Abstract
Objectives
Slow afternoon cortisol decline may be a marker of aging. We hypothesize that lower socioeconomic status (SES) and African American race are associated with lower waking cortisol and slower afternoon decline.
Method
Six salivary cortisol samples, collected within a 24-hr period from 566 cohort participants aged 56–78 years, were examined in random-effects models. SES measures included socioeconomic vulnerability (household income and assets <500% of poverty) and education (≥college, some college, and ≤high school). African Americans were compared with all others.
Results
Adjusting for age and sex, intermediate, but not low, education was associated with approximately 17% lower average waking cortisol and 1% slower decline, compared with high education. Socioeconomic vulnerability was not associated with waking cortisol or linear decline. Accounting for African American race/ethnicity, socioeconomic vulnerability was associated with a 3% faster decline, and education was not associated with cortisol. African Americans had 26% lower average waking cortisol and 1% slower decline than others.
Discussion
African American race/ethnicity, but not lower SES, was associated with lower waking cortisol and slower afternoon decline in middle-aged and older adults. This pattern is likely a marker of earlier biological aging in vulnerable groups. Race/ethnicity may compete with SES as a measure of cumulative vulnerability.
Keywords: Earlier aging, Education, Hypothalamic pituitary adrenal axis, Wealth
Individuals facing social adversity, including those of low socioeconomic status (SES) or racial minorities, often experience earlier biological aging than their peers (Crimmins, Kim, & Seeman, 2009; Seeman et al., 2004). This increases their risk of cognitive impairment, disability, and chronic diseases associated with the aging process (Almeida, Neupert, Banks, & Serido, 2005; Karlamangla, Singer, McEwen, Rowe, & Seeman, 2002; Lee et al., 2007; McEwen, 1998; Sabbah, Watt, Sheiham, & Tsakos, 2008; Seeman, McEwen, Rowe, & Singer, 2001). Biological aging may also partially account for vast disparities in mortality (Seeman et al., 2004). This effect is believed to accumulate over time, producing the greatest disparities in older adults (Dannefer, 2003).
Differential exposure to stressful experiences is believed to contribute to earlier biological aging in low SES and racial minority groups (Kessler, 1979; Pearlin, 1989). There is good evidence that individuals of low SES and African Americans are more frequently exposed to stressful experiences than their peers (Almeida et al., 2005; Turner & Avison, 2003). Repeated stressful experiences may accelerate biological aging in the hypothalamic pituitary adrenal (HPA) axis (McEwen, 1998). It has been hypothesized that flattened diurnal cortisol trajectories and high late-day cortisol nadir are HPA axis markers of aging (Piazza, Almeida, Dmitrieva, & Klein, 2010) because late-day nadir increases with age (Deuschle et al., 1997; Ferrari et al., 2001; Karlamangla, Friedman, Seeman, Stawksi, & Almeida, 2013; Kern, Dodt, Born, & Fehm, 1996) and flattened trajectories predict mortality (Kumari, Shipley, Stafford, & Kivimaki, 2011). High cortisol nadir likely represents loss of homeostatic regulation (Chrousos, 2009), since it appears to be due to decreased HPA axis inhibitory feedback (Wilkinson, Peskind, & Raskind, 1997). Therefore, individuals of low SES or African Americans may demonstrate slower late-day diurnal cortisol decline, compared with their peers, a marker of biological aging in the HPA axis.
Several studies have tested associations between SES, race, and late-day cortisol patterns, when HPA axis inhibition should be the most pronounced. Results are more consistent for race than SES. Middle-aged and older African Americans show slower afternoon and evening cortisol decline and higher late-day nadir than whites (Hajat et al., 2010; Karlamangla et al., 2013). However, the literature provides less insight into socioeconomic differences in late-day cortisol patterns. In the Coronary Artery Risk Development in Young Adult Study, young adults provided up to three of six samples after noon; low education, low income, and African American race were each associated with slower diurnal decline and a higher cortisol nadir (Cohen et al., 2006). These differences should be more pronounced in older adults, who have a longer duration of exposure to SES (Dannefer, 2003), but cortisol nadir has not been found to differ according to level of occupation (Gustafsson, Janlert, Theorell, & Hammarström, 2010; Steptoe et al., 2003) or financial strain (Steptoe, Brydon, & Kunz-Ebrecht, 2005). This evidence is based on data from racially homogeneous samples (e.g., Whitehall). Results may differ in racially diverse samples, where minority status itself may be a surrogate for unmeasured features of SES as well as race-associated adversity and stress (Geronimus, Hicken, Keene, & Bound, 2006; Kaufman & Cooper, 1999; LaVeist, 2005). In such settings, both low SES and minority race may contribute to HPA axis dysfunction in an additive fashion. In racially diverse samples of middle-aged and older adults (e.g., Multi-Ethnic Study of Atherosclerosis and Midlife in the United States Study), low education (Karlamangla et al., 2013) and low wealth (Hajat et al., 2010) have been associated with lower waking cortisol and slower decline in the morning hours but not afternoon and evening hours, after adjusting for race (Hajat et al., 2010; Karlamangla et al., 2013). These studies may have been under-powered to detect difference in late-day cortisol patterns by collecting only one or two samples after noon. Therefore, there is a gap in testing associations between SES and late-day cortisol decline in racially diverse samples of middle-aged and older adults, and evaluating whether SES or race is more relevant to disparities in HPA axis aging.
One critical consideration of such research is that standard SES indicators may be prone to misclassification in older adults (Grundy & Glaser, 2000). After retirement, income and occupation may not reflect SES as well as household wealth, which is not captured in most studies. Education, although generally fixed throughout adulthood, may not capture current SES. Few studies have examined a fuller range of multidimensional measures to evaluate how each may be differentially sensitive to exposure to psychosocial hazards.
The purpose of this study was to evaluate associations between SES and African American race and diurnal cortisol trajectories in middle-aged and older adults. Since race associations have been previously documented, this study focuses on SES associations. In this study, five of the six salivary cortisol samples were collected after noon, facilitating precise modeling of cortisol trajectories during the nadir phase. We hypothesized that low SES and African American race would be associated with lower waking cortisol and slower afternoon diurnal decline as a result of less robust responsiveness of the HPA axis.
Methods
Study Design and Sample
The Baltimore Memory Study (BMS) is a cohort study that enrolled 1,140 adults aged 50–70 years residing in 65 contiguous Baltimore City neighborhoods in 2001 and 2002 using random household phone sampling methods, representing a 48% response rate among those deemed eligible (Schwartz et al., 2004). A detailed examination of salivary cortisol was undertaken during the fourth study visit, which took place between February 2009 and May 2010. At that time, 624 participants were re-consented and came to a clinic visit. The study was approved by the Committee for Human Research of the Johns Hopkins Bloomberg School of Public Health. Participants provided written, informed consent.
Data Collection
All data collection was performed by trained research assistants. Baseline data used in these analyses include SES, age, sex, and race/ethnicity (analyzed as African American vs. all others). The remainder of the data used in these analyses was collected during the fourth study visit. Participants provided detailed medication data, including current use of exogenous hormones or corticosteroids, and recorded the number of alcoholic drinks consumed and the number of cigarettes smoked (0, 1–5, 6–10, 10–15, 16–20, and >20 cigarettes) in the 48hr prior to the clinic visit. Participants also indicated whether they had experienced any of 25 potentially stressful life events in the past week, and the severity of the perceived distress associated with those events, which was summed to create a stressful life event index. Awakening time for the day of the clinic visit was estimated based on reported time going to bed and number of hours slept. Body mass index (BMI) was computed based on measured height and weight. A medical condition summary score assigned one point for each of 15 different chronic diseases or medical events.
Saliva Collection and Cortisol Measurement
Each participant collected two saliva samples at home prior to the clinic visit. One was collected at bedtime the night before the visit and the second was collected immediately upon awakening the morning of the visit. Participants were asked to chew lightly on a salivette swab (Sarstedt, Inc., Newton, NC) or place it under their tongue for 45s. Participants were instructed to document the date and time for both samples and to refrigerate them in their original vials until their clinic visit. Four additional saliva samples were obtained during the study visit using the same procedure over a span of about 150min, before, during and after cognitive testing and at visit completion. In-clinic salivary collection protocols were initiated between 12 p.m. and 6 p.m. Bedtime saliva was collected the night before the study visit; here we assume that measure to be a surrogate for the cortisol value on the day of the study visit. Other studies have found little day-to-day variation in unprovoked cortisol values (Hajat et al., 2010; Karlamangla et al., 2013). To evaluate the sensitivity of our findings to the bedtime cortisol value, we conducted a planned sensitivity analysis excluding the bedtime measure. Salivary cortisol was measured by the core laboratory of the General Clinical Research Center at the Johns Hopkins Bayview Medical Center campus, Baltimore, using a standard radioimmunoassay (Diagnostic Systems Laboratories, Inc., Webster, TX). The lower and upper limits of detection were 2.76 and 276 nmol/L, respectively, for the assay. The inter-assay coefficient of variation was 3.2%. Due to severe skew, cortisol values were ln-transformed prior to analyses.
Socioeconomic Status
Household wealth and educational achievement were measured at baseline using a 110-item questionnaire developed for this study and described in detail elsewhere (Schwartz et al., 2004). A key feature of this instrument is that it assessed several sources of income (e.g., salaries, bonuses, extra income), transfers (e.g., social security, welfare, supplemental security income) and assets (e.g., homes, businesses, vehicles, and retirement assets) for both the respondent and a spouse/partner. Also, the instrument used bracketed value ranges to reduce missing data on participants who would not report exact dollar amounts. Locally weighted scatterplot smoothing (LOWESS) graphs (Cleveland & McGill, 1985) were used to explore cortisol trajectories across SES groups and collapse SES variables in a manner that ensured comparable trajectories within SES and race groups (see Supplementary Figure 1 which examines alternative classifications). Socioeconomic vulnerability was defined as annual income <500% of the US poverty threshold and insufficient assets to remain above that threshold for more than a year, based on prior work (Caner & Wolff, 2004). A lower, more policy-relevant threshold of 250% was considered, but there were insufficient numbers of whites below this threshold to enable race-adjusted analyses. The poverty threshold for either one- or two-person households was used, depending on whether the participant reported a spouse/partner ($8,860 and $11,940, respectively). Educational attainment was measured by combining self-reported years of education completed with credentials and certifications acquired (e.g., degrees, certificates, trade school). Educational status was categorized as low (high school, GED, or less than high school), intermediate (trade certificate, some college) or high (4 year college degree or higher).
Statistical Analyses
The purpose of this analysis was to evaluate associations between SES, race, and cortisol trajectories over the day. Descriptive analyses of variable distributions were conducted using standard graphing techniques and summary statistics. Bivariate associations with cortisol levels were examined using t-tests and ANOVA, as appropriate, and examined with scatterplots. To test hypotheses, random-effects models were estimated in a sequence of models from simple to more complex, adding suspected confounders. Random-effects models allow for robust hypothesis testing of factors thought to alter trajectories of change across a range of temporal specifications while correctly accounting for the serial correlation of repeated observations within subject (Singer & Willett, 2003; Willett, 1997). First, the average diurnal trajectory over the six cortisol values was modeled in an unrestricted time model by adding fixed, and then random effects for time and time polynomials in succession, evaluating fit using nested likelihood ratio tests. Time was calculated as an elapsed count of 30-min intervals centered on the average reported waking time (7:53 a.m.) across the sample (Time 0), allowing the intercept to be interpreted as the average waking cortisol. A random intercept for each subject was included in all models. The final model included fixed and random effects for time and time2 and a fixed effect for time3. An unstructured covariance matrix was used for the random effects and an autoregressive error structure was used to address heteroscedasticity in Level 1 residuals.
For hypothesis testing, each SES measure was tested in a separate model. To test the hypothesis that average cortisol trajectories differed by SES, we added main effects and any statistically significant interaction terms with time and time polynomials to the baseline model, adjusting for the influence of age (years, grand mean centered) and sex, entered as both main effects and as all statistically significant interaction terms with time and time polynomials (Model 1). Model 2 additionally adjusted for race/ethnicity (African American vs. all others). Model 3 additionally adjusted for the stressful life event index, cigarette use, awakening time, BMI, and medical conditions.
Results
Subjects lost to follow-up between baseline and the fourth study visit were similar in age to those who completed the fourth visit. However, they were less wealthy (median income and assets of $37,648 and $60,000, respectively, vs. $53,000 and $101,500), and less likely to have a college degree (35% vs. 48%), or be women (62% vs. 69%). Eleven participants were excluded from analyses due to current oral corticosteroid use. Another 46 were excluded who reported alcohol intake exceeding recommended limits within 48hr prior to cortisol sampling (>4 servings if men, >2 if women). One person was missing SES data, resulting in an analytic sample of 566. Participants contributed, on average, 5.9 cortisol observations (range 3–6), reflecting very little missing cortisol data. Also, the within-person ln-cortisol correlations for the four in-clinic samples was high (partial correlation ρ ranging from .541 to .854), suggesting minimal within-person variability in response to the in-clinic cognitive testing. African Americans had lower waking and higher bedtime cortisol than others (Table 1). Also, an intermediate, but not a low level of education was associated with lower waking cortisol. African Americans were more likely than whites to be women, have socioeconomic vulnerability, and lower levels of education.
Table 1.
Selected Sample Characteristics, Baltimore Memory Study, 2001–2002 and 2009–2010
| Mean ln-cortisol, nmol/L (SD) | Sample characteristics by race | |||
|---|---|---|---|---|
| Waking | Bedtime | White (%) (n = 336) | African American (%) (n = 230) | |
| Race/ethnicity | ||||
| White | 3.22 (0.73)* | 1.84 (0.79)* | ||
| African American | 3.00 (0.72)* | 2.05 (0.79)* | ||
| Other | 3.17 (0.75)* | 1.80 (0.72)* | ||
| Socioeconomically vulnerablea | ||||
| No | 3.13 (0.69) | 1.89 (0.79) | 296 (88)* | 142 (62)* |
| Yes | 3.15 (0.86) | 2.01 (0.81) | 40 (12)* | 88 (38)* |
| Education | ||||
| High | 3.20 (0.71)* | 1.89 (0.80) | 208 (62) * | 56 (24)* |
| Intermediate | 3.00 (0.65)* | 1.94 (0.69) | 58 (17)* | 97 (42)* |
| Low | 3.14 (0.83)* | 1.95 (0.89) | 70 (21)* | 77 (33)* |
| Age | ||||
| Mean (SD) | 66.76 (5.74) | 67.14 (6.08) | ||
| 56–66 years (%) | 3.09 (0.76) | 1.92 (0.84) | 188 (56) | 119 (52) |
| 67–78 years (%) | 3.18 (0.68) | 1.93 (0.75) | 148 (44) | 111 (48) |
| Sex | ||||
| Men | 3.19 (0.82) | 1.95 (0.78) | 120 (36)* | 58 (25)* |
| Women | 3.10 (0.69) | 1.91 (0.80) | 216 (64)* | 172 (75)* |
aDefined as household annual income <500% US poverty threshold and insufficient assets to remain above that threshold for more than 1 year.
*Subgroup differences p < .05.
Since cortisol was ln-transformed, regression coefficients represent the proportion of expected change in cortisol. In all models, cortisol declined linearly after waking (Tables 2 and 3). The coefficient for time2 was also negative in all models suggesting that the decline in cortisol accelerated across the day. The coefficient for time3 was positive, reflecting a trajectory that flattened as the diurnal pattern approaches its evening nadir. Unadjusted diurnal cortisol trajectories within SES and race strata are depicted in Figure 1.
Table 2.
Associations Between Socioeconomic Vulnerability and Both Waking Ln-Cortisol and 24-hr Ln-Salivary Cortisol Trajectories From Random Effects Models, Baltimore Memory Study
| Model 1a (n = 566) | Model 2b (n = 566) | Model 3c (n = 549) | ||||
|---|---|---|---|---|---|---|
| Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | |
| Time | −.02 | .012* | −.02 | .003* | −.03 | <.001* |
| Time2 | −.00 | <.001* | −.00 | <.001* | −.00 | <.001* |
| Time3 | .00 | <.001* | .00 | <.001* | .00 | <.001* |
| Socioeconomically vulnerabled | −.02 | .738 | .07 | .320 | .07 | .322 |
| Vulnerable × time | −.02 | .073 | −.02 | .022* | −.03 | .015* |
| Vulnerable × time2 | .00 | .013* | .00 | .014* | .00 | .010* |
| Vulnerable × time3 | −.00 | .029* | −.00 | .033* | −.00 | .022* |
| AA race | −.26 | <.001* | −.24 | <.001* | ||
| AA race × time | .01 | <.001* | .01 | <.001* | ||
Notes: All models included random intercept, fixed, and random effects for time and time2 and fixed effect for time3 and used an unstructured covariance matrix and a first-order autoregressive error structure.
aModel adjusted for the fixed effects socioeconomic vulnerability, mean-centered age, sex, and their statistically significant interactions with time and time polynomials.
bModel additionally adjusted for the fixed effects of race/ethnicity and its interaction with time.
cModel additional adjusted for the fixed effects of the stressful life event index, cigarette use, awakening time, body mass index and its interaction with time, and medical conditions.
dDefined as household annual income and assets <500% US poverty threshold.
*p < .05.
Table 3.
Associations Between Educational Achievement and Both Waking Ln-Cortisol and 24-hr Ln-Salivary Cortisol Trajectories From Random Effects Models, Baltimore Memory Study
| Model 1a (n = 566) | Model 2b (n = 566) | Model 3c (n = 549) | ||||
|---|---|---|---|---|---|---|
| Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | |
| Time (30min) | −.02 | <.001* | −.02 | <.001* | −.04 | <.001* |
| Time2 | −.00 | <.001* | −.00 | <.001* | −.00 | <.001* |
| Time3 | .00 | <.001* | .00 | <.001* | .00 | <.001* |
| Intermediate education vs. highd | −.17 | .009* | −.09 | .214 | −.05 | .438 |
| Low education vs. highd | −.11 | .089 | −.05 | .452 | −.04 | .571 |
| Intermediate education × time | .01 | .016* | .00 | .439 | .00 | .832 |
| Low education × time | .00 | .310 | −.00 | .920 | −.00 | .551 |
| AA race/ethnicity | −.21 | <.001* | −.21 | .001* | ||
| AA race/ethnicity × time | .01 | <.001* | .01 | <.001* | ||
Notes: All models included random intercept, and fixed and random effects for time and time2 and fixed effect for time3 and used an unstructured covariance matrix and a first-order autoregressive error structure.
*p < .05.
aModel adjusted for the fixed effects of educational level, mean-centered age, sex, and their statistically significant interactions with time and time polynomials.
bModel additionally adjusted for the fixed effects of race/ethnicity and its interaction with time.
cModel additionally adjusted for the fixed effects of the stressful life event index, cigarette use, awakening time, body mass index and its interaction with time, and medical conditions.
dCategorized as high (≥college), intermediate (some college), or low (≤high school).
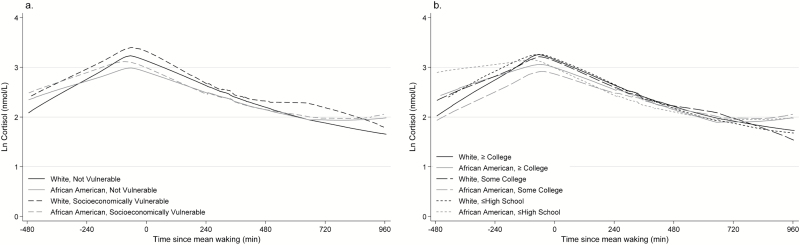
Figure 1.
Unadjusted locally weighted scatterplot smoothing graphs (LOWESS) of ln-transformed salivary cortisol trajectories, stratified by race/ethnicity, categorized as African Americans and all others and (a) socioeconomic vulnerability, defined as annual household income <500% US poverty threshold and insufficient assets to remain above that threshold for more than 1 year, and (b) education, categorized as high (referent, ≥college), intermediate (some college or trade school), or low (≤high school). Time was centered at sample mean waking time (7:53 a.m.).
Table 2 presents results of models examining socioeconomic vulnerability. After adjusting for the fixed effects of age and sex and their associations with cortisol decline (Model 1), socioeconomic vulnerability was not associated with waking cortisol (main effect β = −.02, p > .05) or the linear change in diurnal cortisol decline (β = −.02, p > .05) (Table 2). However, socioeconomic vulnerability was associated with slower acceleration in average diurnal cortisol decline (indicated by time2 interaction β = .002, p < .05) and a slightly less pronounced late-day flattening of the diurnal trajectory (indicated by time3 interaction β = −.0001, p < .05). Also, each additional year of age was associated with an almost 1% higher average waking cortisol level (p < .05), but there were no age-based differences in the rate of diurnal cortisol decline (see Supplementary Tables 1 and 2 for complete regression model results). There were no sex differences in waking cortisol, but women had more rapid decline compared with men (β = −.02, p < .05) (Supplementary Tables 1 and 2).
In Model 2, African Americans had approximately 26% lower average waking cortisol levels compared with the others and a 1% slower diurnal decline (African American race by time interaction). After adjusting for the fixed effect of African American race/ethnicity, socioeconomic vulnerability was associated with a 2% faster diurnal decline in addition to a slower acceleration in decline (time2 interaction β = .002, p < .05) and a slightly less pronounced late-day flattening (time3 interaction β = −.00005, p < .05) (Model 2). Further adjustment by the stressful life event index, cigarette use, awakening time, BMI, and medical conditions did not alter inferences (Model 3).
Table 3 presents results examining three levels of educational attainment. After accounting for age and sex (Model 1), intermediate education (some college or trade school) was associated with approximately 17% lower average waking cortisol (p < .05) and a 1% slower diurnal decline (p < .05 for intermediate education × time), compared with high education (college or more) (Table 3). Low educational attainment was not associated with waking cortisol or rate of decline. After adjustment for African American race/ethnicity (Model 2) educational attainment was not associated with cortisol trajectories.
Finally, we conducted a planned sensitivity analyses excluding bedtime cortisol sample values (i.e., using only the waking and four in-clinic samples) to assess the impact of our assumption that previous bedtime cortisol could be used as a surrogate for bedtime cortisol on the day of the clinic visit. In those models, not presented here, inferences were largely unchanged regarding associations for waking cortisol and rate of afternoon decline for education models. For socioeconomic vulnerability models, coefficients were in the expected direction, but were not statistically significant. Separately, we adjusted for the study visit time, prior diagnosis of anxiety or depression, and both anti-anxiety or anti-depression medications, but these were trimmed from the final models because they did not alter inferences.
Discussion
This study examined associations of SES and African American race with cortisol trajectories measured by six salivary samples in a cohort of community-dwelling middle- and older-aged adults. Based on our conceptual framework, we hypothesized that low SES and African American race contributed to greater stress exposures, resulting in earlier biological aging in these groups. In multilevel regression models of change in ln-transformed cortisol, African American race/ethnicity was associated with approximately 24% lower waking cortisol and 1% slower rate of afternoon cortisol decline in fully adjusted models. However, socioeconomic vulnerability was associated with 3% faster diurnal decline after accounting for race/ethnicity. These results suggest that race, more than SES, is associated with diminished late-day cortisol inhibition. As reviewed earlier, prior work suggests this cortisol trajectory may be a marker for earlier aging in vulnerable groups.
Our results failed to corroborate other studies showing that low SES is associated with lower waking cortisol. After adjusting for age and sex, socioeconomic vulnerability was not associated with waking cortisol or afternoon cortisol decline, and intermediate, but not the lowest level of education, was associated with lower waking cortisol and slower afternoon cortisol decline. After further adjustment for race/ethnicity, socioeconomic vulnerability was associated with faster afternoon decline and no associations were noted between education and cortisol. Results for socioeconomic vulnerability are contrary to our hypothesis of a slower afternoon decline. These results suggest a convex pattern to the diurnal cortisol trajectory in socioeconomically vulnerable individuals (rather than the typical concave pattern), with faster decline and slower acceleration, as depicted in Figure 1. These results add to the growing literature suggesting complex associations between SES and diurnal cortisol trajectories, as reviewed earlier and summarized by Dowd (2009). These results also suggest that low household wealth is associated with faster, rather than slower, diurnal cortisol decline. Although faster decline may also be a marker for HPA axis dysfunction (McEwen, 1998), this pattern has not been linked with earlier aging.
Results from this study suggest a more consistent link between African American race/ethnicity and diminished late-day cortisol inhibition in middle-aged and older adults, corroborating other findings. Associations between SES and cortisol were altered after adding African American race/ethnicity to models. This adds to an ongoing discussion regarding the complex interplay of race/ethnicity and SES (Glymour, Weuve, & Chen, 2008; Kaufman, Cooper, & McGee, 1997; Vanderweele & Robinson, 2014). Causal associations between SES, race/ethnicity and cortisol patterns are ambiguous in observational studies. To the extent that African American race/ethnicity determines individual SES, it may act as a powerful confounder of this association and should be adjusted for. On the other hand, race/ethnicity itself may act as a surrogate for unmeasured aspects of SES not well captured in our data. Self-reported race/ethnicity may capture long-term exposure to stressful experiences. This implies that race/ethnicity (Vanderweele & Robinson, 2014) and SES (Oakes & Rossi, 2003) are not separate causal processes, but are describing different aspects of cumulative adversity and vulnerability (Kawachi, Daniels, & Robinson, 2005). In this scenario, race/ethnicity is a competing measure of the exposure of interest or a surrogate for the effect of causal forces that lie between SES and HPA axis dysregulation. Under this scenario, adjustment for race/ethnicity increases rather than reduces bias. It is possible that our specification is incorrect and that Model 1 represents a better characterization of the causal effect of SES. As seen in Table 1, race and SES are highly correlated and race has been associated with salivary cortisol patterns in this study and others, as reviewed earlier. We followed previous studies that treat race as a confounder. However, questions have been raised about what exactly is being measured by self-reported race/ethnicity, because it represents the joint effect of physical phenotype, psychosocial exposures, and cultural and historical factors such as residential segregation (Vanderweele & Robinson, 2014). Some argue that race/ethnicity cannot be considered a confounder, or a causal factor at all, since it is not subject to plausible counterfactual manipulation (Kaufman & Cooper, 1999; Kaufman et al., 1997).
In addition, adjustment for race/ethnicity may produce biased estimates of the SES associations due to differential and dependent measurement error of SES by race/ethnicity. Measures of household income, wealth, and education may not perform equally well in blacks and whites (Caner & Wolff, 2004). Race/ethnicity may also be linked to measurement error of salivary cortisol. Physiologic factors, such as amount of binding proteins and medication use, can influence salivary cortisol measurement (Kudielka, Gierens, Hellhammer, Wüst, & Schlotz, 2012) and may be associated with race/ethnicity due to racial disparities in chronic diseases. However, our inferences remained unchanged after adjusting for BMI and medical conditions. Also, this study utilized a salivary cortisol assay with a very low coefficient of variation, and a wide-ranging limit of detection, providing evidence to support measurement reliability.
Salivary cortisol was measured as a biomarker for HPA axis dysregulation. However, the stress response system is complex. HPA axis dysregulation may be better captured with additional biomarkers (McEwen & Gianaros, 2010) or with additional cortisol samples, such as the 30–45min post-awakening sample to capture cortisol awakening response. Some (Steptoe et al., 2005; Wright & Steptoe, 2005), but not all (Hajat et al., 2010; Karlamangla et al., 2013) prior studies have found that low SES is associated with heightened cortisol awakening response, and this may account for steeper declines in the socioeconomically vulnerable group. Also, salivary and serum cortisol have a nonlinear correlation, so changes in salivary cortisol may not be proportional to changes in bioactive cortisol (Hellhammer, Wüst, & Kudielka, 2009). Cortisol samples were collected during a study visit that also included a battery of cognitive testing, which may not reflect basal cortisol patterns, although we found no evidence of an HPA axis response to cognitive testing. Also, SES was measured during the first study visit, and may not reflect changes in income at time of cortisol sampling, although household assets and education are likely stable over time. However, this study was strengthened by use of a population-based sample, multidimensional measures of SES with little missing data, and six repeated measures of cortisol, including five during the afternoon and evening hours.
In conclusion, in this study of middle aged and older adults, socioeconomic vulnerability was associated with faster diurnal decline in models accounting for age, sex, and African American race/ethnicity. African Americans had lower waking cortisol and slower afternoon decline, compared with others. This may contribute to health declines in African Americans, since lack of afternoon decline is considered to be a biological aging marker. Race/ethnicity may be a competing measure of cumulative vulnerability in this sample.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This work was supported by the National Institute of Aging (R01AG19604 to B. S. S. and T32AG000247 to L.J.S.).
Supplementary Material
Acknowledgments
L. J. S. conceived the research question, analyzed the data and led manuscript writing. T. A. G. and D. L. R. assisted with analyses and T. A. G. supervised the analyses. B. S. S. and T. A. G. coordinated the parent study, including planning instrumentation. All authors contributed to manuscript writing.
References
- Almeida D. M. Neupert S. D. Banks S. R., & Serido J (2005). Do daily stress processes account for socioeconomic health disparities?The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 60, 34–39. doi:10.1093/geronb/60.Special_Issue_2.S34 [DOI] [PubMed] [Google Scholar]
- Caner A., & Wolff E. N (2004). Asset poverty in the United States, 1984–99: Evidence from the Panel Study of Income Dynamics. Review of Income and Wealth, 50, 493–518. doi:10.1111/j.0034-6586.2004.00137.x [Google Scholar]
- Chrousos G. P. (2009). Stress and disorders of the stress system. Nature Reviews. Endocrinology, 5, 374–381. doi:10.1038/nrendo.2009.106 [DOI] [PubMed] [Google Scholar]
- Cleveland W. S., & McGill R (1985). Graphical perception and graphical methods for analyzing scientific data. Science, 229, 828–833. doi:10.1126/science.229.4716.828 [DOI] [PubMed] [Google Scholar]
- Cohen S., Schwartz J. E., Epel E., Kirschbaum C., Sidney S., Seeman T. (2006). Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosomatic Medicine, 68, 41–50. doi:10.1097/01.psy.0000195967.51768.ea [DOI] [PubMed] [Google Scholar]
- Crimmins E. M. Kim J. K., & Seeman T. E (2009). Poverty and biological risk: The earlier “aging” of the poor. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 64, 286–292. doi:10.1093/gerona/gln010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannefer D. (2003). Cumulative advantage/disadvantage and the life course: Cross-fertilizing age and social science theory. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 58, S327–S337. doi:10.1093/geronb/58.6.S327 [DOI] [PubMed] [Google Scholar]
- Deuschle M. Gotthardt U. Schweiger U. Weber B. Korner A. Schmider J., … Heuser I (1997). With aging in humans the activity of the hypothalamus-pituitary-adrenal system increases and its diurnal amplitude flattens. Life Sciences, 61, 2239–2246. doi:10.1016/S0024-3205(97)00926-0 [DOI] [PubMed] [Google Scholar]
- Dowd J. B., Simanek A. M., Aiello A. E. (2009). Socio-economic status, cortisol and allostatic load: A review of the literature. International Journal of Epidemiology, 38, 1297–1309. doi:10.1093/ije/dyp277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari E. Cravello L. Muzzoni B. Casarotti D. Paltro M. Solerte S. B., … Magri F (2001). Age-related changes of the hypothalamic-pituitary-adrenal axis: pathophysiological correlates. European Journal of Endocrinology, 144, 319–329. doi:10.1530/eje.0.1440319 [DOI] [PubMed] [Google Scholar]
- Geronimus A. T. Hicken M. Keene D., & Bound J (2006). “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health, 96, 826–833. doi:10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour M. M. Weuve J., & Chen J. T (2008). Methodological challenges in causal research on racial and ethnic patterns of cognitive trajectories: Measurement, selection, and bias. Neuropsychology Review, 18, 194–213. doi:10.1007/s11065-008-9066-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy E., & Glaser K (2000). Socio-demographic differences in the onset and progression of disability in early old age: a longitudinal study. Age and Ageing, 29, 149–57. doi:10.1093/ageing/29.2.149 [DOI] [PubMed] [Google Scholar]
- Gustafsson P. E. Janlert U. Theorell T., & Hammarström A (2010). Life-course socioeconomic trajectories and diurnal cortisol regulation in adulthood. Psychoneuroendocrinology, 35, 613–623. doi:10.1016/j.psyneuen.2009.09.019 [DOI] [PubMed] [Google Scholar]
- Hajat A. Diez-Roux A. Franklin T. G. Seeman T. Shrager S. Ranjit N., … Kirschbaum C (2010). Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The multi-ethnic study of atherosclerosis. Psychoneuroendocrinology, 35, 932–943. doi:10.1016/j.psyneuen.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellhammer D. H. Wüst S., & Kudielka B. M (2009). Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology, 34, 163–171. doi:10.1016/j.psyneuen.2008.10.026 [DOI] [PubMed] [Google Scholar]
- Karlamangla A. S. Friedman E. M. Seeman T. E. Stawksi R. S., & Almeida D. M (2013). Daytime trajectories of cortisol: Demographic and socioeconomic differences—Findings from the National Study of Daily Experiences. Psychoneuroendocrinology, 38, 2585–2597. doi:10.1016/j.psyneuen.2013.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlamangla A. S. Singer B. H. McEwen B. S. Rowe J. W., & Seeman T. E (2002). Allostatic load as a predictor of functional decline. MacArthur Studies of Successful Aging. Journal of Clinical Epidemiology, 55, 696–710. doi:10.1016/S0895-4356(02)00399-2 [DOI] [PubMed] [Google Scholar]
- Kaufman J. S., & Cooper R. S (1999). Seeking causal explanations in social epidemiology. American Journal of Epidemiology, 150, 113–120. doi:10.1093/oxfordjournals.aje.a009969 [DOI] [PubMed] [Google Scholar]
- Kaufman J. S. Cooper R. S., & McGee D. L (1997). Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology, 8, 621–628. doi:10.1097/00001648-199711000-00006 [PubMed] [Google Scholar]
- Kawachi I. Daniels N., & Robinson D. E (2005). Health disparities by race and class: why both matter. Health Affairs, 24, 343–352. doi:10.1377/hlthaff.24.2.343 [DOI] [PubMed] [Google Scholar]
- Kern W. Dodt C. Born J., & Fehm H. L (1996). Changes in cortisol and growth hormone secretion during nocturnal sleep in the course of aging. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 51, M3–M9. doi:10.1093/gerona/51A.1.M3 [DOI] [PubMed] [Google Scholar]
- Kessler R. C. (1979). Stress, social status, and psychological distress. Journal of Health and Social Behavior, 20, 259–272. doi:10.2307/2136450 [PubMed] [Google Scholar]
- Kudielka B. M., Gierens A., Hellhammer D. H., Wüst S., Schlotz W. (2012). Salivary cortisol in ambulatory assessment—Some dos, some don’ts, and some open questions. Psychosomatic Medicine, 74, 418–431. doi:10.1097/PSY.0b013e31825434c7 [DOI] [PubMed] [Google Scholar]
- Kumari M., Shipley M., Stafford M., Kivimaki M. (2011). Association of diurnal patterns in salivary cortisol with all-cause and cardiovascular mortality: Findings from the Whitehall II study. The Journal of Clinical Endocrinology and Metabolism, 96, 1478–1485. doi:10.1210/jc.2010-2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVeist T. A. (2005). Disentangling race and socioeconomic status: A key to understanding health inequalities. Journal of Urban Health: Bulletin of the New York Academy of Medicine, 82, iii26–iii34. doi:10.1093/jurban/jti061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. K., Glass T. A., McAtee M. J., Wand G. S., Bandeen-Roche K., Bolla K. I., Schwartz B. S. (2007). Associations of salivary cortisol with cognitive function in the Baltimore memory study. Archives of General Psychiatry, 64, 810–818. doi:10.1001/archpsyc.64.7.810 [DOI] [PubMed] [Google Scholar]
- McEwen B. S. (1998). Protective and damaging effects of stress mediators. The New England Journal of Medicine, 338, 171–179. doi:10.1056/NEJM199801153380307 [DOI] [PubMed] [Google Scholar]
- McEwen B. S., & Gianaros P. J (2010). Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences., 1186, 190–222. doi:10.1111/j.1749-6632.2009.05331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. M., & Rossi P. H (2003). The measurement of SES in health research: Current practice and steps toward a new approach. Social Science & Medicine (1982), 56, 769–784. doi:10.1016/s0277-9536(02)00073-4 [DOI] [PubMed] [Google Scholar]
- Pearlin L. I. (1989). The sociological study of stress. Journal of Health and Social Behavior, 30, 241–256. doi:10.2307/2136956 [PubMed] [Google Scholar]
- Piazza J. R. Almeida D. M. Dmitrieva N. O., & Klein L. C (2010). Frontiers in the use of biomarkers of health in research on stress and aging. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 65, 513–525. doi:10.1093/geronb/gbq049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbah W. Watt R. G. Sheiham A., & Tsakos G (2008). Effects of allostatic load on the social gradient in ischaemic heart disease and periodontal disease: Evidence from the Third National Health and Nutrition Examination Survey. Journal of Epidemiology and Community Health, 62, 415–420. doi:10.1136/jech.2007.064188 [DOI] [PubMed] [Google Scholar]
- Schwartz B. S. Glass T. A. Bolla K. I. Stewart W. F. Glass G. Rasmussen M., … Bandeen-Roche K (2004). Disparities in cognitive functioning by race/ethnicity in the Baltimore Memory Study. Environmental Health Perspectives, 112, 314–20. doi:10.1289/ehp.6727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman T. E. Crimmins E. Huang M. H. Singer B. Bucur A. Gruenewald T. … Reuben D. B (2004). Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Social Science & Medicine (1982), 58, 1985–1997. doi:10.1016/S0277-9536(03)00402-7 [DOI] [PubMed] [Google Scholar]
- Seeman T. E. McEwen B. S. Rowe J. W., & Singer B. H (2001). Allostatic load as a marker of cumulative biological risk: MacArthur Studies of Successful Aging. Proceedings of the National Academy of Sciences of the United States of America, 98, 4770–4775. doi:10.1073/pnas.081072698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. D., & Willett J. B (2003). Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press. [Google Scholar]
- Steptoe A., Brydon L., Kunz-Ebrecht S. (2005). Changes in financial strain over three years, ambulatory blood pressure, and cortisol responses to awakening. Psychosomatic Medicine, 67, 281–287. doi:10.1097/01.psy.0000156932.96261.d2 [DOI] [PubMed] [Google Scholar]
- Steptoe A. Kunz-Ebrecht S. R. Owen N. Feldman Pamela J. Willemsen G. Kirschbaum C., & Marmot M (2003). Socioeconomic status and stress-related biological responses over the working day. Psychosomatic Medicine, 65, 461–470. doi:10.1097/01.PSY.0000035717.78650.A1 [DOI] [PubMed] [Google Scholar]
- Turner R. J., & Avison W. R (2003). Status variations in stress exposure: Implications for the interpretation of research on race, socioeconomic status, and gender. Journal of Health and Social Behavior, 44, 488–505. doi:10.2307/1519795 [PubMed] [Google Scholar]
- Vanderweele T. J., & Robinson W. R (2014). On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology, 25, 473–484. doi:10.1097/EDE.0000000000000105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson C. W. Peskind E. R., & Raskind M. A (1997). Decreased hypothalamic-pituitary-adrenal axis sensitivity to cortisol feedback inhibition in human aging. Neuroendocrinology, 65, 79–90. doi:10.1159/000127167 [DOI] [PubMed] [Google Scholar]
- Willett J. B. (1997). Measuring change: What individual growth modeling buys you. In Amsel E., & Renninger K. A. (Eds.), Change and development: Issues of theory, method, and application (pp. 213–243). Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Wright C. E., & Steptoe A (2005). Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology, 30, 582–90. doi:10.1016/j.psyneuen.2005.01.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.