Abstract
Objectives
There is today ample evidence that negative aging stereotypes impair healthy older adults’ performance on cognitive tasks. Here, we tested whether these stereotypes also decrease performance during the screening for predementia on short cognitive tests widely used in primary care.
Method
An experiment was conducted on 80 healthy older adults taking the Mini Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) under Threat or Reduced-threat condition.
Results
Stereotype threat significantly impaired older adults’ performance on both tests, resulting in 40% of older adults meeting the screening criteria for predementia, compared with 10% in Reduced-threat condition (MMSE and MoCA averaged).
Discussion
Our research highlights the influence of aging stereotypes on short cognitive tests used to screen for predementia. It is of critical importance that physicians provide a threat-free testing environment. Further research should clarify whether this socially induced bias may also operate in secondary care by generating false positives.
Keywords: Aging stereotypes, Dementia, Mild Cognitive Impairment, MMSE, MoCA
Introduction
Because of the relatively high rates of reversion from mild cognitive impairment (MCI) to normal cognitive functioning, false positives during the screening for prodromal state of Alzheimer’s disease (AD) are a debated issue within the medical community. Many individuals diagnosed with MCI do not convert to AD, some remaining stable and others even reversing back to normal (Edmonds et al., 2015; Petersen et al., 2013; Summers & Saunders, 2012). Because the rates of reversion to normal can vary from 4.5% to as high as 53% (Sachdev et al., 2013), false positive diagnostic errors deserve special attention. As pointed out by Le Couteur, Doust, Creasey, and Brayne (2013), these errors have dramatic consequences such as unnecessary patient and family stress, risk of side effects from inappropriate therapies, and undue financial costs. Brown (2015) emphasized that false positives may be amplified due to an increasing pressure on health professionals to routinely screen for dementia based on minimal history and the use of rapid devices such as short cognitive tests (Borson et al., 2013). Several explanations have been provided to account for false positives such as an undetected temporary state of depression, mild psychiatric conditions, lack of motivation, or acute stress during testing (Sachdev et al., 2013).
Here, we argue that an important source of stress during clinical cognitive assessments comes from negative aging stereotypes that can permeate the testing situation and lower performance (Siebert, Wahl, & Schröder, 2016). There is now ample evidence that culturally shared beliefs that aging inescapably causes severe cognitive decline and diseases such as AD typically impair healthy older adults’ performance on cognitive tasks (for a recent meta-analysis, see Lamont, Swift, & Abrams, 2015). There is also evidence that stereotype threat (ST) may operate outside conscious awareness (Levy, 1996). In addition to the normal stress associated with taking cognitive tests, negative aging stereotypes create an extra pressure that interferes with intellectual functioning and leads older adults to perform below their true abilities, a phenomenon, called stereotype threat (Steele & Aronson, 1995). Simply informing older adults about the presence of younger participants can decrease their controlled access to memory and intensify automatic responses (Mazerolle, Régner, Morisset, Rigalleau, & Huguet, 2012) that are often incorrect on complex tasks (Zajonc, 1965). This pattern is typical of social-evaluative threats both in humans and in nonhuman primates (Belletier et al., 2015; Huguet, Barbet, Belletier, Monteil, & Fagot, 2014). Finally, ST effects can be alleviated and sometimes removed when the memory component of the test is de-emphasized (Desrichard & Köpetz, 2005; Kang & Chasteen, 2009) or when the test is presented as age-fair (Mazerolle, Régner, Rigalleau, & Huguet, 2015).
To date, only two laboratory studies investigated whether ST can also affect performance on the short cognitive tests typically used in primary care. Haslam and coworkers (2012) showed that 70% of healthy older adults who self-categorized as older (vs. younger) and expected widespread (vs. specific) cognitive decline scored below the cutoff of the Addenbrooke’s Cognitive Examination–Revised (ACE-R), compared with an average of 14% in the other condition. Similarly, Barber, Mather, and Gatz (2015) found that ST lowered older participants’ ACE-R score; however, this effect did not change the proportion of participants meeting diagnostic criteria for predementia. It is noteworthy that participants from Barber et al.’s study were highly educated and on average 69 years old, whereas most people at this age believe that “old age” starts at 75 (Taylor, 2014), suggesting that these participants did not experience enough threat to fall below the cutoff. These findings suggest further studies to clarify ST effects during the screening for predementia on short cognitive tests used in primary care.
In the present study, we tested ST effects in adults aged around 75 years when using two well-known short cognitive tests: the classic Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975) and the Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005).
Method
Participants
Participants were 80 adults aged 60 to 93 (M = 75 years old; SD = 8.31) from a French community sample with an average of 7.82 years of education (SD = 3.63, range = 4 to 18 years), corresponding to sixth grade. (To reflect the general population in France, where more than 50% of the individuals aged 65 years and older had an educational level corresponding to primary school or lower (INSEE, 2014), our participants’ educational level corresponded to the sixth grade [on average].) They were recruited from local community associations, for a study on the impact of emotions on various cognitive tasks and questionnaires. During recruitment, no mention was made of either memory or age. All participants lived at home, had no history of significant trauma or chronic illness, had normal or corrected-to-normal vision and hearing, and had neither current depression (Geriatric Depression Scale, Yesavage et al., 1982) nor abnormal anxiety (State-Trait Anxiety Inventory; Spielberger, 2010).
Short Cognitive Tests Measures
The MMSE (Folstein et al., 1975) and MoCA (Nasreddine et al., 2005) are brief cognitive screening tests used for initial cognitive screening by frontline physicians and practitioners in clinical settings. Both tests consist in a variety of questions are administered in approximately 10min and are rated on a 30-point scale (see Supplementary Methods 1). Cutoff scores for detecting MCI are typicaly ≤24–26 (depending on patients’ age and education, Tombaugh & McIntyre, 1992) for the MMSE. Using a cutoff score of 26, the MoCA is considered to have a better sensitivity (90%) than the MMSE (18%) for detecting MCI (Gauthier et al., 2006; Howe, 2007).
Procedure
Participants were tested individually at home or in local community associations’ premises by a graduate student trained at neuropsychological testing. Each participant took both the MMSE and the MoCA (in a counterbalanced order). Before the first test, participants provided informed consent and were randomly assigned (with a constraint on gender) to one of the two experimental conditions: Threat versus Reduced-threat (see Supplementary Methods 2). In the Threat condition, the experimenter told participants that they would perform a memory task and that both younger and older adults were taking part in the study. Participants in the Reduced-threat condition received the same instructions and were also told that there is typically no age difference on this task (i.e., the task is age-fair). Before taking Test 2 (either the MMSE or the MoCA depending upon which test was taken first), participants were debriefed about ST. Regardless of the condition (Threat vs. Reduced-threat) under which they performed the first test, participants were told that Test 2 was under construction and were taught about ST (as also did Johns, Schmader, & Martens, 2005) to decrease evaluative pressure (see Supplementary Methods 3). Finally, participants performed Test 2.
Results
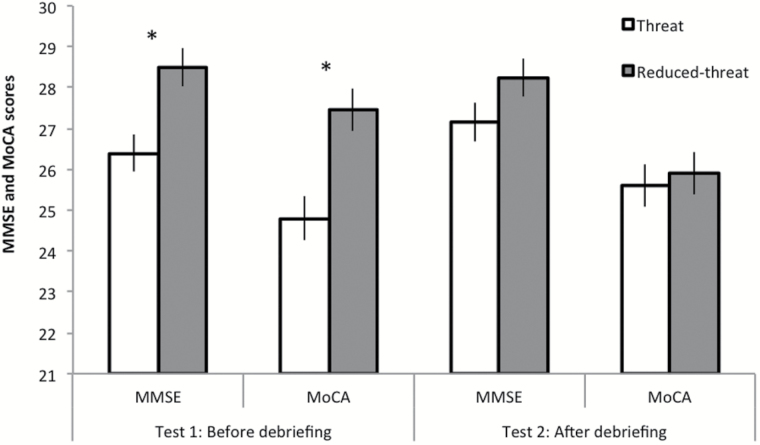
A 2 (ST condition: Threat vs. Reduced-threat) × 2 (Test: MMSE, MoCA [Following Nasreddine and coworker’s (2005) recommendation, one point was added for participants with 12 years of education or less on their total MoCA score.]) × 2 (Order: MMSE then MoCA vs. MoCA then MMSE) mixed analysis of variance with Test as repeated measure revealed significant main effects of Test, F(1, 76) = 41.829, p = .000, = .355, and Threat conditions, F(1, 76) = 12.788, p = .001, = .144. Performance was higher on the MMSE (M = 27.57; SE = .23) than on the MoCA (M = 25.94; SE = .26), and more importantly, participants in the Threat condition (M = 25.99; SE = .30) underperformed compared with those in the Reduced-threat condition (M = 27.52; SE = .30). As expected, the three-way interaction, F(1, 76) = 10.942, p = .001, η2p = .126, indicated that this ST pattern only occurred on Test 1 before the debriefing. When the MMSE was taken first, participants performed lower in the Threat condition (M = 26.40; SE = .43) than in the Reduced-threat condition (M = 28.50; SE = .47), F(1, 76) = 12.506, p = .001, = .141. Likewise, when the MoCA was first proposed, participants performed lower in the Threat condition (M = 24.80; SE = .53) than in the Reduced-threat condition (M = 27.45; SE = .53), F(1, 76) = 10.153, p = .002, = .118). Further analyses indicated that these ST effects were mainly located on the visuospatial/executive and delayed recall subtests for the MoCA and on recall, attention/calculation, and language subtests for the MMSE (see Supplementary Analyses 2 and Supplementary Table 1a and b). After debriefing (Test 2), no ST effects were found (see Figure 1).
Figure 1.
Participants’ scores on MMSE and MoCA tests before and after debriefing as a function of Threat condition. Bars represents standard errors of the mean. The asterisk indicates significantly different group means below .05.
We also examined the proportion of participants meeting the clinical criteria on MoCA and MMSE taken as Test 1. For comparison purpose, we used a cutoff at 26 for both tests (Nasreddine et al., 2005; see Supplementary Methods 1). On the MoCA, 50% of the participants fell below the cutoff score of 26 in the Threat condition, against 15% in Reduced-threat condition during Test 1 (p = .041, Fischer’s exact test). Likewise, 30% of the participants in the Threat condition scored below 26 on the MMSE, against 5% in Reduced-threat condition (p = .038, Fisher’s exact test).
Discussion
The present study shows that ST, a neglected source of stress in many testing situations, can lower performance and result in 40% of participants meeting the MCI clinical criteria on short cognitive tests (MMSE and MoCA averaged). This is an important finding, given the current political pressure on general practitioners to screen for predementia based on minimal history and short cognitive tests (Brown, 2015; Le Couteur et al., 2013). Our research suggests extreme caution when routinely using these tests that seems easily biased by negative aging stereotypes.
Simply mentioning that younger adults also took the memory test (Threat condition) lead older adults to score below the cutoff compared with a condition where the task was presented as age-fair (Reduced-threat condition). This stereotype-based induction particularly alters performance on subtests (of both MMSE and MoCA) that heavily require executive processes. This is consistent with Lamont and coworkers (2015), who argue that such inductions are especially damaging because they create ambiguity about the presence of threat and affect cognitive load and in turn alter performance on tasks that heavily rely on working memory capacity. Future work should more directly focus on the underlying mechanisms of ST in older adults, including working memory resources, changes in motivational regulatory foci (Barber & Mather, 2013), perceived subjective abilities such as hearing, memory self-efficacy (Chasteen, Pichora-Fuller, Dupuis, Smith, & Singh, 2015; Levy, 1996), or stereotype self-relevance (Kornadt, Voss, & Rothermund, 2015; Levy, Zonderman, Slade, & Ferrucci, 2012).
Importantly, our findings also show that ST can easily be removed using appropriate interventions, such as pointing similarities in test performance between older and younger people or informing participants about ST effects and reducing evaluative pressure through the test description. Other interventions such as expressive writing (asking participants to write down their thoughts and feelings about the upcoming tests) have also proved efficient to reduce evaluative pressure (Ramirez & Beilock, 2011). Future work should be conducted to determine which interventions are most effective to reduce ST in older adults facing a cognitive screening.
Although the present findings were obtained on two short cognitive tests, they did raise the question of whether ST effects can also be found during the diagnosis of MCI in secondary care, which relies on neuropsychological batteries in memory clinics and not exclusively on one or two short cognitive tests. Given that false positive diagnosis errors of MCI can be as high as 53% even when using neuropsychological batteries (Sachdev et al., 2013), we believe it is likely that ST is playing a role. This may be particularly true when one considers the context of the assessments in memory clinics, where older patients are often highly fearful of being diagnosed with AD. Future research should directly test the impact of ST in genuine clinical assessment while controlling for AD biomarkers (using β-amyloid tracer, magnetic resonance imaging hippocampal volume, and tau protein levels in the cerebrospinal fluid). Poor cognitive test performance in the absence of any biomarker evidence of neurodegeneration would strengthen the view that ST effects can lead to false positive errors, although there is preliminary evidence that older people holding more negative age stereotypes earlier in life had significantly steeper hippocampal volume loss and significantly greater accumulation of neurofibrillary tangles and amyloid plaques, adjusting for relevant covariates (Levy et al., 2016).
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences online.
Funding
This work was supported by Plan Alzheimer Foundation on a Humanities and Social Sciences grant (AAP SHS 2013: “Sociocognitive aspects of Alzheimer disease” to F. Rigalleau and M. Mazerolle), and writing of this manuscript was in part supported by a grant from the National Institute on Aging (grant number R01-AG046464 to S. Barber).
Supplementary Material
References
- Barber S. J., & Mather M (2013). Stereotype threat in older adults: When and why does it occur, and who is most affected? In Verhaeghen P., Hertzog C. (Eds.), The Oxford Handbook of Emotion, Social Cognition, and Everyday Problem Solving During Adulthood. (pp. 302–320). Oxford, UK: Oxford University Press. doi:org/10.1093/oxfordhb/9780199899463.013.008 [Google Scholar]
- Barber S. J. Mather M., & Gatz M (2015). How stereotype threat affects healthy older adults’ performance on clinical assessments of cognitive decline: The key role of regulatory fit. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 70, 891–900. doi:10.1093/geronb/gbv009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belletier C. Davranche K. Tellier I. S. Dumas F. Vidal F. Hasbroucq T., & Huguet P (2015). Choking under monitoring pressure: Being watched by the experimenter reduces executive attention. Psychonomic Bulletin & Review, 22, 1410–1416. doi:10.3758/s13423-015-0804-9 [DOI] [PubMed] [Google Scholar]
- Borson S. Frank L. Bayley P. J. Boustani M. Dean M. Lin P. J., … Ashford J. W (2013). Improving dementia care: The role of screening and detection of cognitive impairment. Alzheimer’s & Dementia, 9, 151–159. doi:10.1016/j.jalz.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. (2015). The use and misuse of short cognitive tests in the diagnosis of dementia. Journal of Neurology, Neurosurgery, and Psychiatry, 86, 680–685. doi:10.1136/jnnp-2014-309086 [DOI] [PubMed] [Google Scholar]
- Chasteen A. L. Pichora-Fuller M. K. Dupuis K. Smith S., & Singh G (2015). Do negative views of aging influence memory and auditory performance through self-perceived abilities? Psychology and Aging, 30, 881–893. doi:10.1037/a0039723 [DOI] [PubMed] [Google Scholar]
- Desrichard O., & Köpetz C (2005). A threat in the elder: The impact of task-instructions, self-efficacy and performance expectations on memory performance in the elderly. European Journal of Social Psychology, 35, 537–552. doi:org/10.1002/ejsp.249 [Google Scholar]
- Edmonds E. C. Delano-Wood L. Clark L. R. Jak A. J. Nation D. A. McDonald C. R., … Bondi M. W; Alzheimer’s Disease Neuroimaging Initiative (2015). Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s & Dementia, 11, 415–424. doi:10.1016/j.jalz.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F. Folstein S. E., & McHugh P. R (1975). Mini-Mental State. Journal of Psychiatric Research, 12, 189–198. doi:org/10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Gauthier S. Reisberg B. Zaudig M. Petersen R. C. Ritchie K. Broich K., … Winblad B; International Psychogeriatric Association Expert Conference On Mild Cognitive Impairment. (2006). Mild cognitive impairment. Lancet, 367, 1262–1270. doi:org/10.1016/S0140-6736(06)68542–5 [DOI] [PubMed] [Google Scholar]
- Haslam C. Morton T. A. Haslam S. A. Varnes L. Graham R., & Gamaz L (2012). “When the age is in, the wit is out”: Age-related self-categorization and deficit expectations reduce performance on clinical tests used in dementia assessment. Psychology and Aging, 27, 778–784. doi:10.1037/a0027754 [DOI] [PubMed] [Google Scholar]
- Howe E. (2007). Initial screening of patients for Alzheimer’s disease and minimal cognitive impairment. Psychiatry, 4, 24–27. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2880929/ [PMC free article] [PubMed] [Google Scholar]
- Huguet P. Barbet I. Belletier C. Monteil J. M., & Fagot J (2014). Cognitive control under social influence in baboons. Journal of Experimental Psychology General, 143, 2067–2073. doi:10.1037/xge0000026 [DOI] [PubMed] [Google Scholar]
- Institut National de la Statistique et des Etudes Economiques (INSEE). . 2014. Enquêtes emploi Retrieved from http://www.insee.fr/fr/themes/tableau.asp?reg_id=0&ref_id=nattef07232.
- Johns M. Schmader T., & Martens A (2005). Knowing is half the battle: Teaching stereotype threat as a means of improving women’s math performance. Psychological Science, 16, 175–179. doi:10.1111/j.0956-7976.2005.00799.x [DOI] [PubMed] [Google Scholar]
- Kang S. K., & Chasteen A. L (2009). The moderating role of age-group identification and perceived threat on stereotype threat among older adults. The International Journal of Aging and Human Development, 69, 201–220. doi:org/10.2190/AG.69.3.c [DOI] [PubMed] [Google Scholar]
- Kornadt A. E. Voss P., & Rothermund K (2015). Age stereotypes and self-views revisited: patterns of internalization and projection processes across the life span. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. Advance online publication. doi:10.1093/geronb/gbv099 [DOI] [PubMed] [Google Scholar]
- Lamont R. A. Swift H. J., & Abrams D (2015). A review and meta-analysis of age-based stereotype threat: negative stereotypes, not facts, do the damage. Psychology and Aging, 30, 180–193. doi:10.1037/a0038586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur D. G. Doust J. Creasey H., & Brayne C (2013). Political drive to screen for pre-dementia: not evidence based and ignores the harms of diagnosis. BMJ, 347, f5125. doi:org/10.1136/bmj.f5125 [DOI] [PubMed] [Google Scholar]
- Levy B. (1996). Improving memory in old age through implicit self-stereotyping. Journal of Personality and Social Psychology, 71, 1092–1107. doi:org/10.1037/0022-3514.71.6.1092 [DOI] [PubMed] [Google Scholar]
- Levy B. R. Ferrucci L. Zonderman A. B. Slade M. D. Troncoso J., & Resnick S. M (2016). A culture–brain link: Negative age stereotypes predict Alzheimer’s disease biomarkers. Psychology and Aging, 31, 82–88. Retrieved from http://dx.doi.org/10.1037/pag0000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy B. R. Zonderman A. B. Slade M. D., & Ferrucci L (2012). Memory shaped by age stereotypes over time. Journal of Gerontology: Psychological Sciences, 67, 432–436. doi:10.1093/geronb/gbr120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazerolle M. Régner I. Morisset P. Rigalleau F., & Huguet P (2012). Stereotype threat strengthens automatic recall and undermines controlled processes in older adults. Psychological Science, 23, 723–727. doi:10.1177/0956797612437607 [DOI] [PubMed] [Google Scholar]
- Mazerolle M. Régner I. Rigalleau F., & Huguet P (2015). Stereotype threat alters the subjective experience of memory. Experimental Psychology, 62, 395–402. doi:10.1027/1618-3169/a000303 [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S. Phillips N. A. Bédirian V. Charbonneau S. Whitehead V. Collin I., … Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. doi:10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Petersen, R. C., Aisen, P., Boeve, B. F., Geda, Y. E., Ivnik, R. J., Knopman, D. S., ... & Weigand, S. (2013). Mild cognitive impairment due to Alzheimer disease in the community. Annals of Neurology, 74, 199–208. doi:10.1002/ana.23931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez G., & Beilock S. L (2011). Writing about testing worries boosts exam performance in the classroom. Science, 331, 211–213. doi:org/10.1126/science.1199427 [DOI] [PubMed] [Google Scholar]
- Sachdev P. S. Lipnicki D. M. Crawford J. Reppermund S. Kochan N. A. Trollor J. N., … Brodaty H; Sydney Memory, Ageing Study Team. (2013). Factors predicting reversion from mild cognitive impairment to normal cognitive functioning: a population-based study. PloS One, 8, e59649. doi:10.1371/journal.pone.0059649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert J. S. Wahl H. W., & Schröder J (2016). The role of attitude toward own aging for fluid and crystallized functioning: 12-Year evidence from the ILSE study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. Advance online publication. doi:10.1093/geronb/gbw050 [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. (2010). Test Anxiety Inventory. The Corsini Encyclopedia of Psychology. doi:10.1002/9780470479216.corpsy0985 [Google Scholar]
- Steele C. M., & Aronson J (1995). Stereotype threat and the intellectual test performance of African Americans. Journal of Personality and Social Psychology, 69, 797–811. doi:org/10.1037/0022-3514.69.5.797 [DOI] [PubMed] [Google Scholar]
- Summers M. J., & Saunders N. L (2012). Neuropsychological measures predict decline to Alzheimer’s dementia from mild cognitive impairment. Neuropsychology, 26, 498–508. doi:10.1037/a0028576 [DOI] [PubMed] [Google Scholar]
- Taylor P. (2014). The Next America. New York: PublicAffairs Press. [Google Scholar]
- Tombaugh T. N., & McIntyre N. J (1992). The Mini-Mental State Examination: A comprehensive review. Journal of the American Geriatrics Society, 40, 922–935. doi:org/10.1111/j.1532–5415.1992.tb01992.x [DOI] [PubMed] [Google Scholar]
- Yesavage J. A. Brink T. L. L. Rose T. L. Lum O. Huang V. Adey M., & Leirer V. O (1982). Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research, 17, 37–49. doi:org/10.1016/0022-3956(82)90033–4 [DOI] [PubMed] [Google Scholar]
- Zajonc R. B. (1965). Social facilitation. Science, 149, 269–274. doi:10.1126/science.149.3681.269 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.