:iii–iv. ix. doi: 10.3310/hta11490. [DOI] [PubMed] [Google Scholar]
- 86.Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O’Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE, for the COURAGE Investigators Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 87.Beanlands RS, Nichol G, Huszti E, Humen D, Racine N, Freeman M, Gulenchyn KY, Garrard L, deKemp R, Guo A, Ruddy TD, Benard F, Lamy A, Iwanochko RM, PARR-2 Investigators F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2) J Am Coll Cardiol. 2007;50:2002–2012. doi: 10.1016/j.jacc.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 88.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, Masoudi FA, Peterson ED, Shaw LJ. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129:2329–2345. doi: 10.1161/CIR.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 89.Drummond M, Weatherly H, Ferguson B. Economic evaluation of health interventions. BMJ. 2008;337:a1204. doi: 10.1136/bmj.a1204. [DOI] [PubMed] [Google Scholar]
- 90.Stern RG. Diagnostic imaging: powerful, indispensable, and out of control. Am J Med. 2012;125:113–114. doi: 10.1016/j.amjmed.2011.07.037. [DOI] [PubMed] [Google Scholar]
- 91.Mark DB, Federspiel JJ, Cowper PA, Anstrom KJ, Hoffmann U, Patel MR, Davidson-Ray L, Daniels MR, Cooper LS, Knight JD, Lee KL, Douglas PS, PROMISE Investigators Economic outcomes with anatomical versus functional diagnostic testing for coronary artery disease. Ann Intern Med. 2016;165:94–102. doi: 10.7326/M15-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steg PG, Greenlaw N, Tendera M, Tardif JC, Ferrari R, Al-Zaibag M, Dorian P, Hu D, Shalnova S, Sokn FJ, Ford I, Fox KM, Prospective Observational Longitudinal Registry of Patients With Stable Coronary Artery Disease (CLARIFY) Investigators Prevalence of anginal symptoms and myocardial ischemia and their effect on clinical outcomes in outpatients with stable coronary artery disease: data from the International Observational CLARIFY Registry. JAMA Intern Med. 2014;174:1651–1659. doi: 10.1001/jamainternmed.2014.3773. [DOI] [PubMed] [Google Scholar]
- 93.ISCHEMIA trial website. http://www.ischemiatrial.org. Accessed September 21, 2017.
- 94.Centers for Medicare & Medicaid Services. Quality Payment Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/MACRA-MIPS-and-APMs/MACRA-Quality-Payment-Program-webinar-slides-10-26-16.pdf. Accessed June 27, 2017.
- 95.Centers for Medicare & Medicaid Services. The Quality Payment Program overview fact sheet. https://www.qpp.cms.gov/docs/Quality_Payment_Program_Overview_Fact_Sheet.pdf. Accessed June 27, 2017.
- 96.Rubin BB, Drenth B, Beanlands RS. Appropriate, quality imaging tests through linkage of payment to guidelines, accreditation and training. CMAJ. 2015;187:E126–E127. doi: 10.1503/cmaj.140638. [DOI] [PMC free article] [PubMed] [Google Scholar]


