Abstract
We investigated the long-term safety and disease-control data obtained with intravenous busulfan (Bu) combined with clofarabine (Clo) in patients with acute lymphoblastic leukemia (ALL) undergoing allogeneic hematopoietic stem cell transplantation (SCT). 107 patients with median age 38 years (range 19–64 years) received a matched sibling (n=52), or matched unrelated donor transplant (n=55) for ALL in first complete remission (n=62), second complete remission (n=28), or more advanced disease (n=17). Nearly half of the patients had high-risk cytogenetic profiles as defined by the presence of t(9;22) (n=34), t(4;11) (n=4), or complex cytogenetics (n=7). Clo 40 mg/m2 was given once daily, each dose followed by pharmacokinetically-dosed Bu infused over three hours daily for 4 days, followed by hematopoietic cell infusion after two rest days. The Bu dose was based upon the drug clearance determined by a test Bu dose, 32 mg/m2, given 48 hours prior to the high dose regimen. The target daily area under the curve (AUC) was 5,500 microMol-min for patients less than 60 years of age and 4000 microMol-min for patients older than 59 years of age. With a median follow-up of 3.3 years among surviving patients (1–5.8 years), the 2-year progression-free survival (PFS) rates for patients transplanted in CR1, CR2, or more advanced disease were 62%, 34%, and 35%, respectively. The regimen was well tolerated with non-relapse mortality (NRM) rates of 10% and 31% and at 100 days and 2 years, respectively. The incidence of grades II–IV and III–IV acute graft versus host disease (GVHD) were 35% and 10%, respectively; 18% patients developed extensive chronic GVHD. The 2-year overall survival (OS) rates for patients transplanted in CR1, CR2, or more advanced disease were 70%, 57%, and 35%, respectively. Among 11 patients older than 59 years treated with reduced dose Bu in CR1 (n=7) or CR2 (n=4), 4 remain alive and disease-free with a median follow up of 2.6 years (2–4.7 years). Only the presence of MRD at time of transplant was associated with significantly worse PFS and OS on multivariate analysis. The Clo-Bu combination provides effective disease control while maintaining a favorable safety profile. Overall survival and NRM rates compare favorably with traditional myeloablative TBI-based conditioning regimens.
Keywords: Acute lymphoblastic leukemia, Allogeneic hematopoietic stem cell transplantation, Transplant conditioning regimens
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (SCT) is an effective, potentially curative treatment option for adults with acute lymphoblastic leukemia (ALL), but may be associated with significant morbidity. Non-relapse mortality (NRM) rates between 20% and 45% have been reported for patients receiving a standard, total body irradiation (TBI)-based, myeloablative preparative regimen(1–3). In efforts to improve NRM, reduced intensity conditioning (RIC) regimens have been investigated, with improvements in acute NRM, but resulting in increased risk of relapse, especially for patients beyond first complete remission(4–6). In attempts to limit the toxicities associated with TBI-based, myeloablative regimens, we replaced radiation with a chemotherapy-only, double alkylator regimen consisting of intravenous (i.v.), pharmacokinetically (PK)-dosed busulfan (Bu), and melphalan (Mel)(7). We showed comparable disease control to radiation-based regimens, while decreasing acute regimen-related toxicities, but long-term NRM, primarily related to graft versus host disease (GVHD), remained substantial (55% at 2 years for patients older than 40 years(7).
We, and others, have shown good disease control and decreased toxicity when a second alykylator (Melphalan or cyclophosphamide) was replaced by the nucleoside analogue (NA) fludarabine (Flu) in the transplant conditioning regimen in children and adults with leukemia(8–15). We further hypothesized that replacing the NA Flu with the second generation NA clofarabine (Clo), which has single agent activity in refractory relapsed ALL(16, 17), would provide particularly good disease control in patients with ALL. The i.v. Clo-Bu combination was used for patients undergoing allogeneic SCT for ALL, and the early published results in 51 patients were encouraging, showing a low 100-day TRM of 6%, and a projected 1-year disease-free survival rate for patients transplanted in CR1 of 64% (18).
We have completed the trial and accrued 107 patients. This report presents the long-term follow-up results of this trial.
PATIENTS AND METHODS
Patient eligibility and study treatment
This was a prospective, phase II single arm study investigating the combination of Bu and Clo in patients with ALL. Enrollment began in October 2009 and completed in July 2015, and we are reporting the outcomes for adult patients treated consecutively during this time period. Patient eligibility and study methods were detailed in our previous publication (18). Briefly, patients were between 18 and 65 years of age, with an available human leukocyte antigen (HLA) matched related donor or 8/8 unrelated donor undergoing first allogeneic SCT. Additional eligibility criteria included a Zubrod performance status of 0 or 1, adequate organ function, and absence of active infection. Patients with active CNS disease were excluded.
The transplant conditioning regimen consisted of Clo 40 mg/m2 infused over one hour followed by pharmacokinetically-dosed Bu infused over 3 hours once daily for 4 days followed by hematopoietic cell infusion after 2 rest days. The therapeutic dose was determined by the drug clearance determined from a pharmacokinetic test dose of IV Bu, 32 mg/m2 infused over 45 minutes two days before the first therapeutic Bu dose. The therapeutic i.v. Bu dose targeted an average daily AUC of 5,500 microMol-min for patients less than 60 years of age or 4,000 microMol-min for patients older than 59 years. Collection of blood and methods for PK analyses were performed as previously reported (7, 19).
Phenytoin 600 mg orally was used during and one day after completion of i.v. Bu therapy, starting the evening before the first dose (20). Graft versus host disease (GVHD) prophylaxis consisted of a combination of tacrolimus and mini-dose methotrexate. Patients who received unrelated donor products additionally received rabbit anti-thymocyte globulin for a total 4 mg/kg infused over three days beginning three days prior to SCT. Institutional transplant guidelines for antimicrobial, antifungal, and antiviral prophylaxis were followed as previously reported(21). Patients with a prior history of CNS involvement received craniospinal XRT immediately prior to transplant conditioning or post transplant intrathecal pre-emptive therapy, as feasible; patients without history of CNS involvement of leukemia did not receive any CNS therapy beyond completing their recommended primary treatment(22). Finally, patients whose leukemia was positive for the Philadelphia chromosome (Ph) were started on maintenance therapy with tyrosine kinase inhibitor (TKI) upon normalization of blood counts following SCT to continue for up to 5 years.
Definitions and clinical outcome variables
The disease stage at transplantation was defined using established criteria based on bone marrow morphology. Criteria for complete response included normal cytogenetics, the absence of circulating blasts, less than 5% marrow blasts, and normalization of complete blood counts (CBC). Response was documented as best response occurring by day 30 following SCT. Standard morphologic criteria were used to diagnose recurrent disease. Molecular response measured by quantitative polymerase chain reaction (PCR) analysis for BCR-ABL rearrangement was obtained when possible. Multiparameter flow cytometry, with a sensitivity of 0.01%, was used to further assay for minimal residual disease (MRD). MRD and PCR analyses were not used to assign disease stage or document relapse. Hematologic recovery was defined as the first day the patient had an absolute neutrophil count of 0.5 × 109/L or higher for 3 consecutive days. Platelet recovery was defined as occurring on the first of 7 consecutive days with a platelet count of 20 × 109/L or higher without transfusion support. Failure to engraft by day +30 was considered primary engraftment failure. Hematopoietic chimerism was evaluated in peripheral blood (with myeloid and T- lineage sorting) by restriction fragment length polymorphisms using PCR methods to determine donor engraftment. Mixed chimerism was defined as the presence of any detectable (≥1%) recipient DNA in addition to donor-derived DNA in myeloid or T-lineage cells.
Overall survival (OS) was estimated from the time of SCT until death from any cause, and patients still alive at last follow-up were administratively censored. Progression-free survival (PFS) was estimated from SCT until the date of relapse or death from any cause. Patients alive and disease-free at last follow-up were censored. Non-relapse mortality (NRM) was defined as death from any cause other than disease progression or relapse. Acute and chronic GVHD were graded based on standard criteria (23–24).
Statistical methods
The trial completed accrual in July 2015, and this is the final report of the 107 adult patients treated with matched related or unrelated donors on this study. Patients undergoing transplant with syngeneic donors were excluded from this analysis. The primary outcomes for this single-arm trial were safety and overall survival. Bayesian early stopping rules based on the observed rates of these 2 outcomes, as compared to historical data, were implemented (25). The methods of Gooley, Fine, and Gray were used to compare the cumulative incidence of NRM vs. the competing risk of relapse, separately by age group (<40 vs ≥ 40) and by disease stage. Overall survival and PFS were analyzed using the Kaplan-Meier estimator (26), and univariate and multivariate Cox proportional hazards models. The factors age (≥40 vs. <40 years), cytogenetic risk (high vs. intermediate), immune phenotype (T- vs. B-lineage), donor relation (MUD vs. sib), and presence of MRD in patients with morphologic remission (CR1, CR2, or CR3) were investigated in univariate and multivariate analyses for OS and PFS. Survival curves were generated and the log-rank test was used to compare groups. Hazard ratios and 95% confidence intervals were estimated. With respect to cytogenetic risk group, only the subset of patients in high and intermediate groups were compared. Descriptive statistics were used to summarize patient demographics. The cumulative incidence of GVHD and relapse were calculated with death as a competing risk.
RESULTS
Patient and treatment characteristics
Patient demographics and baseline disease characteristics are listed in Table 1. One hundred and seven patients with median age of 37 years (range 19–64 years), with 11 patients older than 59 years, were evaluated on this study. The median time from diagnosis to transplant was 9.2 months (range 2.3–118.2 months). The majority of patients had high risk features at diagnosis with 32% (n=34) having an elevated WBC count and 43% (n=45) having high-risk cytogenetics, defined as presence of the t(9;22), t(4;11), or complex karyotype, defined as ≥5 cytogenetic abnormalities. Additionally, 8 patients presented with CNS involvement and 14 patients presented with lymph node involvement at time of diagnosis. At time of transplant, 58% (n=62) were in CR1, 26% (n=28) were in CR2, and 16% of patients had more advanced disease (CR3, n=2; incomplete recovery of counts, n=9; blasts >5%, n=6). Among the patients in CR, 30% had MRD present (n=28). Among 34 patients with the t(9;22) translocation, 15 patients (44%) were started on TKI maintenance therapy at a median of 2.2 months (range 1.3–14.4) following SCT. The majority of patients received dasatinib (n=10) at a median dose of 100 mg; four patients received imatinib and one patient received ponatinib. All of the Ph+ patients received TKI therapy with their chemotherapy prior to being referred for transplant.
Table 1.
Patient characteristics at diagnosis, N=107
| Patient Characteristic | No. |
|---|---|
| Median age (range) | 37 (19–64) years |
| Greater than 59 years | 11 |
| Sex, male/female | 64/43 |
| Disease lineage (%) | |
| B-lineage | 91 (85) |
| T-lineage | 16 (15) |
| WBC count at diagnosis (%) | |
| <30,000/µL | 58 (54) |
| 30,000/µL–100,000/µL | 13 (12) |
| >100,000/µL | 21 (20) |
| Unknown | 15 (14) |
| Cytogenetics at diagnosis (%) | |
| Diploid | 27 (25) |
| Other | 27 (25) |
| 9;22 | 34 (32) |
| 4;11 | 4 (4) |
| Complex | 7 (7) |
| Unknown | 8 (7) |
| Time to achieve CR (%) | |
| Within 4 weeks | 43 (40) |
| >4 weeks | 59 (55) |
| Unknown | 5 (5) |
| Disease status at transplant (%) | |
| CR1 | 62 (58) |
| CR2 | 28 (26) |
| CR3 | 2 (2) |
| Incomplete recovery of counts | 9 (8) |
| >5% blasts | 6 (6) |
| CR, n=92 | |
| MRD present (%) | 28 (30) |
| MRD absent (%) | 64 (70) |
| Med chemo. regimens pre-SCT (range) | 1 (1–5) |
| Median months to SCT (range) | 9.2 (2.3–118.2) |
Graft content and engraftment
Stem cell graft characteristics and hematopoietic recovery data are summarized in Table 2. Approximately half of the patients received a matched related donor transplant (49%) and half matched unrelated donor SCT (51%). The source of stem cells was peripheral blood for the majority of patients. The median days to neutrophil and platelet recovery were 11 (range 10–25 days) and 14 (range 8–109 days), respectively. Two patients died before day 30 following transplant due to infection and ensuing end-organ failure, leaving 105 patients evaluable for chimerism assessment. Full donor chimerism by day 30 was achieved in 70% of patients; 92% of patients eventually achieved full donor chimerism defined as 100% donor T-cells and myeloid cells. Eight patients remained mixed chimera in their T-cell component, ranging from <50% to 99% donor T cells, and 4 of these patients eventually relapsed. One of these patients remained severely cytopenic and required a stem cell boost. Of note, among the 11 patients 60 years and older, who received reduced dose Bu, 10 attained full donor chimerism at median of 44 days; 1 patient remained mixed chimera in the T cell component at 84% and had disease progression at 2 months post SCT.
Table 2.
Graft characteristics at transplant, and hematopoietic recovery
| Characteristic | No. |
|---|---|
| Donor type (%) | |
| Matched related | 52 (49) |
| Matched unrelated | 55 (51) |
| Stem cell source (%) | |
| Bone marrow | 34(32) |
| Peripheral blood | 73 (68) |
| Median days to ANC recovery (range)1 | 11 (10–25) |
| Median days to platelet recovery (range)1, 2 | 13 (8–109) |
Count recovery defined in text of manuscript.
Eight patients did not have platelet recovery.
Response, relapse, and progression-free survival
Among 105 patients evaluable for response (2 patients not evaluable due to early death), 13 patients cleared their disease, 91 patients maintained their remission, and one patient remained with active disease. Thirty-eight patients progressed at a median of 5.6 months (range 1.9–50.2) following SCT, with a 2-year progression or death probability of 38% for patients transplanted in CR1, 66% for patients transplanted in CR2, and 65% for patients transplanted with more advanced disease. Three patients had isolated CNS relapse, with two of these patients having a prior history of CNS involvement. One patient relapsed in the CNS nearly two years after SCT, was re-induced into remission with XRT and intrathecal therapy followed by consolidation with a second transplant and remains in remission. One patient relapsed in the CNS 2.7 months post SCT, was re-induced into remission with XRT and intrathecal therapy, and died 4 years later due to GVHD complications. The last patient relapsed in the CNS nearly one year post SCT, was re-induced into remission with intrathecal chemotherapy, consolidated with systemic chemotherapy, and died 3 years later from infection.
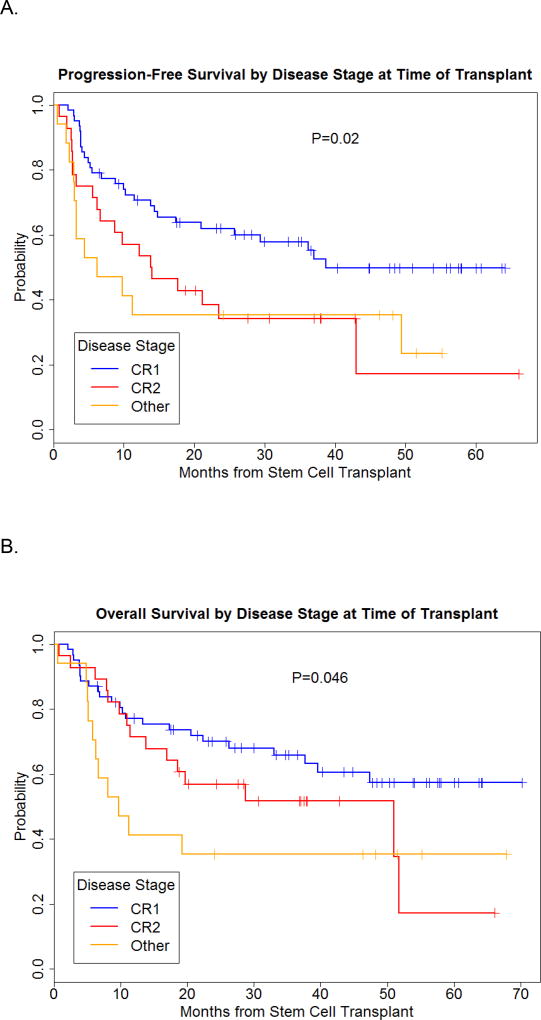
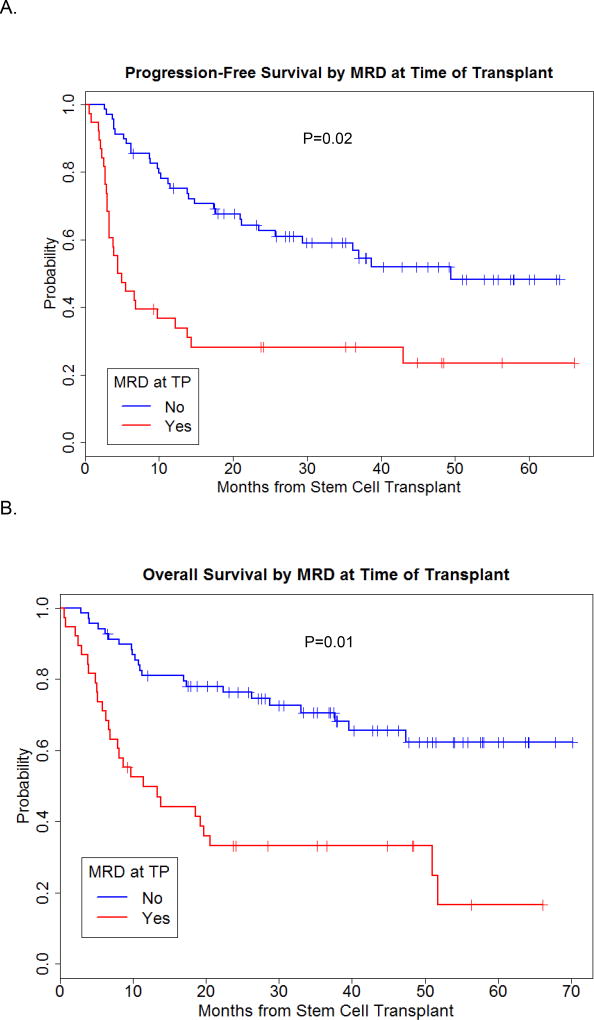
The 2-year PFS probability for the entire group was 51%. As expected disease stage had an impact on PFS, with 2-year PFS probability of 62%, 34% and 35% for patients transplanted in CR1, CR2, and with more advanced disease, respectively (Figure 1A). Only the presence of MRD at time of transplant was significantly associated with lower PFS, with a hazard ratio of 2.15, p=0.02. (Figure 2A).
Figure 1.
Progression-free and overall survival by disease stage at time of transplant.
Figure 2.
Progression-free and overall survival by MRD at time of transplant.
Overall survival
With a median follow-up of 3.3 years among surviving patients (range 1–5.8 years), the 2-year OS rates for patients transplanted in CR1, CR2, or more advanced disease were 70%, 57%, and 35%, respectively (Figure 1B). The same factors investigated for PFS were evaluated for their impact on OS, and again, only the presence of MRD at time of transplant was highly associated with worse survival in mulitvariate analysis with a hazard ratio of 2.54, p=0.01 (Figure 1B).
Toxicity, NRM, and GVHD
Regimen-related toxicities assessed by the NCI CTCAE version 3 are detailed in Table 3. The most commonly observed toxicities involved the GI tract, and were mild grades 1 or 2 nausea (100%) and/or diarrhea (48%), and grade II mucositis (65%). Reversible elevation in liver enzymes was commonly noted (85%), and reversible elevation in creatinine was noted in 9% of patients. Six patients developed sinusoidal obstructive syndrome or veno-occlusive disease (VOD), with median time to onset 25.5 days post SCT (range 7–37 days). The majority of these patients had a prolonged period of treatment prior to transplant, median time to transplant 17.5 months (range 3.7–118.2 months), with one fatality due to irreversible VOD. Non-relapse mortality rates at 100 days and 2 years were 6% and 18%, respectively. There was no regimen-related death within the first 100 days of transplant in the 11 patients 60-years and older. However, there was a trend for significantly higher NRM in patients greater than 40 years-old (p=0.07, Figure 3) on univariate analysis. There were 44 deaths: infection (n=7), GVHD (n=10), VOD (n=1), metastatic colon cancer at 10.4 months post SCT (n=1), and relapse (n=25).
Table 3.
Regimen related toxicity in 107 patients
| Toxicity, n | Gr I | Gr II | Gr III | Gr IV | Gr V |
|---|---|---|---|---|---|
| Liver | |||||
| Bilirubin elevation | 7 | 22 | 9 | 0 | 0 |
| Transaminitis | 36 | 23 | 32 | 0 | 0 |
| VOD | 0 | 0 | 5 | 0 | 1 |
| Gastrointestinal tract | |||||
| Diarrhea | 38 | 13 | 5 | 0 | 0 |
| Nausea | 6 | 61 | 1 | 0 | 0 |
| Mucositis | 9 | 70 | 26 | 0 | 0 |
| Urinary tract/kidney | |||||
| Creatinine elevation | 5 | 5 | 1 | 0 | 0 |
| Skin | |||||
| Rash | 11 | 7 | 3 | 1 | 0 |
| Neurologic | |||||
| Headache | 4 | 2 | 0 | 0 | 0 |
Figure 3.
Non-relapse mortality by age at time of transplant
The cumulative incidence of grades II–IV and III–IV acute GVHD were 35% and 10%, respectively; there was no statistically significant difference between patients receiving grafts from matched related- and unrelated donors. The cumulative incidence of chronic GVHD was 29%, with 18% experiencing extensive GVHD. Again, no difference was noted between allotypes (Table 4).
Table 4.
Cumulative incidence of acute and chronic GVHD
| No. (%) | |
|---|---|
| Acute GVHD | |
| Grades II–IV | 19/54 (35) |
| Grades III–IV | 5/50 (10) |
| Chronic | |
| Limited and/or extensive | 8/28 (29) |
| Extensive | 4/22 (18) |
DISCUSSION
The availability of an effective, TBI-free pre-transplant conditioning regimen for ALL patients is necessary to avoid the long-term toxicities documented with the use of radiation, in particular those including impaired growth and cognitive function (27, 28), the increased incidence without plateau in secondary malignancies(29, 30), and the increased incidence of a cardiometabolic trait leading to diabetes and to accelerated atherosclerotic cardiovascular disease (31, 32). The combination of busulfan and cyclophosphamide (Bu-Cy) has historically been an alternative to TBI. We previously explored Bu-Mel as an alternative to BuCy2 (Bu 16 mg/kg and Cy 120 mg/kg) in lymphoid malignancies. However, the double alkylator regimen had similarly high NRM and GVHD rates compared with standard TBI-based regimens (7). The replacement of Cy with the NA Flu has shown good efficacy with reduced toxicity. We demonstrated excellent results with the Flu-Bu combination in patients undergoing transplant for AML(8), and our results were corroborated in prospective, multicenter, randomized studies of Flu-Bu vs. Bu- Cy(33)(13) in AML, in which the patients treated with Flu-Bu had a significantly lower rate of NRM rate compared with Bu-Cy2, with similar overall and disease-free survival in the two groups. The Flu-Bu combination has also been tested in ALL. Santarone and colleagues demonstrated encouraging results in patients with ALL treated with PK- guided i.v. Flu-Bu with an 2-year OS rate of 63% for patients transplanted in first complete remission (CR1)(12); importantly long-term NRM was only 18% at 2 years(12). In an updated report on their series, with 65 patients transplanted with Flu-Bu in CR1 for ALL, the 2-year OS was 65%, and NRM remained low at 14%(14). Based on the single agent activity of clofarabine in refractory or relapsed ALL, we hypothesized that a combination of clofarabine and i.v. busulfan would be particularly effective in in this setting. The in vitro data obtained in our cell line models of ALL supported this, provided that attention was paid to optimizing the sequence and timing of the two agents(34–36) Indeed our early reported results were encouraging(18). Now, with a median follow-up time of 3.3 years among survivors, the 2-year OS and NRM rates for patients transplanted in CR 1 are 68% and 18%, respectively. These results compare favorably with reports of adult patients treated with myelo-ablative, TBI-based regimens in CR1, with reported survival rates of approximately 45%–55% and NRM rates of 20–35% (2, 37,38).
The administration of PK-guided busulfan allows for accurate dose delivery within a tighter range in systemic drug exposure (7), and contributed to the low toxicity profile of the regimen. Transient transaminitis was common, as was also reported by Magenau and colleagues(39). Six patients developed VOD, with one patient having fatal VOD, and this was also comparable to the Flu-Bu experience reported by others (8, 11, 14). All of these patients had extensive therapy prior to HCT with median time from diagnosis to HCT of 17.5 months. Interestingly, none of the 13 refractory patients who were treated with inotuzumab ozogamicin developed VOD. Inotuzumab is a CD22 monoclonal antibody conjugated to the toxin calecheamicin that is very effective in relapsed ALL(40) but associated with hepatic toxicity, and has been noted to be associated with increased VOD rates across our various transplant regimens(41). The 100-day NRM rate of 6% was comparable to what was reported for Flu-Bu(14), and markedly lower than the 19% reported by Doney and colleagues using TBI-based regimens(42).
The cumulative incidence of acute GVHD, grades 2–4 35% and grades 3–4 10% (Table 4), is comparable to reports from radiation-based(37) and non-radiation based regimens(12), and is substantially lower than that observed in our prior combination of Bu with melphalan(43). Reducing the incidence of GVHD, especially in older patients, may significantly benefit the risk of late NRM, since 7 (14%) of our patients died of GVHD- related complications. Notably, the 2-year NRM rate ranged from 12% to 27% based on age greater than or less than 40 years (Figure 2), and again NRM compares favorably to data reported using myelo-ablative TBI-based regimens with 2-year NRM 20–35% based on age (35 years)(2). Importantly, the generally low NRM rate for all patients translates into an equal survival rate for younger vs. older patients (Fig 3). This observation is critical to studies that don’t show benefit to transplant versus chemotherapy due to higher NRM(2, 44).
We(8, 20, 45), and others(10–12) have shown the optimal daily Bu AUC dose to be within the window of 3800 microMol-min (when used with low-dose TBI) to 6080 microMol-min when combined with NA. Indeed, in the study by Kunter et al, patients with daily Bu AUC targeting 5300 microMol-min had a better OS compared to those targeting >6000 microMol-min (p=.04)(14). Thus, we targeted a (median) daily AUC of 5500 microMol-min for our younger patients for maximum disease control, and 4000 microMol-min for our patients greater than 59 years in efforts to minimize toxicity. We have shown reliable engraftment with this lower dose Bu in combination with fludarabine in patients with myeloproliferative disorders receiving allogeneic SCT(46). The rate of 100% engraftment at day 30 was similar between the low and high Bu AUC dose. Five of the 11 older patients remain alive and disease-free, with 3 patients dying from disease progression and one from GVHD; one patient had pre-existing cardiac disease and died from cardiac failure and one died from pre-existing metastatic colon cancer.
Despite a high CR rate following SCT, relapse remains the major cause of treatment failure. This is strikingly evident when patients are evaluated based on the presence of MRD at time of SCT; patients with positive MRD have significantly lower PFS and OS (Figure 2). Thus, the regimen needs to be modified to improve disease control. We have published data in AML that the addition of a second NA results in added synergy and reduced relapse(47). Furthermore, we have published data that the addition of biologic modifiers, such as histone deacetylase inhibitors or DNA methyltransferase inhibitors, add synergy to cytotoxic chemotherapy combinations in lymphoma and leukemia cells lines(36) (48). Importantly, biologic modifiers have non-overlapping toxicities with cytotoxic agents, and thus would not be expected to increase regimen-related toxicity. Therefore, our current trial for advanced ALL patients is a combination of Bu-Clo-Flu + the histone deacetylase inhibitor vorinostat, and early results are encouraging.
In conclusion, the Clo-Bu preparative regimen produces good disease outcomes in patients with high-risk disease and/or older age, and provides patients with an alternative to TBI-based regimens. Importantly, the regimen’s excellent toxicity profile provides a platform to study novel additional approaches to improve disease control which remains the main reason for treatment failure.
Highlights.
Long-term outcomes using a radiation-free SCT conditioning regimen for ALL.
Study results are comparable to radiation-containing regimens.
Myeloablative clofarabine plus busulfan is an effective SCT regimen for adult ALL.
Acknowledgments
Dr. Borje Andersson is a consultant to Otsuka America Pharmaceuticals, Inc. (OAPI).
Funding source: This work supported in part by the Cancer Center Support Grant (NCI Grant P30 CA016672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sutton L, Kuentz M, Cordonnier C, Blaise D, Devergie A, Guyotat D, et al. Allogeneic bone marrow transplantation for adult acute lymphoblastic leukemia in first complete remission: factors predictive of transplant-related mortality and influence of total body irradiation modalities. Bone Marrow Transplant. 1993 Dec;12(6):583–9. Epub 1993/12/01. [PubMed] [Google Scholar]
- 2.Goldstone AH, Richards SM, Lazarus HM, Tallman MS, Buck G, Fielding AK, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008 Feb 15;111(4):1827–33. doi: 10.1182/blood-2007-10-116582. Epub 2007/12/01. [DOI] [PubMed] [Google Scholar]
- 3.Oliansky DM, Larson RA, Weisdorf D, Dillon H, Ratko TA, Wall D, et al. The Role of Cytotoxic Therapy with Hematopoietic Stem Cell Transplantation in the Treatment of Adult Acute Lymphoblastic Leukemia: Update of the 2006 Evidence-Based Review. Biol Blood Marrow Transplant. Jul 29; doi: 10.1016/j.bbmt.2011.09.002. Epub 2011/08/02. [DOI] [PubMed] [Google Scholar]
- 4.Mohty M, Labopin M, Tabrizzi R, Theorin N, Fauser AA, Rambaldi A, et al. Reduced intensity conditioning allogeneic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Haematologica. 2008 Feb;93(2):303–6. doi: 10.3324/haematol.11960. Epub 2008/02/05. [DOI] [PubMed] [Google Scholar]
- 5.Hamaki T, Kami M, Kanda Y, Yuji K, Inamoto Y, Kishi Y, et al. Reduced- intensity stem-cell transplantation for adult acute lymphoblastic leukemia: a retrospective study of 33 patients. Bone Marrow Transplant. 2005 Mar;35(6):549–56. doi: 10.1038/sj.bmt.1704776. Epub 2005/03/10. [DOI] [PubMed] [Google Scholar]
- 6.Martino R, Giralt S, Caballero MD, Mackinnon S, Corradini P, Fernandez-Aviles F, et al. Allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning in acute lymphoblastic leukemia: a feasibility study. Haematologica. 2003 May;88(5):555–60. Epub 2003/05/15. [PubMed] [Google Scholar]
- 7.Kebriaei P, Madden T, Kazerooni R, Wang X, Thall PF, Ledesma C, et al. Intravenous busulfan plus melphalan is a highly effective, well-tolerated preparative regimen for autologous stem cell transplantation in patients with advanced lymphoid malignancies. Biol Blood Marrow Transplant. 2011 Mar;17(3):412–20. doi: 10.1016/j.bbmt.2010.07.016. Epub 2010/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004 Aug 1;104(3):857–64. doi: 10.1182/blood-2004-02-0414. Epub 2004/04/10. [DOI] [PubMed] [Google Scholar]
- 9.Russell JA, Duan Q, Chaudhry MA, Savoie ML, Balogh A, Turner AR, et al. Transplantation from matched siblings using once-daily intravenous busulfan/fludarabine with thymoglobulin: a myeloablative regimen with low nonrelapse mortality in all but older patients with high-risk disease. Biol Blood Marrow Transplant. 2008 Aug;14(8):888–95. doi: 10.1016/j.bbmt.2008.05.010. Epub 2008/07/22. [DOI] [PubMed] [Google Scholar]
- 10.Russell JA, Savoie ML, Balogh A, Turner AR, Larratt L, Chaudhry MA, et al. Allogeneic transplantation for adult acute leukemia in first and second remission with a novel regimen incorporating daily intravenous busulfan, fludarabine, 400 CGY total-body irradiation, and thymoglobulin. Biol Blood Marrow Transplant. 2007 Jul;13(7):814– 21. doi: 10.1016/j.bbmt.2007.03.003. Epub 2007/06/21. [DOI] [PubMed] [Google Scholar]
- 11.Russell JA, Tran HT, Quinlan D, Chaudhry A, Duggan P, Brown C, et al. Once-daily intravenous busulfan given with fludarabine as conditioning for allogeneic stem cell transplantation: study of pharmacokinetics and early clinical outcomes. Biol Blood Marrow Transplant. 2002;8(9):468–76. doi: 10.1053/bbmt.2002.v8.pm12374451. Epub 2002/10/11. [DOI] [PubMed] [Google Scholar]
- 12.Santarone S, Pidala J, Di Nicola M, Field T, Alsina M, Ayala E, et al. Fludarabine and pharmacokinetic-targeted busulfan before allografting for adults with acute lymphoid leukemia. Biol Blood Marrow Transplant. 2011 Oct;17(10):1505–11. doi: 10.1016/j.bbmt.2011.02.011. Epub 2011/03/10. [DOI] [PubMed] [Google Scholar]
- 13.Liu H, Zhai X, Song Z, Sun J, Xiao Y, Nie D, et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: a prospective and multicenter study. Journal of hematology & oncology. 2013;6:15. doi: 10.1186/1756-8722-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunter G, Perkins JB, Pidala J, Nishihori T, Kharfan-Dabaja MA, Field T, et al. Pharmacokinetically-targeted BU and fludarabine as conditioning before allogeneic hematopoietic cell transplantation for adults with ALL in first remission. Bone Marrow Transplant. 2014 Jan;49(1):11–6. doi: 10.1038/bmt.2013.121. [DOI] [PubMed] [Google Scholar]
- 15.Bartelink IH, van Reij EM, Gerhardt CE, van Maarseveen EM, de Wildt A, Versluys B, et al. Fludarabine and exposure-targeted busulfan compares favorably with busulfan/cyclophosphamide-based regimens in pediatric hematopoietic cell transplantation: maintaining efficacy with less toxicity. Biol Blood Marrow Transplant. 2014 Mar;20(3):345–53. doi: 10.1016/j.bbmt.2013.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Jeha S, Gandhi V, Chan KW, McDonald L, Ramirez I, Madden R, et al. Clofarabine, a novel nucleoside analog, is active in pediatric patients with advanced leukemia. Blood. 2004 Feb 1;103(3):784–9. doi: 10.1182/blood-2003-06-2122. Epub 2003/10/11. [DOI] [PubMed] [Google Scholar]
- 17.Jeha S, Gaynon PS, Razzouk BI, Franklin J, Kadota R, Shen V, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006 Apr 20;24(12):1917–23. doi: 10.1200/JCO.2005.03.8554. Epub 2006/04/20. [DOI] [PubMed] [Google Scholar]
- 18.Kebriaei P, Basset R, Ledesma C, Ciurea S, Parmar S, Shpall EJ, et al. Clofarabine combined with busulfan provides excellent disease control in adult patients with acute lymphoblastic leukemia undergoing allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012 Dec;18(12):1819–26. doi: 10.1016/j.bbmt.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madden T, de Lima M, Thapar N, Nguyen J, Roberson S, Couriel D, et al. Pharmacokinetics of once-daily IV busulfan as part of pretransplantation preparative regimens: a comparison with an every 6-hour dosing schedule. Biol Blood Marrow Transplant. 2007 Jan;13(1):56–64. doi: 10.1016/j.bbmt.2006.08.037. Epub 2007/01/16. [DOI] [PubMed] [Google Scholar]
- 20.Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, Chow DS, et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant. 2000;6(5A):548–54. doi: 10.1016/s1083-8791(00)70064-4. Epub 2000/11/09. [DOI] [PubMed] [Google Scholar]
- 21.Kebriaei P, Saliba R, Rondon G, Chiattone A, Luthra R, Anderlini P, et al. Long-Term Follow-up of Allogeneic Hematopoietic Stem Cell Transplantation for Patients with Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia: Impact of Tyrosine Kinase Inhibitors on Treatment Outcomes. Biol Blood Marrow Transplant. 2011 Aug 23; doi: 10.1016/j.bbmt.2011.08.011. Epub 2011/08/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabbour E, Thomas D, Cortes J, Kantarjian HM, O'Brien S. Central nervous system prophylaxis in adults with acute lymphoblastic leukemia: current and emerging therapies. Cancer. 2010 May 15;116(10):2290–300. doi: 10.1002/cncr.25008. [DOI] [PubMed] [Google Scholar]
- 23.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995 Jun;15(6):825–8. Epub 1995/06/01. [PubMed] [Google Scholar]
- 24.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401. e1. doi: 10.1016/j.bbmt.2014.12.001. Epub 2014/12/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thall PF, Wathen JK. Covariate-adjusted adaptive randomization in a sarcoma trial with multi-stage treatments. Stat Med. 2005 Jul 15;24(13):1947–64. doi: 10.1002/sim.2077. Epub 2005/04/05. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]
- 27.Bushhouse S, Ramsay NK, Pescovitz OH, Kim T, Robison LL. Growth in children following irradiation for bone marrow transplantation. Am J Pediatr Hematol Oncol. 1989 Summer;11(2):134–40. Epub 1989/01/01. [PubMed] [Google Scholar]
- 28.Sanders JE. The impact of marrow transplant preparative regimens on subsequent growth and development. The Seattle Marrow Transplant Team. Seminars in hematology. 1991 Jul;28(3):244–9. Epub 1991/07/01. [PubMed] [Google Scholar]
- 29.Mody R, Li S, Dover DC, Sallan S, Leisenring W, Oeffinger KC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood. 2008 Jun 15;111(12):5515–23. doi: 10.1182/blood-2007-10-117150. Epub 2008/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meadows AT, Friedman DL, Neglia JP, Mertens AC, Donaldson SS, Stovall M, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009 May 10;27(14):2356–62. doi: 10.1200/JCO.2008.21.1920. Epub 2009/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chow EJ, Simmons JH, Roth CL, Baker KS, Hoffmeister PA, Sanders JE, et al. Increased cardiometabolic traits in pediatric survivors of acute lymphoblastic leukemia treated with total body irradiation. Biol Blood Marrow Transplant. 2010 Dec;16(12):1674–81. doi: 10.1016/j.bbmt.2010.05.016. Epub 2010/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker KS, Ness KK, Steinberger J, Carter A, Francisco L, Burns LJ, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007 Feb 15;109(4):1765–72. doi: 10.1182/blood-2006-05-022335. Epub 2006/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rambaldi A, Grassi A, Masciulli A, Boschini C, Mico MC, Busca A, et al. Busulfan plus cyclophosphamide versus busulfan plus fludarabine as a preparative regimen for allogeneic haemopoietic stem-cell transplantation in patients with acute myeloid leukaemia: an open-label, multicentre, randomised, phase 3 trial. The lancet oncology. 2015 Nov;16(15):1525–36. doi: 10.1016/S1470-2045(15)00200-4. [DOI] [PubMed] [Google Scholar]
- 34.Valdez BC, Andersson BS. Interstrand crosslink inducing agents in pretransplant conditioning therapy for hematologic malignancies. Environmental and molecular mutagenesis. 2010 Jul;51(6):659–68. doi: 10.1002/em.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valdez BC, Li Y, Murray D, Champlin RE, Andersson BS. The synergistic cytotoxicity of clofarabine, fludarabine and busulfan in AML cells involves ATM pathway activation and chromatin remodeling. Biochemical pharmacology. 2011 Jan 15;81(2):222–32. doi: 10.1016/j.bcp.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valdez BC, Murray D, Nieto Y, Li Y, Wang G, Champlin RE, et al. Synergistic cytotoxicity of the DNA alkylating agent busulfan, nucleoside analogs and suberoylanilide hydroxamic acid in lymphoma cell lines. Leuk Lymphoma. 2012 May;53(5):973–81. doi: 10.3109/10428194.2011.634043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marks DI, Wang T, Perez WS, Antin JH, Copelan E, Gale RP, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010 Jul 22;116(3):366–74. doi: 10.1182/blood-2010-01-264077. Epub 2010/04/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004 Oct 15;22(20):4075–86. doi: 10.1200/JCO.2004.10.050. Epub 2004/09/09. [DOI] [PubMed] [Google Scholar]
- 39.Magenau J, Tobai H, Pawarode A, Braun T, Peres E, Reddy P, et al. Clofarabine and busulfan conditioning facilitates engraftment and provides significant antitumor activity in nonremission hematologic malignancies. Blood. 2011 Oct 13;118(15):4258–64. doi: 10.1182/blood-2011-06-358010. Epub 2011/08/16. [DOI] [PubMed] [Google Scholar]
- 40.Kantarjian H, Thomas D, Jorgensen J, Jabbour E, Kebriaei P, Rytting M, et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. The lancet oncology. 2012 Apr;13(4):403–11. doi: 10.1016/S1470-2045(11)70386-2. Epub 2012/02/24. [DOI] [PubMed] [Google Scholar]
- 41.Kebriaei P, Wilhelm K, Ravandi F, Brandt M, de Lima M, Ciurea S, et al. Feasibility of allografting in patients with advanced acute lymphoblastic leukemia after salvage therapy with inotuzumab ozogamicin. Clinical lymphoma, myeloma & leukemia. 2013 Jun;13(3):296–301. doi: 10.1016/j.clml.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doney K, Gooley TA, Deeg HJ, Flowers ME, Storb R, Appelbaum FR. Allogeneic hematopoietic cell transplantation with full-intensity conditioning for adult acute lymphoblastic leukemia: results from a single center, 1998–2006. Biol Blood Marrow Transplant. 2011 Aug;17(8):1187–95. doi: 10.1016/j.bbmt.2010.12.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kebriaei P, Madden T, Wang X, Thall PF, Ledesma C, de Lima M, et al. Intravenous BU plus Mel: an effective, chemotherapy-only transplant conditioning regimen in patients with ALL. Bone Marrow Transplant. 2013 Jan;48(1):26–31. doi: 10.1038/bmt.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolach O, Stevenson KE, Wadleigh M, DeAngelo DJ, Steensma DP, Ballen KK, et al. Allogeneic transplantation is not superior to chemotherapy in most patients over 40 years of age with Philadelphia-negative acute lymphoblastic leukemia in first remission. American journal of hematology. 2016 May 6; doi: 10.1002/ajh.24410. [DOI] [PubMed] [Google Scholar]
- 45.Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.v. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8(9):477–85. doi: 10.1053/bbmt.2002.v8.pm12374452. Epub 2002/10/11. [DOI] [PubMed] [Google Scholar]
- 46.Popat BSA Uday, Bassett Roland, Ciurea Stefan, Rondon Gabriela, Alousi Amin M, Anderlini Paolo, Hosing Chitra, Jones Roy, Kebriaei Partow, Nieto Yago, Khouri Issa, Shpall Elizabeth J, Verstovsek Srdan, Qazilbash Muzaffar, de Lima Marcos J, Champlin Richard., editors. American Society of Hematology. San Diego, California: 2011. Allogeneic Hematopoietic Stem Cell Transplantation for Myelofibrosis: PK Guided IV Busulfan Dose Intensity Results in Improved Event Free Survival. [Google Scholar]
- 47.Andersson BS, Valdez BC, de Lima M, Wang X, Thall PF, Worth LL, et al. Clofarabine +/− fludarabine with once daily i.v. busulfan as pretransplant conditioning therapy for advanced myeloid leukemia and MDS. Biol Blood Marrow Transplant. 2011 Jun;17(6):893–900. doi: 10.1016/j.bbmt.2010.09.022. Epub 2010/10/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valdez BC, Li Y, Murray D, Corn P, Champlin RE, Andersson BS. 5-Aza-2'-deoxycytidine sensitizes busulfan-resistant myeloid leukemia cells by regulating expression of genes involved in cell cycle checkpoint and apoptosis. Leukemia research. 2010 Mar;34(3):364–72. doi: 10.1016/j.leukres.2009.08.014. Epub 2009/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]