Regulator of Calcineurin 1 (RCAN1) is an endogenous inhibitor of the Ca2+-activated protein phosphatase calcineurin. It has been known for some time that deletion of RCAN1 in the mouse exacerbates ischemia/reperfusion-induced infarction in heart1 and brain.2 In this issue of Circulation Research, Parra et al3 elucidate an underlying mechanism.
Calcineurin is a widely expressed serine/threonine phosphatase consisting of one catalytic and one regulatory subunit.4 This enzyme is activated by binding of Ca2+ to EF-hand motifs in the regulatory subunit and by calmodulin binding to the catalytic domain. Calcineurin exerts pleiotropic effects including T-lymphocyte activation, neurite outgrowth, heart valve formation, skeletal myocyte differentiation, and cardiac and skeletal muscle hypertrophy. These effects are attributable primarily to calcineurin-mediated dephosphorylation of transcription factors (e.g. Nuclear Factor of Activated T-cells (NFAT)5, 6) resulting in their nuclear translocation and activation of various gene expression programs. Calcineurin, however, also acts on other substrates including structural proteins, receptors, channels, and signaling molecules.7
RCAN1, encoded by a gene in the Down syndrome critical region 1 on human chromosome 21, is enriched in striated muscle and brain.4 RCAN1 inhibits calcineurin through direct binding to its catalytic subunit. As might be expected from the actions of calcineurin, cardiomyocyte-specific overexpression of RCAN1 in mice inhibits cardiac hypertrophy elicited by pathological or physiological stimuli.8 Less expected are effects of RCAN1 on ischemia/reperfusion injury. Cardiomyocyte-specific transgenic overexpression ameliorates myocardial damage in vivo, while the opposite is observed in mice with generalized RCAN1 knockout.1 Moreover, pharmacological inhibition of calcineurin reverses this RCAN1-deficient phenotype showing that these effects of RCAN1 depletion are mediated by unleashing of calcineurin.
So, what mechanisms connect RCAN1-calcineurin signaling with myocardial ischemia/reperfusion injury (Figure)? Parra et al postulated that mitochondria may be involved. One molecule known to link calcineurin with mitochondria is Dynamin Related Protein (DRP1), a GTPase involved in mitochondrial fission. Calcineurin-mediated dephosphorylation of serine 637 in human DRP1 triggers DRP1 translocation from cytosol to mitochondria, where it promotes outer mitochondrial membrane constriction events involved in mitochondrial fission.9 In fact, the investigators observed that RCAN1-depletion induces mitochondrial fragmentation in a variety of cell types including mouse embryonic fibroblasts and primary neonatal and adult rat cardiomyocytes, an effect that was reversed by pharmacological inhibition of calcineurin or DRP1. Moreover, DRP1-mediated mitochondrial fission may not be the only mechanism responsible for mitochondrial fragmentation as RCAN1-knockdown also results in selective decreases in Optic Atrophy 1 (OPA1) and, under some experimental conditions, Mitofusin 2 (MFN2) – proteins that mediate mitochondrial fusion. While the mechanisms linking RCAN1 with OPA1 and MFN2 are not known, these observations suggest that RCAN1 maintains mitochondrial connectivity through coordinate inhibition of fission and promotion of fusion.
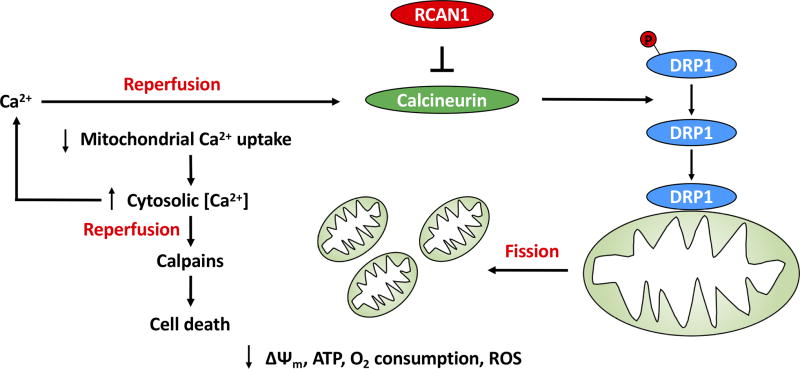
Figure. RCAN1 modulates tissue damage during myocardial ischemia-reperfusion.
Depletion of RCAN1 disinhibits the Ca2+-activated protein phosphatase calcineurin resulting in calcineurin-mediated dephosphorylation of serine 637 in human DRP1. This stimulates the translocation of DRP1 from cytosol to the outer mitochondrial membrane where it promotes mitochondrial fission. Depletion of RCAN1 may also promote mitochondrial fragmentation through decreases in mitochondrial fusion proteins OPA1 and MFN2 (not shown). Fragmented mitochondria manifest decreased Ca2+ uptake, which may reflect their loss of electrical potential difference across the inner mitochondrial membrane (Δψm) or perhaps a more direct action of calcineurin on the mitochondrial Ca2+ import machinery. Impaired uptake of Ca2+ results in elevation of cytosolic Ca2+ concentrations, thereby predisposing to the activation of calpains, Ca2+-activated proteases. Calpains cleave signaling and structural proteins to induce cell death. Activation of calcineurin and calpains in this schema takes place during reperfusion because the activities of these enzymes are inhibited in the acidic environment of ischemia.
The relationship between mitochondrial dynamics (fission and fusion) and cell death is poorly understood. BAX is a BCL-2 protein that mediates permeabilization of the outer mitochondrial membrane (OMM) and cytochrome c release during apoptosis. Although complex interactions have been reported between DRP1 and BAX,10 the role of mitochondrial fission in apoptosis remains unclear.11 An additional wrinkle is that other studies suggest that promotion of mitochondrial connectivity (whether by increasing fusion or decreasing fission) sensitizes cells to Ca2+-induced opening of the permeability transition pore (mPTP) on the inner mitochondrial membrane (IMM) and necrotic cell death12 - arguably of more relevance in myocardial ischemia-reperfusion injury than apoptosis.
To gain insights beyond mitochondrial dynamics, Parra et al chose to examine the role of Ca2+ itself in the death process and made an interesting observation: RCAN1-deficient cardiomyocytes exhibit a deficit in mitochondrial Ca2+ uptake and concomitant elevation of cytosolic Ca2+ concentrations. The mechanism by which RCAN1 depletion dampens mitochondrial Ca2+ uptake is not clear, but may reflect observed decreases in polarization across the IMM (Δψm), a driving force in the movement of Ca2+ into the mitochondrial matrix through the mitochondrial calcium uniporter.13 This begs the question, however, as to why Δψm is decreased in RCAN1-depleted cardiomyocytes. One possibility is that decreases in Δψm reflect merely the poor health of the fragmented mitochondria, which also exhibit decreased ATP levels. Another possibility is that RCAN1 deficiency somehow causes a primary defect in mitochondrial Ca2+ uptake which, in turn, results in decreased catabolism of nutrients. In fact, lower rates of oxygen consumption and ROS production were observed in RCAN1-depleted cells. Further study will be needed to test whether the RCAN1-calcineurin axis directly regulates mitochondrial Ca2+ uptake.
While decreased mitochondrial Ca2+ uptake could compromise substrate oxidation in mitochondria, it would be expected to be protective against Ca2+-induced opening of the mPTP and necrosis. What then would account for the increased sensitivity to cell death in RCAN1-deficient cardiomyocytes? Given the relative increases in cytosolic Ca2+, the investigators considered Ca2+-dependent mechanisms that may be operating in this cellular compartment. In fact, they observed activation of calpains, Ca2+-stimulated proteases whose substrates include signaling and structural molecules involved in apoptotic and necrotic cell death14, 15. Importantly, combined knockdown of calpains 1 or 2 or pharmacological inhibition of these proteases reversed the increased sensitization to simulated ischemia-reperfusion-induced cell death resulting from RCAN1 knockdown.
Thus, in essence, what RCAN1 deficiency appears to be doing is to shift the dominant cell death mechanism during ischemia-reperfusion away from the mPTP and towards cytosolic calpain activation. The augmentation of cell killing resulting from this shift may reflects the fact that high Ca2+ concentrations are less well tolerated in the cytosol than in the mitochondrial matrix.
The investigators also explored the effects of increasing RCAN1 levels. High levels of RCAN1 overexpression from adenoviral-mediated transduction of cardiomyocytes resulted in hyper-connected mitochondria with augmented rates of oxygen consumption and ROS levels. Moreover, in keeping with the cardioprotection afforded by transgenic overexpression in cardiomyocytes in vivo, RCAN1 overexpression ameliorated cardiomyocyte death elicited by simulated ischemia-reperfusion. Similar findings were obtained with more mild overexpression of RCAN1 as observed in cardiomyocytes derived from trisomic 21 human induced pluripotent stem (iPS) cells compared with cardiomyocytes derived the same iPS cells in the disomic state (the extra chromosome 21 being spontaneously lost with passage in culture). Importantly, the phenotype in the trisomic cells is attributable specifically to RCAN1 – rather than the extra copy of multiple other genes on chromosome 21 – as it is reversed by RCAN1 knockdown. Moreover, these findings suggest that excess RCAN1 is an important factor in the high degrees of oxidative stress present in the cells of Down syndrome patients.
In summary, this study establishes the importance of RCAN1 in maintaining a connected mitochondrial network. In addition, it delineates a critical mechanism by which the RCAN1-calcineurin axis regulates the susceptibility of the myocardium to damage from ischemia-reperfusion (Figure). Future work is needed to elucidate the precise molecular connections by which calcineurin impacts mitochondrial Ca2+ uptake. In addition, the existence of this mechanism does not preclude the possibility that other calcineurin substrates contribute to the set-point of tissue damage resulting from ischemia-reperfusion. Finally, the possibility that this mechanism also operates during ischemia-reperfusion in the brain merits further investigation.
Acknowledgments
We thank Drs. John Elrod and Wendy McKimpson for critical reading of the manuscript, and Dulguun Amgalan for assistance with figures.
Sources of Funding
RNK is supported by grants from the National Institutes of Health (R01HL128071, R01HL130861, R01HL138475, R01CA170911), DOD (PR151134P1), AHA (15CSA26240000), Fondation Leducq (RA15CVD04), and the Dr. Gerald and Myra Dorros Chair in Cardiovascular Disease. We thank the Wilf Family for their generous support.
Footnotes
Disclosures
None
References
- 1.Rotter D, Grinsfelder DB, Parra V, Pedrozo Z, Singh S, Sachan N, Rothermel BA. Calcineurin and its regulator, RCAN1, confer time-of-day changes in susceptibility of the heart to ischemia/reperfusion. J Mol Cell Cardiol. 2014;74:103–11. doi: 10.1016/j.yjmcc.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sobrado M, Ramirez BG, Neria F, Lizasoain I, Arbones ML, Minami T, Redondo JM, Moro MA, Cano E. Regulator of calcineurin 1 (Rcan1) has a protective role in brain ischemia/reperfusion injury. J Neuroinflammation. 2012;9:48. doi: 10.1186/1742-2094-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parra VM, Altamirano F, Hernandez-Fuentes CP, Tong D, Kyrychenko V, Rotter D, Pedrozo ZR, Hill JA, Eisner V, Lavandero S, Schneider JW, Rothermel BA. Down Syndrome Critical Region 1 Gene, Rcan1, Helps Maintain a More Fused Mitochondrial Network. Circ Res. 2018 doi: 10.1161/CIRCRESAHA.117.311522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parra V, Rothermel BA. Calcineurin signaling in the heart: The importance of time and place. J Mol Cell Cardiol. 2017;103:121–136. doi: 10.1016/j.yjmcc.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crabtree GR. Calcium, calcineurin, and the control of transcription. J Biol Chem. 2001;276:2313–6. doi: 10.1074/jbc.R000024200. [DOI] [PubMed] [Google Scholar]
- 6.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–28. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li H, Rao A, Hogan PG. Interaction of calcineurin with substrates and targeting proteins. Trends Cell Biol. 2011;21:91–103. doi: 10.1016/j.tcb.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothermel BA, McKinsey TA, Vega RB, Nicol RL, Mammen P, Yang J, Antos CL, Shelton JM, Bassel-Duby R, Olson EN, Williams RS. Myocyte-enriched calcineurin-interacting protein, MCIP1, inhibits cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2001;98:3328–33. doi: 10.1073/pnas.041614798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008;105:15803–8. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, Martinou JC. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whelan RS, Konstantinidis K, Wei AC, Chen Y, Reyna DE, Jha S, Yang Y, Calvert JW, Lindsten T, Thompson CB, Crow MT, Gavathiotis E, Dorn GW, 2nd, O'Rourke B, Kitsis RN. Bax regulates primary necrosis through mitochondrial dynamics. Proc Natl Acad Sci U S A. 2012;109:6566–71. doi: 10.1073/pnas.1201608109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mammucari C, Gherardi G, Rizzuto R. Structure, Activity Regulation, and Role of the Mitochondrial Calcium Uniporter in Health and Disease. Front Oncol. 2017;7:139. doi: 10.3389/fonc.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA. Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem. 2001;276:30724–8. doi: 10.1074/jbc.M103701200. [DOI] [PubMed] [Google Scholar]
- 15.Letavernier E, Zafrani L, Perez J, Letavernier B, Haymann JP, Baud L. The role of calpains in myocardial remodelling and heart failure. Cardiovasc Res. 2012;96:38–45. doi: 10.1093/cvr/cvs099. [DOI] [PubMed] [Google Scholar]