Abstract
Arabidopsis inflorescence stems develop a vascular pattern similar to that found in most dicots. The arrangement of vascular tissues within the bundle is collateral, and vascular bundles in the stele are arranged in a ring. Although auxin has been shown to be an inducer of vascular differentiation, little is known about the molecular mechanisms controlling vascular pattern formation. By screening ethyl methanesufonate-mutagenized populations of Arabidopsis, we have isolated an avb1 (amphivasal vascular bundle) mutant with a novel vascular pattern. Unlike the collateral vascular bundles seen in the wild-type stems, the vascular bundles in the avb1 stems were similar to amphivasal bundles, i.e. the xylem completely surrounded the phloem. Furthermore, branching vascular bundles in the avb1 stems abnormally penetrated into the pith, which resulted in a disruption in the ring-like arrangement of vascular bundles in the stele. The avb1 mutation did not affect leaf venation pattern and root vascular organization. Auxin polar transport assay indicated that the avb1 mutation did not disrupt the auxin polar transport activity in inflorescence stems. The avb1 mutation also exhibited pleiotropic phenotypes, including curled stems and extra cauline branches. Genetic analysis indicated that the avb1 mutation was monogenic and partially dominant. The avb1 locus was mapped to a region between markers mi69 and ASB2, which is covered by a yeast artificial chromosome clone, CIC9E2, on chromosome 5. Isolation of the avb1 mutant provides a novel means to study the evolutionary mechanisms controlling the arrangement of vascular tissues within the bundle, as well as the mechanisms controlling the arrangement of vascular bundles in the stele.
Vascular plants appeared on the land in the Silurian period (438–408 million years ago) and they are dominant on the earth. One of the key events for their successful emergence from aquatic environments was the evolution of vascular tissues, which solved the problem of water and food transport on the land (Raven et al., 1992). Although vascular tissues consist of two types of transport tissues, xylem and phloem, diverse arrangements of vascular tissues within the bundles and of vascular bundles in the stele evolved in vascular plants. The occurrence of diverse vascular patterns in vascular plants offers an excellent opportunity to study evolutionary mechanisms controlling pattern formation.
In primary stems vascular patterns are organized at two levels (Esau, 1977; Mauseth, 1988; Fahn, 1990). First, the primary vascular tissues within the bundles are arranged in an orderly pattern. The common arrangement of vascular tissues is collateral, i.e. the primary xylem is located on the inner side of the bundle, and the primary phloem is located on the outer side of the bundle. Some plants develop bicollateral bundles with additional primary phloem interior to the xylem. Many monocots develop amphivasal vascular bundles, i.e. the xylem surrounds the phloem. In contrast to the amphivasal bundles, amphicribral bundles have phloem surrounding xylem.
Second, vascular bundles in the stele of stems are arranged in an orderly pattern. The protostele is arranged in such a way that the xylem is located as a solid mass in the center, and the phloem surrounds this solid mass. However, in siphonosteles, the solid mass of xylem is separated by parenchyma cells. Vascular bundles are organized as a ring in the stems of most gymnosperms and dicots or distributed throughout the ground tissue in the stems of most monocots.
Although the molecular mechanisms determining vascular patterns are largely unknown, the aspects of vascular differentiation have been intensively studied using physiological, biochemical, and molecular approaches. It has been shown that auxin is the major controlling factor for induction of vascular differentiation in both in vitro and in vivo conditions (Aloni, 1987). For in vitro conditions cytokinin and/or Suc, together with auxin, are required for vascular induction. One of the best examples in the study of in vitro xylogenesis is the tracheary-element induction from isolated zinnia mesophyll cells (Fukuda, 1996). Mesophyll cells isolated from zinnia leaves can be induced to transdifferentiate into tracheary elements in the presence of auxin and cytokinin, indicating that these hormones control the formation of xylem cells.
Because of the lack of tools to study vascular patterning, no regulatory genes controlling the organization of vascular tissues have been identified. Nevertheless, a number of genes associated with xylem and phloem differentiation have been characterized. Through differential screening and subtractive hybridization, a number of cDNAs whose mRNAs were differentially expressed during xylogenesis were isolated from in vitro tracheary elements induced from isolated zinnia mesophyll cells (Demura and Fukuda, 1993; Ye and Varner, 1993; Fukuda, 1996). The corresponding genes were shown to be specifically induced during xylogenesis in the plant (Demura and Fukuda, 1994; Ye and Varner, 1994). In terms of phloem differentiation, several genes have been characterized, such as those encoding β-amylase (Wang et al., 1995), P-protein (Tóth et al., 1994) and Arabidopsis H+-ATPase isoform 3 (Dewitt and Sussman, 1995). The proteins are specifically present in either sieve elements (β-amylase and P-protein) or companion cells (Arabidopsis H+-ATPase isoform 3). However, these xylem- or phloem-associated genes appear to be involved in structural formation during differentiation. It seems difficult to find genes determining the arrangement of vascular tissues by biochemical and molecular approaches. Recently, Baima et al. (1995) and Tornero et al. (1996) showed that some homeobox genes were preferentially expressed in vascular tissues of Arabidopsis but their functions are not yet known.
Considering the existence of naturally diverse vascular patterns among plant groups, we can study the genetic control of vascular pattern formation by mutational analysis. A number of mutants affecting formation of midvein or minor veins in leaves have been isolated in grasses (for review, see Nelson and Dengler, 1997). A wilty mutant of maize was found to be defective in metaxylem differentiation (Postlethwait and Nelson, 1957). In Arabidopsis several mutants with alteration of vascular differentiation (Scheres et al., 1995; Przemeck et al., 1996) or leaf venation (Carland and McHale, 1996; Conway and Poethig, 1997) have been characterized. In the monopteros mutant, the alignment and interconnection of tracheary elements were affected without an alteration in the arrangement of vascular tissues (Przemeck et al., 1996). The MONOPTEROS gene has been cloned and shown to encode a member of the Aux/IAA protein family (Guilfoyle, 1998; Hardtke and Berleth, 1998). Mutation of the LOP1 locus caused the formation of a bifurcated and twisted midvein (Carland and McHale, 1996). The xtc1, xtc2, and amp1 mutants had a simple leaf-venation pattern similar to that of cotyledon due to the transformation of leaves into cotyledons (Conway and Poethig, 1997).
In summary, the vascular mutants isolated so far affect vascular differentiation or leaf venation. To our knowledge, no mutants with a global alteration in vascular patterns of Arabidopsis stems have been reported. To better understand the mechanisms controlling vascular patterns, it is important to isolate mutants with a global alteration in vascular patterns.
Intrigued by the diverse patterns of vascular tissues in vascular plants, we have initiated a genetic approach to studying vascular pattern formation in the model dicot Arabidopsis. By screening ethyl methanesulfonate-mutagenized populations of Arabidopsis, we have successfully isolated a novel mutant with a dramatic change of vascular patterns in stems. The most noticeable change in the avb1 mutant was the transformation of the collateral vascular bundles into amphivasal vascular bundles in the stems. Also altered in the avb1 mutant was the arrangement of vascular bundles in the stele. Isolation of the avb1 mutant provided a tool for investigating the evolutionary mechanisms of vascular pattern formation.
MATERIALS AND METHODS
Mutant Screening
M2 plants of ethyl methanesulfonate-mutagenized populations of Arabidopsis ecotype Columbia (Lehle Seeds, Round Rock, TX) were grown in the greenhouse. Inflorescence stems of 7- to 8-week-old plants were free-hand sectioned with a razor blade. Sections were stained with phloroglucinol-HCl or toluidine blue and observed under a dissection microscope. Plants showing altered vascular patterns were saved and allowed to grow out new branches for seed production. Putative mutants were backcrossed with wild-type Columbia three times to reduce unlinked background mutations.
Light Microscopy
Arabidopsis stem segments were fixed overnight in 4% paraformaldehyde at 4°C. After dehydration through a gradient series of ethanol, the stem segments were embedded in paraffin. The embedded segments were then sectioned with a microtome and thin sections were transferred onto poly-l-Lys-coated slides. After deparaffinizing in xylene and rehydration through a gradient series of ethonal, sections were stained with toluidine blue and observed under a compound microscope with bright-field illumination.
Evans Blue Dye Transport
Inflorescence stems were cut under water and the lower ends of stems were then submerged in a 0.1% Evans blue dye solution. After 10 min, serial sections were prepared from the upper part of the stems (not submerged in the solution). The sections were observed immediately under a dissection microscope with dark-field illumination or stained with phloroglucinol-HCl before observation.
Auxin Polar Transport Assay
Inflorescence stems of 6-week-old plants were used for the auxin polar transport activity assay as described previously by Okada et al. (1991). Stem segments were cut into 2.5-cm lengths and the upper ends were submerged in a Murashige and Skoog medium containing 80 nCi mL−1 [1-14C]IAA (American Radiolabeled Chemicals, St. Louis, MO). After 6- or 20-h incubations, 0.5-cm-long parts (not submerged in the solution) were cut from the opposite ends of segments. The cut parts were incubated overnight in a scintillation cocktail and shaken, and the radioactivity was measured in a scintillation counter.
Genetic Analysis
The mutant was backcrossed with wild-type Columbia. Stem sections of the F1 plants were stained with toluidine blue to examine vascular patterns. After the F1 plants were selfed, the F2 plants were analyzed for segregation of the mutation by examining the vascular patterns of stem sections.
For genetic mapping, the mutant was crossed with Arabidopsis ecotype Langsberg erecta. The resulting F1 plants were selfed and the F2 plants were analyzed for segregation of the avb1 mutation. Leaves from the F2 plants with the homozygous avb1 phenotype were collected for isolation of genomic DNA, as described previously by Cocciolone and Cone (1993). Linkage of the mutation with markers on individual chromosomes was determined using CAPS markers developed by Konieczny and Ausubel (1993). Conditions for PCR reactions and restriction enzyme digestions were essentially the same as described previously (Konieczny and Ausubel, 1993). The information on CAPS markers ASB2 (Niyogi et al., 1993), DFR, and LFY3 (Konieczny and Ausubel, 1993) was from the Arabidopsis database. The 91H23 and mi69 markers were developed by Zhong et al. (1997). CAPS markers 34D1, 138D19, MBG8, and MDF20 were developed during our mapping work.
The 34D1 and 138D19 primer sequences originated from the Arabidopsis expressed-sequence tag clones EST34D1 and EST138D19, respectively. The MBG8 and MDF20 primer sequences were derived from DNA sequences of BAC clones MBG8 and MDF20, respectively (website: http://www.kazusa.or.jp/arabi/). The 34D1 primers (5′-CAACAAGCGAAACTAGGGT-3′ and 5′-TTCAAATCCGACTTCGACAT-3′) amplified a 1.7-kb fragment. DdeI digestion of the 34D1-amplified DNA from ecotype Columbia gave three fragments with sizes of 0.7-, 0.6-, and 0.4-kb, and that from Langsberg erecta produced 1.3- and 0.4-kb fragments. The 138D19 primers (5′-GAAGGAAGGCGTCATCTTGT-3′ and 5′-TGGCTATCAGGAGATCCGAT-3′) amplified a 1.8-kb fragment. DraI digestion of the 138D19-amplified DNA from ecotype Columbia produced two 0.9-kb fragments, and that from Langsberg erecta produced 0.9-, 0.5-, and 0.4-kb fragments. The MBG8 primers (5′-CATAGGACCCGATTGGAT-3′ and 5′-CAGATATGGCACTCACACCA-3′) amplified a 3.1-kb fragment. AflII digestion of the MBG8-amplified DNA from ecotype Columbia produced 1.3-, 0.9-, and 0.9-kb fragments, and that from Langsberg erecta produced 2.2- and 0.9-kb fragments. The MDF20 primers (5′-GAGATGTGGCACGTACCAGA-3′ and 5′-GCAGTTGCTGGCTTGATCCA-3′) amplified an 8.5-kb fragment. The XbaI enzyme cut the MDF20-amplified DNA from ecotype Columbia into 7.1- and 1.4-kb fragments but did not cut it from Langsberg erecta.
RESULTS
Vascular Development in Wild-Type Arabidopsis Stems
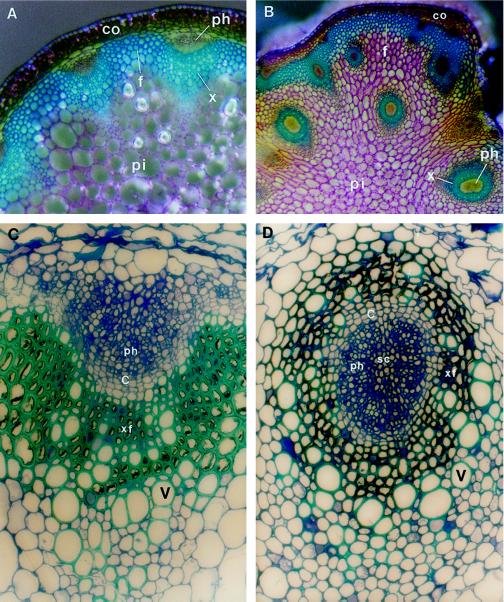
Wild-type Arabidopsis inflorescence stems developed a vascular pattern similar to that found in most dicots (Fig. 1A). In the stele the vascular bundles were arranged in a ring. Typically, about eight discrete vascular bundles developed in the top part of the stem. During the maturation of the stems interfascicular fibers differentiated between vascular bundles to form a continuous ring of sclerified cells (Zhong et al., 1997). Within the vascular bundle, the arrangement of vascular tissues was collateral, i.e. xylem was located on the inner side of the bundle and phloem was located on the outer side of the bundle (Fig. 1, A and C). It was obvious that one to two layers of cambial initials were present in the vascular bundle of an old stem (Fig. 1C), indicating that the bundle retained the potential for further growth. The arrangements of vascular tissues within the bundle and of vascular bundles in the stele were consistent throughout the development of stems.
Figure 1.
Vascular patterns in inflorescence stems of the wild type and the avb1 mutant of Arabidopsis. Stem sections of 8-week-old (A and B) or 10-week-old (C and D) plants were stained with toluidine blue. Xylem and fiber cells stained blue because of the presence of lignin in walls. A, Cross-section of the wild-type stem. The individual vascular bundle was collateral, and collectively the vascular bundles were arranged in a ring. B, Cross-section of the avb1 stem. Xylem formed a circle in most bundles, and extravascular bundles were placed in the pith. Arrows indicate the collateral bundles at the periphery of the stele. C, Collateral vascular bundle in the wild type. Xylem was located on the inner side of the bundle, and phloem was located on the outer side of the bundle. One to two layers of cambial initials were evident between xylem and phloem. D, Amphivasal vascular bundle in the avb1 mutant. It was obvious that the phloem was completely surrounded by vessels, a pattern similar to that seen in an amphivasal vascular bundle. In addition, there were one to two layers of cambial initials between xylem and phloem. c, Cambium; co, cortex; f, interfascicular fibers; ph, phloem; pi, pith; sc, sclerified cell; v, vessel element; x, xylem; and xf, xylary fiber. Magnifications, ×102 (A and B) and ×770 (C and D).
Isolation of a Mutant with an Altered Vascular Pattern in Stems
To understand the genetic control of vascular pattern formation, we screened ethyl methanesulfonate-mutagenized M2 populations of Arabidopsis ecotype Columbia for mutants with altered vascular patterns. Inflorescence stems of 8-week-old plants were free-hand sectioned and stained with toluidine blue to reveal vascular patterns. After screening 100,000 M2 plants, we isolated an avb1 (amphivasal vascular bundle) mutant with a dramatic change of vascular patterns in stems (Fig. 1B).
Alteration of vascular patterns in the avb1 mutant occurred at two levels. First, the arrangement of vascular tissues within the bundle was altered. Unlike the collateral vascular bundles seen in the wild type (Fig. 1C), the mutant had phloem enclosed by xylem (Fig. 1B). In the mutant, the vessel elements were distributed all around the phloem (Fig. 1D), an arrangement resembling amphivasal bundles seen in some monocots (Mauseth, 1988). A ring of one to two layers of cambial initials was also seen in the bundle, (Fig. 1D) in contrast to the half-circle-shaped cambial initials seen in the wild type (Fig. 1C). We additionally noticed that some vascular bundles, mainly those connected with interfascicular fibers, were still collateral, indicating that the mutation did not result in transformation of all collateral bundles into amphivasal bundles (Fig. 1B).
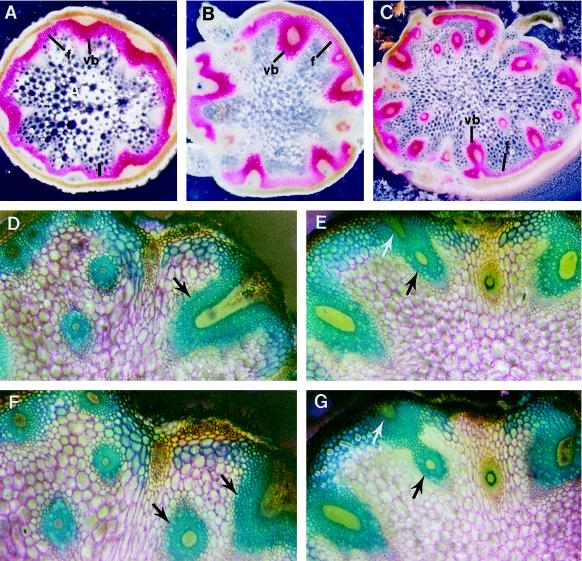
Second, the arrangement of vascular bundles in the stele was altered in the avb1 stems. In addition to a ring of vascular bundles at the periphery of the stele, extra bundles extended through the pith (Fig. 1B). In contrast to approximately 8 bundles seen in the wild-type stem (Fig. 2A), as many as 20 bundles were present in the mutant stem (Fig. 2C; Table I). Because extra bundles were abnormally positioned in the pith, the normal ring-like arrangement of vascular bundles seen in the wild-type stems was disrupted.
Figure 2.
Abnormal branching and penetration of vascular bundles into the pith of avb1 stems. Serial sections of the internodes were prepared and stained with phloroglucinol-HCl (A–C) or toluidine blue (D–G). It was obvious that the presence of extravascular bundles in the pith was due to abnormal branching and penetration of vascular bundles into the pith (D–G). Arrows point to the branching bundles. A to C, Vascular patterns in stems of the wild type (A), heterozygote (B), and homozygote (C) of the avb1 mutant. D and F, Two adjacent sections of an avb1 internode. E and G, Two adjacent sections of an avb1 internode. f, Interfascicular fibers; vb, vascular bundle. Magnifications, ×52 (A–C) and ×154 (D–G).
Table I.
The number of total bundles and amphivasal bundles in stems of the wild type, heterozygote, and homozygote of the avb1 mutant
| Bundle | Phenotype
|
||
|---|---|---|---|
| Wild type | Heterozygote | Homozygote | |
| Total | 8 ± 1 | 10 ± 3 | 15 ± 5 |
| Amphivasal | 0 | 2 ± 1 | 11 ± 4 |
| Extra in pith | 0 | 0 | 5 ± 2 |
Data are the means ± se from 50 plants.
It is interesting that a cluster of cells at the center of the bundle (enclosed by the phloem) was stained red with the lignin-staining dye phloroglucinol-HCl and they were birefringent under polarized optics (data not shown), indicating that these cells were sclerified. It was also evident that the sclerified cells had walls as thick as xylem fibers (Fig. 1D). No sclerified cells were observed at the center of the phloem in the wild type (Fig. 1C), although a few phloem fibers occasionally scattered outside the phloem (data not shown).
Origin of the Extra Bundles in the Pith
To investigate the origin of the abnormally positioned bundles, we prepared serial free-hand sections from the avb1 internodes and traced the distribution of the bundles (Fig. 2, D–G). It appeared that the vascular bundles (indicated by arrows) at the periphery of the stele branched out new bundles, and these new bundles abnormally penetrated into the pith. This resulted in vascular bundles seen as scattered in the stele (Fig. 2C).
Vascular Organization in Leaves and Roots of the avb1 Mutant
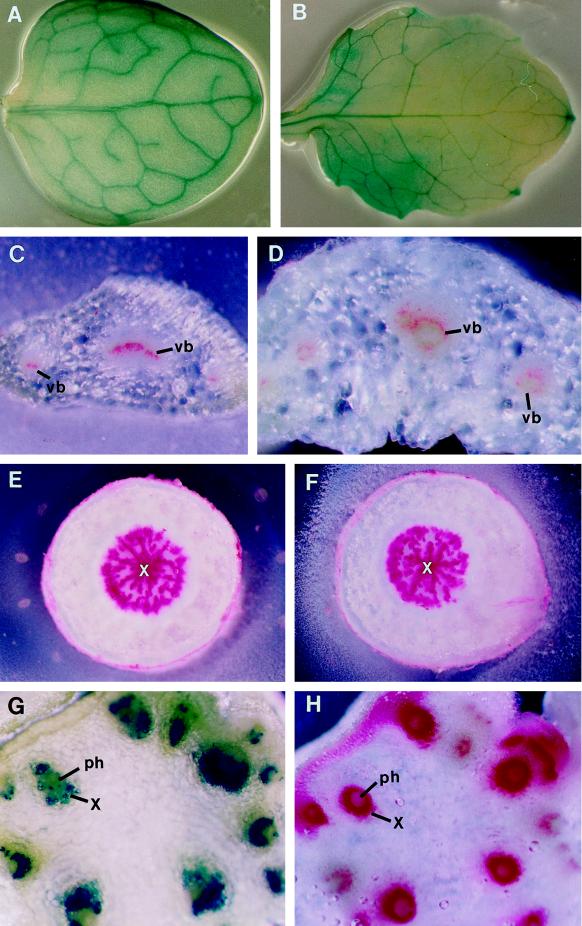
We examined further the vascular patterns in leaves and roots. Veins in the wild-type leaves formed a continuous reticulum (Fig. 3A). A similar venation pattern was seen in the avb1 mutant (Fig. 3B), indicating that the avb1 mutation did not alter the leaf venation pattern. However, the avb1 mutation altered the arrangement of vascular tissues within leaf veins. The organization of vascular tissues within the bundles of wild-type leaves was collateral (Fig. 3C), a pattern similar to that in the wild-type stems (Fig. 1C). In the vascular bundles of avb1 leaves, a layer of vessel elements formed a ring (Fig. 3D), a pattern similar to that seen in the vascular bundles of the avb1 stems (Fig. 1D). This indicated that the avb1 mutation affected the arrangement of vascular tissues within the bundles in both stems and leaves.
Figure 3.
Leaf and root vascular patterns and Evans blue transport in stems. Leaves were cleared in ethanol and stained with toluidine blue to show venation pattern (A and B). Leaf and root sections (C–F) were stained with phloroglucinol-HCl, and lignified walls of xylem cells were stained red. For Evans blue transport assay, the basal end of a stem was submerged in the Evans blue solution. After 10 min, a series of sections of the upper part of the stem were cut and immediately observed under a dissection microscope (G and H). A and B, Leaves of the wild type (A) and the avb1 mutant (B) showing the same venation pattern. C, Cross-section of a wild-type leaf showing xylem cells in the bundles. D, Cross-section of an avb1 leaf showing the arrangement of xylem cells in a ring in the bundles. E and F, Cross-sections of the roots of the wild type (E) and the avb1 mutant (F) showing the same root vascular pattern. G, Section showing Evans blue dye in vascular bundles. All bundles appeared to be functional for dye transport. H, An adjacent section stained with phloroglucinol-HCl showing distribution of vascular bundles. ph, Phloem; vb, vascular bundle; and x, xylem. Magnifications, ×9 (A and B), ×35 (C–F), and ×125 (G and H).
In wild-type roots the vascular pattern was distinct from that seen in stems. The xylem was located as a solid mass at the center and the phloem surrounded the xylem (Fig. 3E), which is a protostele. The vascular pattern in the avb1 roots (Fig. 3F) was the same as that in the wild-type roots.
Functional Analysis of Vascular Bundles in the Mutant
Because some vascular bundles were abnormally positioned in the avb1 internodes, we examined whether they were all functional for transport. To do this, a stem of the avb1 mutant was cut and the basal end was immersed in an Evans blue solution. After a 10-min incubation the upper part of the stem, which was not submerged in the dye, was sectioned to examine the presence of the dye in vascular bundles. If the vessel elements were connected to each other, the Evans blue dye should have been transported to the upper part of the stem through the vessels. The blue dye was seen in every vascular bundle in the stem section of the avb1 mutant (Fig. 3G). The Evans blue-staining pattern was the same as the distribution pattern of vascular bundles (Fig. 3H), indicating that vessels in each vascular bundle of the mutant are functional for transport and that the vascular strands are interconnected.
Morphology of the avb1 Mutant
The avb1 mutation changed the morphology of the plant (Fig. 4). The wild-type inflorescence stems were straight (Fig. 4A). The lower part of the stems had regularly spaced cauline leaves and branches (Fig. 4, A and C). No difference was observed at the top part of the stems between the wild type and the avb1 mutant (Fig. 4, A and B). However, the morphology of the lower part of the stems was altered in the mutant. In the avb1 mutant, the main inflorescence stem was curled and more cauline leaves and branches were produced with irregular spacing along the lower part of the stem (see arrow in Fig. 4D). Frequently, several branches emerged next to each other on the stem (Fig. 4D), and occasionally the cauline leaf blade was fused along the internode to the next node (data not shown).
Figure 4.
Morphology of the avb1 mutant. A and C, Wild-type inflorescence stems were straight in shape and had regularly spaced cauline leaves and branches. B and D, The avb1 mutant stems curled in the lower internodes. The upper parts of the mutant stems, which bore siliques, did not curl. The arrow points to the area that abnormally produced six branches.
Auxin Polar Transport in avb1 Stems
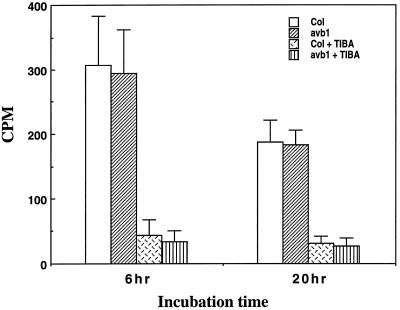
Auxin has been shown to be required for vascular differentiation (Aloni, 1987) and several mutants with defects of vascular differentiation were shown to be associated with reduced auxin polar transport in stems (Carland and McHale, 1996; Przemeck et al., 1996). Because the avb1 mutant showed a dramatic alteration in vascular patterns, it is important to investigate whether the mutation affected the auxin polar transport activity in the stems. As shown in Figure 5, the auxin polar transport assay showed that the basipetal auxin transport activity in the stems of the avb1 mutant was the same as that in the stems of the wild type. The auxin transport activity in both mutant and wild-type stems was effectively inhibited by the auxin polar transport inhibitor 2,3,5-triiodobenzoic acid. These results indicated that the alteration in vascular patterns in the avb1 mutant was not accompanied by a disruption in auxin polar transport activity.
Figure 5.
Auxin polar transport in stems of the wild type and the avb1 mutant. Upper ends of stem segments were immersed in a solution containing labeled IAA. After 6 or 20 h of incubation, the opposite ends were cut and counted for the presence of labeled IAA. Auxin polar transport inhibitor 2,3,5-triiodobenzoic acid was included in the solution in one set of experiments. Both the wild type and the avb1 mutant showed the same rate of auxin polar transport. Data are the means ± se of 15 plants.
Genetic Analysis of the avb1 Mutant
We tested whether the mutation was dominant or recessive. The avb1 mutant was backcrossed with wild-type Arabidopsis ecotype Columbia. The stems of F1 plants (total of 50 plants) were examined for vascular patterns. In the stems of F1 plants, one to three bundles were amphivasal; the other bundles were the same as those seen in the wild type. No extra bundles were seen in the pith (Fig. 2B; Table I). A minor alteration of vascular patterns occurred in the F1 heterozygotes, suggesting that the mutation is partially dominant.
We also analyzed the segregation pattern of the mutation. The F1 plants were selfed and the vascular patterns in the stems of F2 plants were examined. Of the 1432 plants examined, 378 showed a wild-type vascular pattern, 726 showed a heterozygote vascular pattern, and 328 avb1 showed a mutant vascular pattern, giving a segregation ratio of 1:2:1 and indicating that the mutation was monogenic.
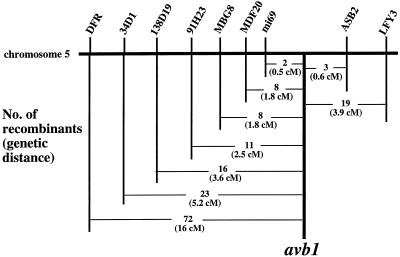
To map the chromosomal location of the avb1 mutation, the avb1 mutant was crossed with the Arabidopsis ecotype Landsberg erecta. The resulting F1 plants were selfed and the F2 plants were analyzed for segregation of the avb1 mutation. F2 plants with the homozygous avb1 mutant phenotype were used to analyze for linkage with CAPS markers located on each of the five chromosomes (Konieczny and Ausubel, 1993). No linkage was found with markers on chromosomes 1 to 4. However, a close linkage was observed with CAPS markers LFY3 and DFR on chromosome 5. Of 241 plants analyzed, 19 plants showed crossovers between LFY3 and the avb1 locus. All of the remaining plants showed Columbia LFY3 genotype. This placed the avb1 locus in a region that was 3.9 centimorgans away from LFY3 (Fig. 6). Further mapping of the avb1 locus with DFR indicated that the avb1 locus was located between DFR and LFY3. The mapping data with DFR indicated that the avb1 locus was 16 centimorgans from DFR (Fig. 6).
Figure 6.
Genetic mapping of the avb1 locus. A total of 241 F2 mapping plants were used for mapping with markers on the right side of the avb1 locus, and a total of 223 F2 mapping plants were used with markers on the left side of the avb1 locus. All markers used for mapping were CAPS markers. The markers 34D1, 138D19, MBG8, and MDF20 were developed during our mapping work. The markers shown on chromosome 5 were not positioned on scale. CM, Centimorgan.
Using sequences from Arabidopsis BAC clones and expressed-sequence tag clones, we have developed four new CAPS markers: 34D1, 138D19, MBG8, and MDF20. Further mapping with these markers showed that the avb1 locus was located between mi69 and ASB2 (Fig. 6). These two markers are covered by a single yeast artificial chromosome clone CIC9E2. In addition, a complete BAC contig has been constructed between these two markers by the Japanese Arabidopsis genome sequencing group (website: http://www.kazusa.or.jp/arabi/). The available resources will allow us in the near future to narrow the avb1 locus to an individual BAC clone and then to clone the gene.
DISCUSSION
Stems of most dicots have a similar vascular organization, i.e. vascular tissues are collateral within the bundle and vascular bundles collectively are arranged in a ring. This type of vascular pattern was conserved during the evolution of dicot plants. However, other vascular patterns have developed in some dicots. For example, bicollateral bundles are common in the families Apocynaceae, Asclepiadaceae, Compositae, Conolvulaceae, Cucurbitaceae, Myrtaceae, and Solanaceae. In dicots such as Begonia, Mesembryanthemum, Rheum, and Rumex, amphicribral bundles occur as medullary bundles, which run through the pith (Mauseth, 1988). Little is known about the mechanisms underlying the evolution of such diverse vascular patterns. Isolation of the avb1 mutant with a global alteration in vascular patterns provides a novel means to study the mechanisms underlying the arrangement of vascular tissues within the bundle.
We have screened 100,000 ethyl methanesulfonate-mutagenized M2 populations of Arabidopsis, and avb1 is the only type of mutant found with such a global alteration in vascular patterns. Two plants with the avb1 phenotype were recovered and were shown to be allelic. We do not know why we did not find other mutants with global alterations in stem vascular patterns. Our mutant-screening method may not be ideal and we may have missed some mutants. It may also be that genes controlling vascular patterns are redundant; therefore, a loss-of-function mutation would not lead to an alteration in vascular patterns and only dominant mutants would show phenotypic changes.
Dominant mutants have been widely used to elucidate developmental pathways in nonplant systems. Similarly, many dominant mutants, such as etr1 (Chang et al., 1993), Kn1 (Hake, 1992), Hooded (Müller et al., 1995), Tkn2 (Chen et al., 1997; Parnis et al., 1997), and art (Grbic and Bleecker, 1996), have been isolated in plants and are instrumental for dissecting plant developmental pathways. For example, analysis of the dominant mutant Kn1 indicated that KN1 is involved in meristem maintenance. Recent results (Kerstetter et al., 1997) concerning the loss-of-function mutant of KN1 confirmed the early conclusion drawn from analysis of the dominant mutant.
Dominant mutants could be the result of ectopic overexpression of a gene (such as Kn1), of mutation of a negative regulator (such as etr1), or of some other mechanism. In any case, the gene may be involved in the observed phenotypes. The analysis of gene functions by ectopic overexpression is also a routine practice, i.e. in some sense, similar to the creation of dominant mutants. Therefore, we suggest that the partially dominant avb1 locus does participate in controlling the organization of vascular patterns; further analysis of the mutant will help us to understand the mechanisms controlling vascular pattern formation.
The Collateral Vascular Bundles Are Transformed into Amphivasal Vascular Bundles in the avb1 Mutant
The most noticeable change in the avb1 mutant is the dramatic alteration of the arrangement of vascular tissues within the bundle (Fig. 1). It was obvious that in the mutant stems the vessel elements completely surrounded the phloem, which is a pattern similar to that seen in the vascular bundles of some monocots, such as Acorus and Dracaena draco (Mauseth, 1988; Bowes, 1996). This transformation could not be due to a displacement in interfascicular fibers, because the vessel elements would not then be distributed all around the phloem. Furthermore, the differentiation of interfascicular fibers and vascular bundles seems to be controlled by two separate pathways, because disruption of interfascicular fiber formation did not affect vascular bundle differentiation or the vascular tissue arrangement (Zhong et al., 1997).
There were several other prominent features in the vascular bundles of the avb1 mutant. First, the cells at the center of the bundle were sclerified (Fig. 1D), similar to the vascular bundles of Acorus, which have the sclerified cells at the center. Second, one to two layers of cambial initials were still present in the vascular bundles of old stems in the avb1 mutant (Fig. 1D). This is somewhat different from the amphivasal bundles seen in Acorus or Dracaena, in which no procambial cells were left when the bundles were fully differentiated (Mauseth, 1988; Bowes, 1996). This is one of the typical differences between dicots and monocots. In most monocots all procambial cells mature and lose the potential for further growth within the bundle. In contrast, vascular bundles in most dicots retain cambial initials after the primary vascular bundles mature excepting a few plants such as Ranunculus in which all of the procambial cells in the vascular bundles differentiate into vascular tissues (Raven et al., 1992). These differences indicate that, although the vascular bundles in the avb1 mutant are similar to the amphivasal bundles seen in some monocots, they retain some of the characteristics seen in most dicots, such as maintenance of cambial initials.
Vascular Bundles Abnormally Penetrate into the Pith in the avb1 Mutant
The other dramatic change in the avb1 mutant is the abnormal positioning of vascular bundles in the pith of stems, which disrupts the normal arrangement of vascular bundles in the stele of stems. This abnormal pattern resulted from branching of the vascular bundles into the pith (Fig. 2). Although it is not clear whether these branching vascular bundles in the avb1 mutant are leaf or branch traces, leaf and branch traces in the wild type are never seen to penetrate into the pith. Thus, it is obvious that the disruption in the ring-like organization of vascular bundles in the stele of mutant stems resulted from mutation of the AVB1 locus.
Since the bundles seen in the pith branch from the normal vascular bundles, they are apparently different from the medullary bundles that usually run through the pith, as seen in families such as Amaranthaceae, Cactaceae, Chenopodiaceae, Melastomataceae, Nyctaginaceae, Piperaceae, and Polygonaceae (Mauseth, 1988). It seems that the abnormal branching pattern seen in the avb1 stems resembles that seen in monocots. Although it appears that the arrangement of vascular bundles in the stems of the majority of monocots is distinct from that of dicots, construction of a three-dimensional structure of vascular bundles revealed ordered patterns in monocots (Esau, 1977; Mauseth, 1988; Fahn, 1990). For example, in the maize stem the scattered distribution of vascular bundles seen in a cross-section is mainly contributed by mid-rib and large lateral leaf traces through deep penetration into the interior of the stem. Thus, the abnormal penetration into the pith of branching bundles seen in the avb1 mutant appeared to share some anatomical features in common with monocots. It will be of interest to examine where the abnormal branching vascular bundles end in the avb1 mutant.
Mutation of the AVB1 locus appeared to have no effect on the arrangement of vasculature in primary roots or on leaf venation pattern. Arabidopsis primary roots have a protostele, whereas stems have a siphonostele. It has been considered that siphonosteles evolved from protosteles (Mauseth, 1988; Raven et al., 1992). Thus, it might be possible that the AVB1 gene is one of the genes evolved to direct the vascular pattern in stems. Once the AVB1 gene is cloned, it will be interesting to examine whether the vascular pattern in primary roots could be altered by overexpression of the AVB1 gene in roots.
Vascular Bundles in the Pith of the avb1 Stems Are Functional for Transport
Two lines of evidence indicate that the bundles that appeared in the pith of the mutant stems are connected with normal vascular bundles. Serial sections of the avb1 stems clearly showed that the bundles seen in the pith branched from normal vascular bundles (Fig. 2). Furthermore, the Evans blue dye-transport experiment showed that the bundles in the pith of mutant stems also functioned for transport of the dye (Fig. 3). These results indicate that appearance of vascular tissues in the pith of mutant stems is not due to random differentiation but is coordinated with the differentiation of bundles at the periphery of the stele.
Auxin Polar Transport Activity Is Not Altered in the avb1 Mutant
It has been demonstrated that the plant hormones auxin and cytokinin are inducers of vascular tissue formation, and the polarity of vascular differentiation is determined by the direction of polar auxin flow (Sachs, 1981; Aloni, 1987). Thus, it is reasonable to question whether the abnormal vascular patterns seen in the avb1 mutant are due to an alteration in auxin polar transport. The auxin polar transport activity in the avb1 stems was indistinguishable from that in the wild type (Fig. 5), indicating that the auxin polar transport activity is not disrupted in the mutant. This is different from the monopteros and lop1 mutants, both of which exhibited defects in auxin polar transport. The lop1 mutant exhibited a bifurcated and twisted midvein in leaves (Carland and McHale, 1996), whereas the monopteros mutant altered the alignment and interconnection of tracheary elements (Przemeck et al., 1996). A number of other mutants with altered auxin polar transport have been isolated (Okada et al., 1991; Bennett et al., 1995; Ruegger et al., 1997). These mutants appeared not to affect the arrangement of vascular tissues, indicating that an alteration in polar auxin transport activity may not result in a defect in vascular pattern formation.
It is not known whether there is any alteration in auxin level in the avb1 mutant. However, several lines of evidence from other studies indicate that the altered vascular patterns might not be due to an alteration in auxin level. First, physiological studies showed that exogenous application of auxin in both in vitro and in vivo organs could induce vascular differentiation but were unable to change vascular patterns (Aloni, 1987). For example, continuous supply of auxin and Suc on a block of Syringa callus induced a continuous ring of vascular bundles, and changing the ratio of auxin and Suc altered the proportion of xylem and phloem in the vascular bundle. However, the ring-like organization of the induced vascular bundles in the Syringa callus was not altered by changing the auxin concentration (Wetmore and Rier, 1963). Second, studies of transgenic plants further demonstrated that changes in the level of auxins or cytokinins only altered the mass but not the organization of vascular tissues (Medford et al., 1989; Romano et al., 1991; Li et al., 1992; Ainley et al., 1993; Tuominen et al., 1995). Third, mutational analysis showed that mutants affecting auxin homeostasis generally exhibited phenotypes associated with the functions of auxins in plant development but no effect on the arrangement of vascular tissues (Boerjan et al., 1995; King et al., 1995).
It has been shown that, in stems with transverse wounds where polar flow of auxin was disrupted, circular vessel rings were induced above the transverse wounds (Sachs and Cohen, 1982; Aloni and Wolf, 1984). However, these circular vessel rings are not individual bundles; instead they are vessels differentiated along the path of auxin flow. This is similar to the formation of normal vascular strands along the path of auxin flow (Sachs, 1981). Therefore, the amphivasal vascular bundles in the avb1 mutant could not be induced by the same mechanism as for circular vessel rings seen in the wounded stems.
Although the avb1 mutation does not alter the overall auxin polar transport activity in stems, the auxin transport pattern within the bundle might be changed. It is likely that the avb1 mutation results in auxin flow all around the phloem in a vascular bundle, instead of the localized auxin flow at the opposite side of the phloem seen in wild-type bundles. The auxin flow around the phloem may thus induce xylem differentiation all around the phloem, forming amphivasal bundles seen in the avb1 mutant. It will be interesting to test this hypothesis by using auxin transport markers.
Because polar auxin flow directs vascular strand differentiation, it is possible that the avb1 mutation results in an abnormal flow of auxin in the pith, which in turn induces vascular strand formation. Therefore, the AVB1 gene may regulate the distribution of the paths of auxin polar flow rather than disrupt auxin polar transport activity, thus determining the pattern of vascular bundle distribution in the stele.
The avb1 Mutant Exhibits Pleiotropic Phenotypes
In addition to the dramatic alteration in vascular patterns, the avb1 mutant exhibited morphological changes such as curled stems and proliferation of branch stems (Fig. 4). It is not clear whether there is any correlation between the alteration of vascular patterns and the morphological changes. It seems unlikely that the morphological changes directly lead to an alteration in vascular patterns. A number of mutants with dramatic morphological changes, such as a mutant with twisted stems (Feldmann, 1991) and mutants with faciated stems (Leyser and Furner, 1992; Clark et al., 1993), have been isolated but no global alteration in vascular patterns were reported in these mutants. One may argue that proliferation of branch stems may be related to production of extra vascular bundles in the pith of the avb1 stems. It is possible that the vascular bundles in the piths of the avb1 stems are branch traces. However, branch and leaf traces in the wild-type stems never penetrate into the pith, indicating that penetration of the extra bundles in the piths of the avb1 stems unlikely results from the production of extra branch stems or leaves.
It is likely that the pleiotropic phenotypes seen in the avb1 mutant result from separate processes controlled by the AVB1 gene. Because these phenotypes are all related to pattern formation and plant forms, it is possible that the AVB1 gene is a transcriptional regulator. Compelling evidence has demonstrated that transcriptional regulators are involved in the evolution of plant forms and their mutations generally result in pleiotropic phenotypes (Doebley and Lukens, 1998). Cloning of the AVB1 gene will be essential for further understanding of the molecular basis of the pleiotropic phenotypes seen in the avb1 mutant. Because the avb1 locus has been mapped to a region covered by a single yeast artificial chromosome clone, it is expected that the AVB1 gene will be isolated soon using the positional cloning approach.
The AVB1 Locus and the Evolution of Amphivasal Vascular Bundles
The angiosperms arose in the early Cretaceous period, some 125 million years ago or more (Heywood, 1993). It is considered that the angiosperms evolved from some primitive gymnosperm (Raven et al., 1992). The ancient angiosperm “ancestor” was thought to precede the divergent evolution of monocots and dicots (Langenheim and Thimann, 1982). Because the gymnosperms typically have collateral bundle patterns in primary vascular tissues of stems, the collateral bundle pattern might be an ancestral type of vascular bundle in the angiosperms. The collateral bundle pattern is still dominant in the angiosperms, and other types of bundles such as amphivasal, amphicribral, or bicollateral bundles arose in a limited numbers of families. For example, the amphivasal vascular bundles have been seen only in some monocots (Mauseth, 1988). Plants such as Acorus, Convallaria, and some Xanthorrhoeaceae have the amphivasal bundles in the primary stems. Plants such as Aloe arborescens and Dracaena develop secondary vascular bundles with the amphivasal arrangement.
Isolation of the Arabidopsis avb1 mutant with an amphivasal vascular bundle phenotype indicates that the AVB1 locus might be involved in the organization of vascular tissues within vascular bundles. It also implicates that the evolution of amphivasal vascular bundles in some monocots might be related to an alteration in the AVB1 locus. One may argue that the AVB1 locus may not be related to vascular development in the wild type because wild-type Arabidopsis stems do not have amphivasal bundles and the avb1 mutation is partially dominant. However, the important message here is that evolution of amphivasal bundles could result from dominant mutation of the AVB1 gene, or at least mutation of the AVB1 gene could result in amphivasal bundles. Once the AVB1 gene is cloned, it will be interesting to test whether expression of the Arabidopsis AVB1 gene in the monocots with amphivasal vascular bundles would be sufficient to convert the amphivasal bundles into collateral bundles.
ACKNOWLEDGMENTS
We thank the Arabidopsis Biological Resource Center (Ohio State University, Columbus) for providing clones EST34D1 and EST138D19; Glenn Freshour for preparation of sections in Figure 1, C and D; and Dr. Roni Aloni for his suggestions concerning the manuscript. J.J.T. was an undergraduate student at Washington University (St. Louis, MO) when she participated in this project.
Abbreviations:
- BAC
bacterial artificial chromosome
- CAPS
codominant cleaved, amplified polymorphic sequence
Footnotes
This work was supported by a faculty research grant from the University of Georgia.
LITERATURE CITED
- Ainley WM, McNeil KJ, Hill JW, Lingle WL, Simpson RB, Brenner ML, Nagao RT, Key JL. Regulated endogenous production of cytokinins up to ‘toxic’ levels in transgenic plants and plant tissues. Plant Mol Biol. 1993;22:13–23. doi: 10.1007/BF00038992. [DOI] [PubMed] [Google Scholar]
- Aloni R. Differentiation of vascular tissues. Annu Rev Plant Physiol. 1987;38:179–204. [Google Scholar]
- Aloni R, Wolf A. Suppressed buds embedded in the bark across the bole and the occurrence of their circular vessels in Ficus religiosa. Am J Bot. 1984;71:1060–1066. [Google Scholar]
- Baima S, Nobili F, Sessa G, Luchetti S, Ruberti I, Morelli G. The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development. 1995;121:4171–4182. doi: 10.1242/dev.121.12.4171. [DOI] [PubMed] [Google Scholar]
- Bennett SRM, Alvarez J, Bossinger G, Smyth D. Morphogenesis in pinoid mutants of Arabidopsis thaliana. Plant J. 1995;8:505–520. [Google Scholar]
- Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Onckelen HV, Montagu MV, Inzé D. superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes BG (1996) A Color Atlas of Plant Structure. Iowa State University Press, Ames
- Carland FM, McHale NA. LOP1: a gene involved in auxin transport and vascular patterning in Arabidopsis. Development. 1996;122:1811–1819. doi: 10.1242/dev.122.6.1811. [DOI] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleeker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–545. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- Chen J-J, Janssen B-J, Williams A, Sinha N. A gene fusion at a homeobox locus: alterations in leaf shape and implications for morphological evolution. Plant Cell. 1997;9:1289–1304. doi: 10.1105/tpc.9.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development. 1993;119:397–418. doi: 10.1242/dev.119.2.397. [DOI] [PubMed] [Google Scholar]
- Cocciolone SM, Cone KC. Pl-Bh, an anthocyanin regulatory gene of maize that leads to variegated pigmentation. Genetics. 1993;135:575–588. doi: 10.1093/genetics/135.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway LJ, Poethig RS. Mutation of Arabidopsis thaliana that transform leaves into cotyledons. Proc Natl Acad Sci USA. 1997;94:10209–10214. doi: 10.1073/pnas.94.19.10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demura T, Fukuda H. Molecular cloning and characterization of cDNAs associated with tracheary element differentiation in cultured zinnia cells. Plant Physiol. 1993;103:815–821. doi: 10.1104/pp.103.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demura T, Fukuda H. Novel vascular cell-specific genes whose expression is regulated temporally and spatially during vascular system development. Plant Cell. 1994;6:967–981. doi: 10.1105/tpc.6.7.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt ND, Sussman MR. Immunocytological localization of an epitope-tagged plasma membrane proton pump (H+-ATPase) in phloem companion cells. Plant Cell. 1995;7:2053–2067. doi: 10.1105/tpc.7.12.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley J, Lukens L. Transcriptional regulators and the evolution of plant form. Plant Cell. 1998;10:1075–1082. doi: 10.1105/tpc.10.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. Anatomy of Seed Plants, Ed 2. New York: John Wiley & Sons; 1977. [Google Scholar]
- Fahn A. Plant Anatomy, Ed 4. Oxford, UK: Butterworth-Heinemann; 1990. [Google Scholar]
- Feldmann KA. T-DNA insertion mutagenesis in Arabidopsis: mutational spectrum. Plant J. 1991;1:71–82. [Google Scholar]
- Fukuda H. Xylogenesis: initiation, progression, and cell death. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:299–325. doi: 10.1146/annurev.arplant.47.1.299. [DOI] [PubMed] [Google Scholar]
- Grbic V, Bleecker AB. An altered body plan is conferred on Arabidopsis plants carrying dominant alleles of two genes. Development. 1996;122:2395–2403. doi: 10.1242/dev.122.8.2395. [DOI] [PubMed] [Google Scholar]
- Guilfoyle TJ. Aux/IAA proteins and auxin signal transduction. Trends Plant Sci. 1998;3:203–204. [Google Scholar]
- Hake S. Unraveling the knots in plant development. Trends Genet. 1992;8:109–114. doi: 10.1016/0168-9525(92)90199-e. [DOI] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17:1405–1410. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood VH (1993) Flowering Plants of the World. Oxford University Press, New York
- Kerstetter RK, Laudencia-Chingcuanco D, Smith LG, Hake S. Loss-of-function mutations in the maize homeobox gene, knotted1, are defective in shoot meristem maintenance. Development. 1997;124:3045–3054. doi: 10.1242/dev.124.16.3045. [DOI] [PubMed] [Google Scholar]
- King JJ, Stimart DP, Fisher RH, Bleeker AB. A mutation altering auxin homeostasis and plant morphology in Arabidopsis. Plant Cell. 1995;7:2023–2037. doi: 10.1105/tpc.7.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny A, Ausubel FM. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313x.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- Langenheim JH, Thimann KV. Botany: Plant Biology and Its Relation to Human Affairs. New York: John Wiley & Sons; 1982. [Google Scholar]
- Leyser HMO, Furner IJ. Characterization of three shoot apical meristem mutants of Arabidopsis thaliana. Development. 1992;116:397–403. [Google Scholar]
- Li Y, Hagen G, Guilfoyle TJ. Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Dev Biol. 1992;153:386–395. doi: 10.1016/0012-1606(92)90123-x. [DOI] [PubMed] [Google Scholar]
- Mauseth JD (1988) Plant Anatomy. The Benjamin/Cummings Publishing Co., Menlo Park, CA
- Medford JL, Horgan R, EI-Sawi Z, Klee HJ. Alterations of endogenous cytokinins in transgenic plants using a chimeric isopentenyl transferase gene. Plant Cell. 1989;1:403–413. doi: 10.1105/tpc.1.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller KJ, Romano N, Gerstner O, Garcia-Maroto F, Pozzi C, Salamini F, Rohde W. The barley Hooded mutation caused by a duplication in a homeobox gene intron. Nature. 1995;374:727–730. doi: 10.1038/374727a0. [DOI] [PubMed] [Google Scholar]
- Nelson T, Dengler N. Leaf vascular pattern formation. Plant Cell. 1997;9:1121–1135. doi: 10.1105/tpc.9.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Last RL, Fink GR, Keith B. Suppressors of trp1 fluorescence identify a new Arabidopsis gene, TRP4, encoding the anthranilate synthase β subunit. Plant Cell. 1993;5:1011–1027. doi: 10.1105/tpc.5.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnis A, Cohen O, Gutfinger T, Hareven D, Zamir D, Lifschitz E. The dominant developmental mutants of tomato, Mouse-ear and Curl, are associated with distinct modes of abnormal transcriptional regulation of a Knotted gene. Plant Cell. 1997;9:2143–2158. doi: 10.1105/tpc.9.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwait SN, Nelson OE. A chronically wilted mutant of maize. Am J Bot. 1957;44:628–633. [Google Scholar]
- Przemeck GKH, Mattsson J, Hardtke CS, Sung ZR, Berleth T. Studies on the role of the Arabidopsis gene MONOPTEROS in vascular development and plant cell axialization. Planta. 1996;200:229–237. doi: 10.1007/BF00208313. [DOI] [PubMed] [Google Scholar]
- Raven PH, Evert RF, Eichhorn SE (1992) Biology of Plants, Ed 5. Worth Publishers, New York
- Romano CP, Hein MB, Klee HJ. Inactivation of auxin in tobacco transformed with the indoleacetic acid-lysine synthetase gene of Pseudomonas savastanoi. Genes Dev. 1991;5:438–446. doi: 10.1101/gad.5.3.438. [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Hobbie L, Brown D, Bernasconi P, Turner J, Muday G, Estelle M. Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell. 1997;9:745–757. doi: 10.1105/tpc.9.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T. The control of the patterned differentiation of vascular tissues. Adv Bot Res. 1981;9:151–262. [Google Scholar]
- Sachs T, Cohen D. Circular vessels and the control of vascular differentiation in plants. Differentiation. 1982;21:22–26. [Google Scholar]
- Scheres B, Laurenzio LD, Willemsen V, Hauser M-T, Janmaat K, Weisbeek P, Benfey P. Mutations affecting the radial organization of the Arabidopsis root display specific defects throughout the embryonic axis. Development. 1995;121:53–62. [Google Scholar]
- Tornero P, Conejero V, Vera P. Phloem-specific expression of a plant homeobox gene during secondary phases of vascular development. Plant J. 1996;9:639–648. doi: 10.1046/j.1365-313x.1996.9050639.x. [DOI] [PubMed] [Google Scholar]
- Tóth KF, Wang Q, Sjölund RD. Monoclonal antibodies against phloem P-protein from plant tissue cultures. I. Microscopy and biochemical analysis. Am J Bot. 1994;81:1370–1377. [Google Scholar]
- Tuominen H, Sitbon F, Jacobsson C, Sandberg G, Olsson O, Sundberg B. Altered growth and wood characteristics in transgenic hybrid aspen expressing Agrobacterium tumefaciens T-DNA indoleacetic acid-biosynthetic genes. Plant Physiol. 1995;109:1179–1189. doi: 10.1104/pp.109.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Monroe J, Sjölund RD. Identification and characterization of a phloem-specific β-amylase. Plant Physiol. 1995;109:743–750. doi: 10.1104/pp.109.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore RH, Rier JP. Experimental induction of vascular tissue in callus of angiosperms. Am J Bot. 1963;50:418–430. [Google Scholar]
- Ye Z-H, Varner JE. Gene expression patterns associated with in vitro tracheary element formation in isolated single mesophyll cells of Zinnia elegans. Plant Physiol. 1993;103:805–813. doi: 10.1104/pp.103.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z-H, Varner JE. Expression of an auxin- and cytokinin-regulated gene in cambial region in zinnia. Proc Natl Acad Sci USA. 1994;91:6539–6543. doi: 10.1073/pnas.91.14.6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Taylor JT, Ye Z-H. Disruption of interfascicular fiber differentiation in an Arabidopsis mutant. Plant Cell. 1997;9:2159–2170. doi: 10.1105/tpc.9.12.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]