Abstract
The Global Burden of Disease (GBD) project quantifies the impact of different health conditions by combining information about morbidity and premature mortality within a single metric, the Disability Adjusted Life Year. One important goal for the GBD project has been to inform decisions about global health priorities. A number of recent studies have used GBD data to argue that global health funding fails to align with the GBD. We argue that these studies’ shared assumption that global health resources should ‘align’ with the burden of disease is unfounded and has troubling implications. First, since the allocation of resources involves difficult trade-offs between different, potentially competing goals, any ‘misalignment’ of allocation and disease burdens need not necessarily indicate that the allocation of funds fails to meet recipient countries’ needs or interests. Second, using alignment as a baseline implicitly makes controversial assumptions about how harms of different magnitudes affecting different numbers of individuals should be aggregated. We discuss two alternative ways in which GBD data could help inform decisions about resource allocation, neither of which gives more than a limited role to GBD data.
The Global Burden of Disease (GBD) project quantifies the impact of different health conditions by combining information about morbidity and premature mortality within a single metric, the Disability-Adjusted Life Year (DALY). First initiated in the 1990s, the most recent version of the GBD, published in 2012, captures the state of global health in 2010.1 From the beginning, the DALY and many aspects of its underlying methodology have been criticized on both technical and normative grounds, including age-weighting (i.e. DALYs lost at different ages were assigned different weights), discounting of future DALY losses and the survey methods used for determining disability weights.2 In response to these concerns, the most recent version of the GBD has abandoned or adapted certain aspects of the original methodology. For example, the GBD no longer incorporates age-weighting in the DALY (Murray et al., 2012c), and it uses a new and more globally representative procedure for determining disability weights (Salomon, 2010; Salomon et al., 2015).
While concerns about the DALY and its methodology remain,3 in this article, we want to set aside the debate about the validity of the DALY to focus on issues arising in the context of its use in priority-setting and resource-allocation decisions. These issues are particularly salient because a central goal for the GBD has been not only to monitor developments in global health but also to inform policy decisions and debates about global health priorities (Murray, 1996; Murray et al., 2012b, 2012c). Concerns about the validity of the DALY of course also challenge its utility for such decisions; however, even if the DALY is accepted as a valid measure, there are separate concerns about the use of DALYs to inform priority-setting and resource-allocation decisions. In this article, therefore, we discuss problems that remain when using the DALY to make decisions about global health priorities and resource allocation, even if the validity of the DALY as a measure of health is confirmed.
We begin by describing how GBD data has been used in some discussions of global health policy. We focus on a number of recent studies that use GBD data to assess the allocation of global health aid and express concern that this allocation is ‘misaligned’ with the disease burden. We argue that the reliance on the idea of ‘alignment’ is problematic in two related respects. First, deviation from alignment does not necessarily indicate a deviation from recipient needs or priorities. Second, the idea that ‘alignment’ is useful in assessing the allocation of global health aid relies on implicit assumptions about how to aggregate harms of different magnitudes. The subsequent section sketches two alternative methods of assessing the allocation of global health aid: directly applying distributive principles to allocation decisions and focusing on the legitimacy of allocation decisions. Neither of these alternatives gives a prominent role to GBD data.
The DALY, Alignment and Priorities for Global Health
Notwithstanding concerns about the GBD’s reliance on estimates where actual data are not available (Byass et al., 2013), the DALY is probably the most powerful metric available for monitoring the distribution of health and illness at the global level, and arguments about global health policy and priority setting often rely on GBD data.4 In particular, a number of recent studies use GBD data to assess the distribution of global health resources across different health conditions and different geographical regions, each concluding that global health funding fails to align with the GBD (Shiffman, 2006; Stuckler et al., 2008; Dieleman et al., 2014; Hanlon et al., 2014). These studies share a common concern about a number of ‘disparities’ in how funding is allocated.
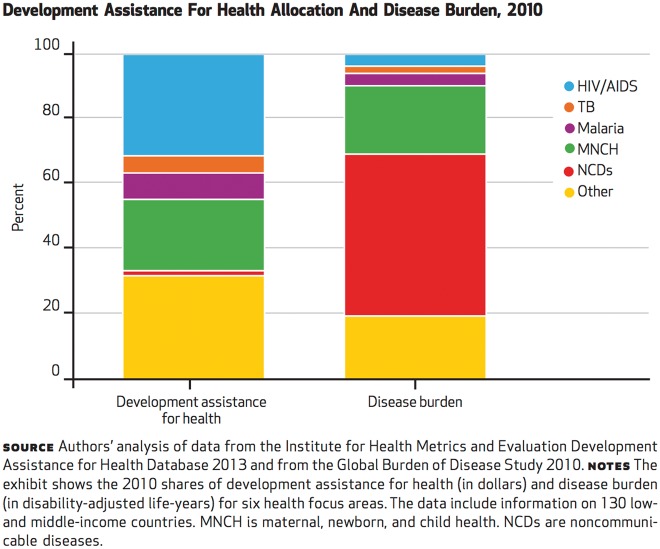
First, they suggest that there is a disparity in the distribution of funds across diseases or disease groups. For example, Dieleman et al. compare the disease burden and development assistance for health (DAH) provided by donors and international agencies for 130 low- and middle-income countries. They find what they call a ‘lack of congruence’: whereas noncommunicable diseases accounted for 49.8 per cent of the total disease burden, they received 2.3 per cent of the development assistance; by contrast, HIV/AIDS and maternal, newborn and child health, which make up 3.7 per cent and 21 per cent of the burden, receive 45.9 per cent and 32.2 per cent of development assistance, respectively (see Figure 1).
Figure 1.
Allocation of DAH relative to disease burden, 2010. Reproduced from Dieleman et al. (2014). Copyrighted and published by Project HOPE/Health Affairs as Dieleman et al., Global health development assistance remained steady in 2013 but did not align with recipients' disease burden. Health Affairs, 33 (5): 878–886. The published article is archived and available online at www.healthaffairs.org. Reprinted with permission.
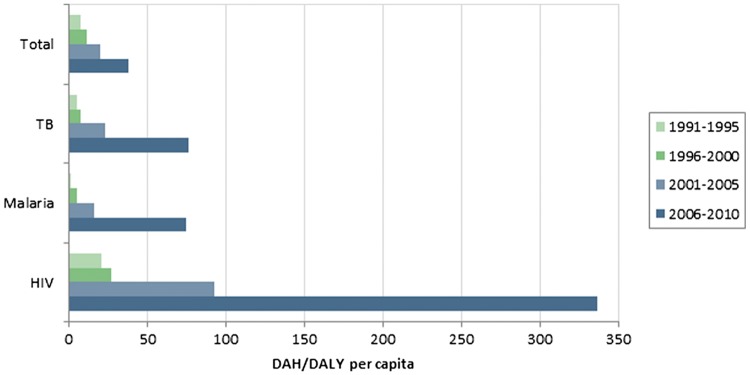
Using a similar methodology, Hanlon et al. find significant variation across disease-specific funding. With respect to development assistance per DALY per capita, they find that ‘donors provide much more development assistance for health per HIV/AIDS DALY than from other burdens’ (compared to total burden, burden caused by tuberculosis (TB) and burden caused by malaria) (Hanlon et al., 2014). Figure 2 illustrates these findings.
Figure 2.
Global DAH per DALY, by disease. Reproduced from Hanlon et al. (2014).
Focusing on funds for health research, Stuckler et al. analyze how the budget of the World Health Organization (WHO) is distributed across different diseases and disease categories, using both mortality and DALY burdens of different diseases. They find that
WHO funds and disease burden were not correlated in 2004–05, irrespective of whether measured in terms of mortality or DALYs … noncommunicable disease accounted for more than half of global mortality and almost half of global DALYs, but received roughly a tenth of all WHO funds. We recorded a similar disparity with injuries, which claimed 9% of global mortality and 12% of global DALYs, but received less than 1% of global funds. (Stuckler et al., 2008: 1566)
Shiffman assesses the allocation of funds across different infectious diseases, using annual donor dollars per DALY caused by different health conditions (Shiffman, 2006). He finds that annual donor dollars vary significantly across diseases; for example, whereas polio receives US$2,454 per DALY in annual donations, acute respiratory infections receive only US$0.58 per DALY. He concludes that ‘direct grants [of funds] correspond little to burden’ (Shiffman, 2006: 415).
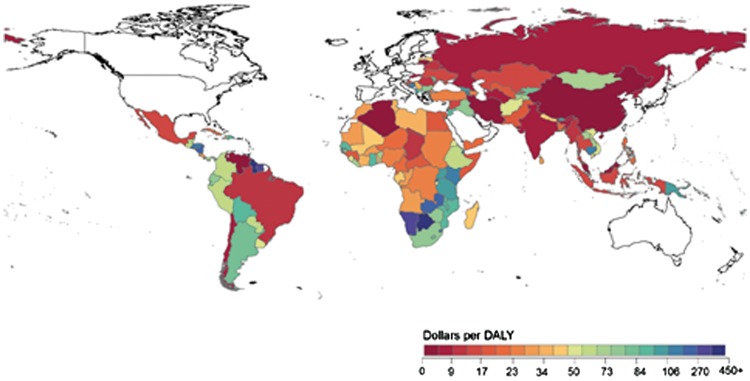
Second, some studies suggest that there is a disparity in funding across geographical regions. Hanlon et al. find considerable variation in funding across geographical areas: South Asia, East Asia and Pacific receive relatively little health-related development assistance per DALY, while Sub-Saharan Africa receives the most. They also provide a map illustrating country-level funding, again showing considerable variation in dollars received per DALY (see Figure 3) (Hanlon et al., 2014).
Figure 3.
Global map of DAH at the country level (2006–2010). Figure reproduced from Hanlon et al. (2014).
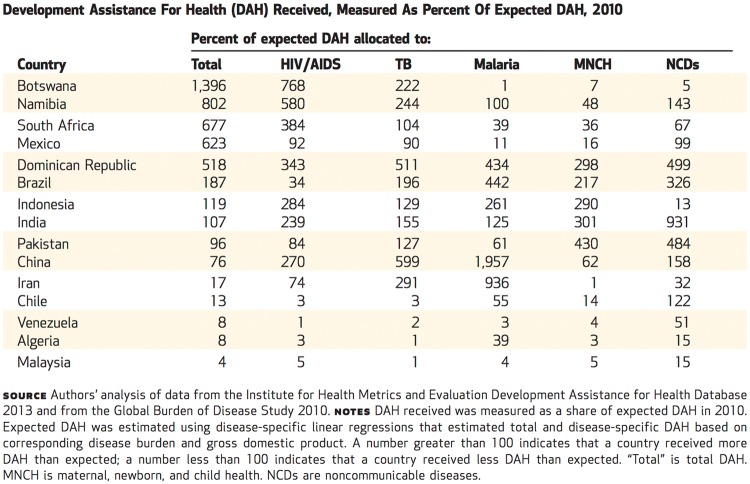
Dieleman et al. also consider how funds were distributed across a sample of 130 low- and middle-income countries. They calculated the amount of ‘expected’ development assistance for each country based on its total disease-specific disease burden and its GDP. Again, the funds actually received differed significantly from the expected amounts, with Botswana, for example, receiving almost 14 times its expected amount and Malaysia only 4 per cent of the expected amount (Figure 4).
Figure 4.
Reproduced from Dieleman et al. (2014). Copyrighted and published by Project HOPE/Health Affairs as Dieleman et al., Global health development assistance remained steady in 2013 but did not align with recipients' disease burden. Health Affairs, 33 (5): 878–886. The published article is archived and available online at www.healthaffairs.org. Reprinted with permission.
Stuckler et al.’s analysis also considers allocations of funds across geographical regions, although with a somewhat different purpose. They compare funds distributed across different diseases in Africa and the Western Pacific regions.5 The disease burdens in these two regions are very different: whereas in Africa, the disease burden is mostly due to infectious disease, in the Western Pacific area, noncommunicable diseases are prominent. They ask whether WHO allocates a greater proportion of funds to noncommunicable diseases in the Western Pacific region compared to the African region and find that ‘in both regions, the resources were heavily skewed towards infectious disease’ (Stuckler et al., 2008: 1566).
While the studies’ normative assumptions are typically not made explicit, the findings are frequently couched in normative language, suggesting that the authors consider their findings problematic. For example, Stuckler et al. speak about a ‘mismatch between the disease burden and allocated funds’ (Stuckler et al., 2008: 1567), a ‘misalignment’ (Stuckler et al., 2008: 1563) and a ‘skew towards infectious diseases’ (Stuckler et al., 2008: 1563), while Dieleman et al. talk about a ‘lack of alignment between disease burden, income [i.e. national income of recipient country], and funding’ (Dieleman et al., 2014: 878) and a striking ‘discrepancy between global burden and DAH health focus areas’ (Dieleman et al., 2014: 884).
Some of the studies suggest that allocation decisions would be better if they more closely tracked the distribution of disease burdens across different diseases and geographical regions. For example, Dieleman et al. suggest that the lack of alignment ‘reveals the potential for improvement in resource allocation’, and that
Tracking … international transfers of funds for health and tying them to disease burden estimates is imperative for policy makers, donors, and health program managers. Comparisons across disease burdens and spending on different diseases provide a necessary input into resource allocation decisions. When such comparisons are combined with information describing the cost, effectiveness, and availability of interventions, analysts can use them to prescribe optimal spending patterns. (Dieleman et al., 2014: 878)
Perhaps most explicitly, Hanlon et al. suggest that the ‘ratio of assistance to burden … is a useful metric that should influence donor behavior’ (Hanlon et al., 2014: 4).
To be sure, none of the studies suggest that disease burden alone should guide resource allocation across diseases and/or regions. Considerations such as preventing the spread of infectious diseases, economic effects, cost-effectiveness of available interventions, national security concerns and non-health factors are mentioned as possible (and appropriate) reasons for ‘deviation’ from funding in direct proportion to disease burden (Shiffman, 2006; Stuckler et al., 2008; Dieleman et al., 2014; Hanlon et al., 2014). These considerations, however, do not capture a more fundamental concern about the implicit assumptions and approach adopted in these studies, which have more thorough-going implications for the project of using GBD data to inform global health policy.
Concerns
While these studies provide important insights into the distribution of global health funding, we are concerned about their normative assumptions and implications. Implicit in these arguments is the idea that ‘alignment’ between allocation of funds and disease burdens (either in terms of its distribution across different kinds of diseases or across different geographical regions) is a self-evident criterion for assessing the allocation of global health funding. Stuckler et al. note explicitly that ‘[i]deally, we would relate trends in global expenditure on research to the burden that is attributable to different categories of disease’ (Stuckler et al., 2008: 1564). For the allocation of funds to be perfectly aligned with disease burdens, the allocation of resources across different health conditions would be in direct proportion to the contribution each makes to the all-cause disease burden. For example, if a third of the all-cause disease burden results from a particular disease group, then one-third of available funds should be directed toward that disease group. We have two concerns about the idea of ‘alignment’.
‘Alignment’ and Recipient Priorities
Several studies suggest that deviation from perfect alignment indicates that current funding is driven at least in part by priorities other than those of the recipients. For example, Shiffman suggests that the relatively high funding for HIV/AIDS and TB may reflect the fact that these diseases are also important contributors to the burden of disease in industrialized countries (Shiffman, 2006: 415–416). Similarly, Stuckler et al. note that the WHO’s regular budget is more closely aligned with the burden of disease than extra-budgetary funds, which reflect the priorities of wealthy donor countries and private donors (Stuckler et al., 2008: 1567).
While it may, of course, be true that the current distribution of global health resources reflects donor rather than recipient priorities, this cannot be concluded from the presence of ‘misalignment’ alone and, by the same token, it does not follow that recipients’ needs would be best met by ensuring that the distribution of funding across different diseases corresponds to the contribution each condition makes to the overall, all-cause disease burden in those countries. While, as we noted earlier, most of the studies acknowledge plausible reasons for deviating from such an allocation—e.g. cost-effectiveness of interventions addressing different diseases, concerns about contagiousness or national security—they generally presume that if no such reasons can be found, the allocation of funds must reflect a departure from the true interests or priorities of recipients.
However, whether or not alignment reflects the interests of recipients is an open question requiring both normative inquiry and empirical research. Allocating resources for health involves difficult trade-offs between different, potentially competing goals. Countries may seek to improve population health as measured by the DALY, but may also seek to reduce health inequalities, or to address poor health outcomes due to specific health conditions or among specific social groups. A distribution of resources that, from the outside, may look like a ‘misalignment’, could reflect different, but entirely reasonable, normative decisions about which objectives to prioritize. Moreover, different communities may differ in their priorities, and a distribution of resources—including one that ‘aligns’ with the burden of disease—that reflects the priorities of one country may not be appropriate for another.
Normative Issues with Aggregation
A second concern is that using alignment as a baseline implies a particular answer to the question of how we should respond to harms of different magnitudes affecting different numbers of individuals. This problem is familiar in philosophical debates (e.g. Hirose, 2014) and the relevance of these concerns to the application of the DALY and other summary measures of health such as the quality-adjusted life-year has been recognized (e.g. Daniels, 1998; Arnesen and Kapiriri, 2004). In the present context, relying on alignment could lead us to prioritize conditions that are less severe but more prevalent over conditions that are more severe but less prevalent. This is because GBD data aggregates DALYs across different individuals who may suffer from conditions of different levels of severity; the total DALYs resulting from any particular condition are a function of the severity weight attached to the condition, life-years typically lost and/or lived with the condition and the number of people affected by the condition.
Consider the following example (which oversimplifies certain aspects of the DALY). Imagine three different conditions, each constituting a total DALY loss of 30. Condition A affects one person, causing her to lose 30 fully healthy life-years; condition B causes 30 people to lose one fully healthy life year each; and condition C causes 600 people to live 1 year with a disability weighted at 0.05. Each of these three conditions results in 30 DALYs lost, or one-third of a total DALY burden of 90.
If we were to perfectly align spending with disease burden, each condition would receive one-third of available resources. However, one can make reasonable cases for ‘misaligned’ funding. For example, one might prioritize C, on the grounds that it will benefit the most individuals; alternatively, one might prioritize A, on the grounds that it will address the most severe condition. Aggregating harms of different magnitudes suffered by different individuals raises important philosophical questions, which are neglected by studies that assume ‘alignment’ as the default distribution.
While many diseases responsible for high DALY burdens are severe conditions that typically lead to premature mortality at a young age, less severe conditions with lower mortality can also make large contributions to the total disease burden if their prevalence is very high. For example, individuals may suffer from depressive disorders over long periods of time, and the high prevalence of these disorders makes them one of the top contributors to the total burden of disease, even though they are not associated with a large mortality rate. Non-communicable diseases such as cancer and diabetes, which make up an increasingly large share of the total burden of disease, typically result in premature mortality at older ages and have varying degrees of severity. Should these conditions receive higher priority than more severe diseases because they are more prevalent? Using ‘alignment’ as a baseline simply assumes, without further argument, that the answer to this question is yes.
Similar concerns arise if we look at how funding relates to the distribution of disease burdens across different countries or geographical regions. Imagine three different countries, each with the same per capita disease burden (let us also assume that these countries are similar in other relevant respects, such as income). In country A, the disease burden is due to a small number of people suffering from very severe conditions; in county B, the same per capita DALY loss is the result of a larger number of people suffering from a moderately disabling condition; in country C, everyone suffers from a mild disease, again resulting in the same per capita DALY loss. The idea of ‘alignment’ would suggest that, at least as a starting point, the total funds should be allocated equally between these three countries. Again, however, there is little reason to assume, certainly not without further argument, that this would be a reasonable starting point, once we take into account that per capita DALY loss averages out differences in disease severity across individuals within a population. One might argue, for example, that no funds should be allocated to country C because the DALY loss is entirely caused by mild diseases.
Alternatives
The DALY was developed to allow monitoring of global health in a way that captures premature mortality, morbidity and disability all within the same measure. This was meant to address the overemphasis on mortality (especially among young children) at the expense of morbidity in global health debates, and the potential for advocacy groups to overstate the mortality rates associated with ‘their’ diseases (Murray, 1996). The creators of the DALY envisaged that GBD data would provide important insights for debates about global health priorities and resource allocation; they also suggested, however, that the data would only be one among several criteria on which global health priorities should be based:
The comparative view provided by summary measures [of health] helps decision-makers, researchers, and citizens understand what the most important problems are and whether they are getting better or worse. This information, along with information on the costs, intervention effectiveness, and equity implications of health interventions and policy options, lays the foundation for a debate on priorities for health policy action and research that is clearly informed by the best available evidence. (Murray et al., 2012c: 2198, emphasis added)
This passage suggests an appreciation of the shortcomings of the DALY in resource allocation and priority-setting decisions, but also leaves policy makers with the task of determining how exactly to use GBD data in their deliberations. Thus, even though the GBD was developed with the goal of informing the debate about global health priorities, it is not clear how exactly it can play this role. In this section, we sketch out two alternative methods of making decisions about allocation of global health resources, and the possible role of GBD data in each method.
Direct Application of Distributive Principles
One way to approach the question of how global health aid should be allocated is to use theories of distributive justice to guide distributive decisions. Political philosophers of course disagree about how precisely the notion of distributive justice should be interpreted. The most prominent approaches in the current debate—egalitarianism, prioritarianism and sufficientarianism—propose rather different ways of thinking about distributions and what makes them just or unjust; moreover, within each of these broad categories, different interpretations of the basic distributive principle are possible and disputed in the literature. Despite these theoretical disagreements, Wolff and de-Shalit (2007) have argued that, for practical, real-world purposes, the goal of improving the condition of the least advantaged is one that advocates of these different accounts of distributive justice can endorse:
provided that there are people in society who have not yet achieved sufficiency, and provided we have in mind limited, or at least finite, budgets and financial resources, then all of these views appear to converge on the same general policy prescription in the short to medium term: identify the worst off and take appropriate steps so that their position can be improved. (Wolff and de-Shalit, 2007: 3, emphasis in original)
One implication of an approach that prioritizes the worst-off in this way could be that, contrary to the assumption that ‘alignment’ represents the best way to distribute resources, we should not direct resources toward mild or even moderately severe diseases at all, including many non-communicable diseases (Sharp and Millum, 2015a, 2015b). Thus, for many normatively appealing ways of allocating resources, it is crucial to distinguish between mortality, disease severity and prevalence—distinctions that the DALY obscures in an effort to provide a way of making different health states quantifiable and comparable.
A similar concern for the worst-off may underlie Stuckler et al.’s decision to assess the distribution of funding not just across diseases with different DALY loss but also to relate the distribution of funding to the mortality burdens caused by different diseases (Stuckler et al., 2008). Whereas DALYs capture both morbidity and mortality, focusing on mortality effectively focuses on diseases that are severe enough to kill. To the extent that this can capture aspects of disease severity, this comparison is more apt than a focus on DALY losses alone. However, we should note that this would be true only for age-adjusted mortality rates: if our goal is to identify the worst-off, it would likely also matter at what age people die—those threatened by a disease that kills at age 15, for example, might be considered worse off than those who face death at age 65.
Importantly, on such an approach, GBD data would play a different and much smaller role in resource allocation decisions than is assumed in much of the literature. An approach such as Wolff and de-Shalit’s requires us to decide which criteria should be used to identify the worst-off. On this issue, a number of different positions can be defended; for example, do we focus on those who are worst off with respect to health only, or are we trying to capture who is worst off, all things considered (Brock, 2012; Sharp and Millum, 2015a)? On the latter interpretation, a focus on improving the situation of the worst-off could justify a funding ‘misalignment’ in favor of funding interventions addressing a very severe but rare disease that is particularly prevalent among the most disadvantaged residents of a country.
For many of the positions we might take on these philosophical questions, GBD data might be helpful in identifying those individuals who should be considered among the worst off. Further, among different interventions that seek to help the worst-off, we may prefer more over less cost-effective interventions; GBD data could again be helpful in making such assessments (though the caveats about aggregation mentioned above still apply), as the DALY has been used to capture the cost-effectiveness of a range of different interventions (Jamison et al., 2006; Laxminarayan et al., 2006). However, questions about the relative contributions different diseases and different geographical regions make to the total disease burden will no longer be directly relevant, and GBD data would play a much smaller role in informing allocation decisions than is assumed in much of the literature.
Legitimacy of Allocation Decisions
An alternative approach for making decisions about research allocation and priorities for global health focuses on the political legitimacy of such decisions. Stuckler et al.’s (2008) analysis hints at these considerations. One of their objectives is to identify discrepancies between the allocation priorities implicit in the regular WHO budget and those of its extra-budgetary funds. There are crucial differences in how these two budgets are allocated. The allocation of the extra-budgetary funds is driven by ‘the wealthy donor countries, industry and philanthropists’ (Stuckler et al., 2008: 1567). By contrast, the allocation of the regular budget is decided by the World Health Assembly, ‘a uniquely representative organization, providing a forum for 193 governments to influence the global health agenda’ (Stuckler et al., 2008: 1564), thus reflecting the views of all countries, including low-income and middle-income countries. Because the regular budget reflects the views of all countries and is not, like the extra-budgetary funds, driven primarily by donor interests, and because the allocation of the regular budget is ‘much more closely aligned with the actual burden of disease’ (Stuckler et al., 2008: 1567) than the extra-budgetary funds, they conclude that ‘extra-budgetary funds are misaligned with the health needs of the main recipients of WHO’s activities’ (Stuckler et al., 2008: 1567).
Stuckler et al. seem to assume a link between the greater legitimacy of the World Health Assembly’s distributive decisions and the fact that its distribution of funds is more closely aligned with the disease burden, compared to the distributive decisions of private donors. They seem to reason that because the WHA’s distribution is closely aligned with disease burden, it reflects recipients’ needs and, therefore, it has greater legitimacy than the distributive decisions of private donors. In principle, however, the WHA’s distributive decisions may have greater legitimacy than those of donors because of how these decisions are made, irrespective of how those decisions align with the disease burden. In other words, the more ‘representative’ World Health Assembly lends a legitimacy to the allocation of the regular budget that the donor-driven allocation of the extra-budgetary funds does not have. Importantly, this line of reasoning does not assume that alignment with the burden of disease reflects an ‘optimal’ allocation of resources. Instead, it compares the actual distribution of resources to a distribution that reflects the commitments and priorities of the WHO—in part because those commitments and priorities to some degree reflect the commitments and priorities of the international community, or at least have a greater claim to legitimacy than the decisions of private donors. What matters, on such an approach, is whether decisions are the result of a political process that can claim some legitimacy in terms of how priorities and allocations are determined.
This approach resonates with a broader development in the debate on resource allocation, which focuses on the legitimacy of decision-making processes regarding particular questions about rationing or resource allocation, rather than arguing for specific principles that are to be applied to these problems. In particular, Daniels and Sabin have developed an influential framework—‘accountability for reasonableness’ (Daniels and Sabin, 2008; Daniels, 2000)—that sets out a range of criteria for fair decision-making procedures that may generate legitimate decisions on controversial questions.
The framework has influenced the decision-making procedures of a number of national bodies, including the United Kingdom’s National Institute for Health and Clinical Excellence (Daniels and Sabin, 2008), and the applicability of this framework to priority-setting in low-income countries has also been explored (Kapiriri and Martin, 2007). To our knowledge, however, there has not been any application at the global level, where decisions affect allocations across rather than within countries.
The accountability for reasonableness framework is intended to facilitate decisions on normative questions on which there is reasonable disagreement: for example, even if we cannot agree on which distributive principles should govern resource allocation decisions, it is possible to agree on a fair process through which legitimate decisions can be reached. The framework requires ‘transparency about the grounds for decisions; appeals to rationales that all can accept as relevant to meeting health needs fairly; and procedures for revising decisions in light of challenges to them’ (Daniels, 2000: 1300). While the decision-making process employed in the World Health Assembly may not meet the requirements of a legitimate decision-making procedure as it is described in the accountability for reasonableness framework, it is not implausible to suggest that it has greater potential for generating legitimate decisions than the decisions of private donors, who need not make the grounds for their decisions explicit and whose decisions are generally not subject to a review process. However, the key point here is that we might prefer the WHA distribution on the grounds of political legitimacy, not because it more closely aligns with the burden of disease.
If we foreground the procedural dimensions of allocation decisions, the precise role of GBD data is variable. Participants in a decision-making process may choose to use GBD data as a benchmark or starting point for distributive decisions, amending it in exceptional circumstances; they may include it as one among a larger toolbox of available metrics that take into account disease severity and prevalence among different socioeconomic groups; or they may dispense with it entirely.
Conclusions
GBD data provide an intuitively appealing and useful means of comparing across health conditions. However, despite its intuitive appeal, it would be a mistake to uncritically assume that global health resources should ‘align’ with the GBD. What may seem like a ‘misalignment’ across diseases, disease groups or geographical regions does not necessarily indicate that resources are allocated suboptimally, illegitimately or unfairly. Since criteria—such as disease severity—that may be crucial to resource allocation are obscured by how GBD data are aggregated, assuming alignment as the starting point for assessing allocations of global health aid is misguided.
We discussed two alternatives for approaching questions about resource allocation for global health: one directly applying distributive principles to such decisions, the other focusing on how such decisions are made and their legitimacy. It is striking that neither of these alternatives gives more than a limited role to GBD data. Given that informing decisions about global health policy has been a central rationale for the GBD study (and, presumably, an important reason for investing significant resources), more work needs to be done to show how the GBD can in fact fulfill this role. While this does not undermine the usefulness of GBD data for the purposes of monitoring global health, it raises difficult questions for the use of GBD data in policy decisions.
Acknowledgements
We would like to thank Sam Harper and the reviewers of this journal for their comments. Some of this material was presented at the Brocher Foundation, the Oxford Global Health and Bioethics International Conference, and the Otago Global Health Initiative Annual Conference. Part of this research was undertaken while Kristin Voigt was a Caroline Miles Visiting Scholar at the Ethox Centre, Oxford University.
Funding
This work was supported by the Canadian Institutes for Health Research (CIHR) (Operating Grant EPP-122908, ‘Measuring Global Health’).
Conflict of Interest
None declared.
Footnotes
2. See, for example, Anand and Hanson (1997, 1998); Nord (2002); Arnesen and Kapiri (2004); Arnesen and Nord (1999); Fox-Rushby and Hanson (2001); Barendregt et al. (1996); Bognar (2008). For overviews of the debate, see Voigt (2012); Chen et al. (2015) and Schroeder (2017).
3. See, for example, Hausman (2012a, 2012b); Nord (2013, 2015); Voigt and King (2014).
4. For example, Yach et al. (2004); Laxminarayan et al. (2006); Geneau et al. (2010); Hunter and Reddy (2013); Binagwaho et al. (2014); Bukhman et al. (2015).
5. Their analysis is based on WHO region classifications. The Western Pacific region includes countries such as China, Japan and Australia as well as a number of Pacific islands.
References
- Anand S., Hanson K. (1997). Disability-Adjusted Life Years: A Critical Review. Journal of Health Economics, 16, 685–702. [DOI] [PubMed] [Google Scholar]
- Anand S., Hanson K. (1998). DALYs: Efficiency Versus Equity. World Development, 26, 307–310. [Google Scholar]
- Arnesen T., Kapiriri L. (2004). Can the Value Choices in DALYs Influence Global Priority-Setting? Health Policy, 70, 137–149. [DOI] [PubMed] [Google Scholar]
- Arnesen T., Nord E. (1999). The Value of DALY Life: Problems with Ethics and Validity of Disability Adjusted Life Years. British Medical Journal, 319, 1423.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendregt J. J., Bonneux L., Van der Maas P. J. (1996). DALYs: The Age-Weights on Balance. Bulletin of the World Health Organization, 74, 439–443. [PMC free article] [PubMed] [Google Scholar]
- Binagwaho A., Muhimpundu M. A., Bukhman G. (2014). 80 Under 40 by 2020: An Equity Agenda for NCDs and Injuries. The Lancet, 383, 3–4. [DOI] [PubMed] [Google Scholar]
- Bognar G. (2008). Age-Weighting. Economics and Philosophy, 24, 167–189. [Google Scholar]
- Brock D. W. (2012) Priority to the Worse Off in Health Care Resource Prioritization In Rhodes R., Battin M., Silvers A. (eds), Medicine and Social Justice. 2nd edn New York, NY: Oxford University Press, pp. 155–164. [Google Scholar]
- Bukhman G., Mocumbi A. O., Horton R. (2015). Reframing NCDs and Injuries for the Poorest Billion: A Lancet Commission. The Lancet, 386, 1221–1222. [DOI] [PubMed] [Google Scholar]
- Byass P., de Courten M., Graham W. J., Laflamme L., McCaw-Binns A., Sankoh O. A., Tollman S. M., Zaba B. (2013). Reflections on the Global Burden of Disease 2010 Estimates. PLoS Medicine, 10, e1001477.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A., Jacobsen K. H., Deshmukh A. A., Cantor S. B. (2015). The Evolution of the Disability-Adjusted Life Year (DALY). Socio-Economic Planning Sciences, 49, 10–15. [Google Scholar]
- Daniels N. (1998) Distributive Justice and the Use of Summary Measures of Population Health Status In Field M., Gold M. (eds), Summarizing Population Health: Directions for the Development and Application of Population Metrics. Washington, DC: Institute of Medicine, pp. 58–72. [Google Scholar]
- Daniels N. (2000). Accountability for Reasonableness. British Medical Journal, 321, 1300–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels N., Sabin J. E. (2008). Setting Limits Fairly. 2nd edn Oxford University Press. [Google Scholar]
- Dieleman J. L., Graves C. M., Templin T., Johnson E., Baral R., Leach-Kemon K., Haakenstad A. M., Murray C. J. (2014). Global Health Development Assistance Remained Steady in 2013 but did Not Align with Recipients' Disease Burden. Health Affairs, 33, 878–886. [DOI] [PubMed] [Google Scholar]
- Fox-Rushby J., Hanson K. (2001). Calculating and Presenting Disability Adjusted Life Years (DALYs) in Cost-Effectiveness Analysis. Health Policy and Planning, 16, 326–331. [DOI] [PubMed] [Google Scholar]
- Geneau R., Stuckler D., Stachenko S., McKee M., Ebrahim S., Basu S., Chockalingham A., Mwatsama M., Jamal R., Alwan A., Beaglehole R. (2010). Raising the Priority of Preventing Chronic Diseases: A Political Process. The Lancet, 376, 1689–1698. [DOI] [PubMed] [Google Scholar]
- Hanlon M., Graves C. M., Brooks B., Haakenstad A., Lavado R., Leach-Kemon K., Dieleman J. L. (2014). Regional Variation in the Allocation of Development Assistance for Health. Globalization and Health, 10, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausman D. M. (2012a). Measuring or Valuing Population Health: Some Conceptual Problems. Public Health Ethics, 55, 229–239. [Google Scholar]
- Hausman D. M. (2012b). Health, Well-Being, and Measuring the Burden of Disease. Population Health Metrics, 1010, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose I. (2014). Moral Aggregation. Oxford: Oxford University Press. [Google Scholar]
- Hunter D. J., Reddy K. S. (2013). Noncommunicable Diseases. New England Journal of Medicine, 369, 1336–1343. [DOI] [PubMed] [Google Scholar]
- Jamison D., Breman J., Measham A., Alleyne G., Claeson M., Evans D. B., Jha P., Mills A., Musgrove P. (2006). Disease Control Priorities in Developing Countries. 2nd edn Washington, DC: The World Bank. [PubMed] [Google Scholar]
- Kapiriri L., Martin D. K. (2007). A Strategy to Improve Priority Setting in Developing Countries. Health Care Analysis, 15, 159–167. [DOI] [PubMed] [Google Scholar]
- Laxminarayan R., Mills A. J., Breman J. G., Measham A. R., Alleyne G., Claeson M., Jha P., Musgrove P., Chow J., Shahid-Salles S., Jamison D. T. (2006). Advancement of Global Health: Key Messages from the Disease Control Priorities Project. The Lancet, 367, 1193–1208. [DOI] [PubMed] [Google Scholar]
- Lim S. S., Vos T., Flaxman A. D. Danaei, G., Shibuya, K., Adair-Rohani, H., Amann, M., Anderson, H. R., Andrews, K. G., Aryee, M., Atkinson, C., Bacchus, L., Bahalim, A., Balakrishnan, K., Balmes, J., Barker-Collo, S., Baxter, A., Bell, M., Blore, J., Blyth, F., Bonner, C., Borges, G., Bourne, R., Boussinesq, M., Brauer, M., Brooks, P., Bruce, N., Brunekreef, B., Bryan-Hancock, C., Bucello, C., Buchbinder, R., Bull, F., Burnett, R., Byers, T., Calabria, B., Carapetis, J., Carnahan, E., Chafe, Z., Charlson, F., Chen, H., Chen, J. S., Cheng, A., Child, J. C., Cohen, A., Colson, K. E., Cowie, B., Darby, S., Darling, S., Davis, A., Degenhardt, L., Dentener, F., Des Jarlais, D., Devries, K., Dherani, M., Ding, E., Dorsey, E. R., Driscoll, T., Edmond, K., Ali, S. E., Engell, R. E., Erwin, P. J., Fahimi, S., Falder, G., Farzadfar, F., Ferrari, A., Finucane, M., Flaxman, S., Fowkes, F. G. R., Freedman, G., Freeman, M. K., Gakidou, E., Ghosh, S., Giovannucci, E., Gmel, G., Graham, K., Grainger, R., Grant, B., Gunnell, D., Gutierrez, H., Hall, W., Hoek, H., Hogan, A., Hosgood III, H. D., Hoy, D., Hu, H., Hubbell, B. J., Hutchings, S. J., Ibeanusi, S. E., Jacklyn, G. L, Jasrasaria, R., Jonas, J. B., Kan, H., Kanis, J. A., Kassebaum, N., Kawakami, N., Khang, Y., Khatibzadeh, S., Khoo, J., Kok, C., Laden, F., Lalloo, R., Lan, Q., Lathlean, T., Leasher, J. L., Leigh, J., Li, Y., Lin, J. K., Lipshultz, S. E., London, S., Lozano, R., Lu, Y., Mak, J., Malekzadeh, R., Mallinger, L., Marcenes, W., March, L., Marks, R., Martin, R., McGale, P., McGrath, J., Mehta, S., Mensah, G. A., Merriman, T. R., Micha, R., Michaud, C., Mishra, V., Mohd Hanafiah, K., Mokdad, A. A., Morawska, L., Mozaffarian, D., Murphy, T., Naghavi, M., Neal, B., Nelson, P. K., Nolla, J. M., Norman, R., Olives, C., Omer, S. B., Orchard, J., Osborne, R., Ostro, B., Page, A., Pandey, K. D., Parry, C. D. H., Passmore, E., Patra, J., Pearce, N., Pelizzari, P. M., Petzold, M., Phillips, M. R., Pope, D., Pope III, C. A., Powles, J., Rao, M., Razavi, H., Rehfuess, E. A., Rehm, J. T., Ritz, B., Rivara, F. P., Roberts, T., Robinson, C., Rodriguez-Portales, J. A., Romieu, I., Room, R., Rosenfeld, L. C., Roy, A., Rushton, L., Salomon, J. A., Sampson, U., Sanchez-Riera, L., Sanman, E., Sapkota, A., Seedat, S., Shi, P., Shield, K., Shivakoti, R., Singh, G. M., Sleet., D. A., Smith, E., Smith, K., Stapelberg, N., Steenland, K., Stöckl, H., Stovner, L. J., Straif, K., Straney, L., Thurston, G., Tran, J., Van Dingenen, R., van Donkelaar, A., Veerman, J. L., Vijayakumar, L., Weintraub, R., Weissman, M., White, R., Whiteford, H., Wiersma, S., Wilkinson, J., Williams, H., Williams, W., Wilson, N., Woolf, A., Yip, P., Zielinski, J., Lopez, A. D., Murray, C. J. L., Ezzati, M. (2012). A Comparative Risk Assessment of Burden of Disease and Injury Attributable to 67 Risk Factors and Risk Factor Clusters in 21 Regions, 1990-2010: A Systematic Analysis for the Global Burden of Disease Study 2010. The Lancet, 380, 2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. J. L. (1996) Rethinking DALYs In Murray C. J. L., Lopez A. (eds), Global Burden of Disease. Boston: Harvard School of Public Health, pp. 1–98. [Google Scholar]
- Murray C. J. L., Ezzati M., Flaxman A. D., Lim S., Lozano R., Michaud C., Naghavi M., Salomon J. A., Shibuya K., Vos T., Lopez A. D. (2012a). GBD 2010: A Multi-Investigator Collaboration for Global Comparative Descriptive Epidemiology. The Lancet, 380, 2055–2058. [DOI] [PubMed] [Google Scholar]
- Murray C. J. L., Ezzati M., Flaxman A. D., Lim S., Lozano R., Michaud C., Naghavi M., Salomon J. A., Shibuya K., Vos T., Wikler D., Lopez A. D. (2012b). GBD 2010: Design, Definitions, and Metrics. The Lancet, 380, 2063–2066. [DOI] [PubMed] [Google Scholar]
- Murray C. J. L., Vos T., Lozano R. Naghavi, M., Flaxman, A., Michaud, C., Ezzati, M., Shibuya, K., Salomon, J. A., Abdalla, S., Aboyans, V., Abraham, J., Ackerman, I., Aggarwal, R., Ahn, S., Ali, M., AlMazroa, M., Alvarado, M., Anderson, H. R., Anderson, L., Andrews, K., Atkinson, C., Baddour, L., Bahalim, A., Barker-Collo, S., Barrero, L., Bartels, D., Basáñez, M., Baxter, A., Bell, M., Benjamin, E., Bennett, D., Bernabé, E., Bhalla, K., Bhandari, B., Bikbov, B., Bin Abdulhak, A., Birbeck, G., Black, J., Blencowe, H., Blore, J., Blyth, F., Bolliger, I., Bonaventure, A., Boufous, S., Bourne, R., Boussinesq, M., Braithwaite, T., Brayne, C., Bridgett, L., Brooker, S., Brooks, P., Brugha, T., Bryan-Hancock, C., Bucello, C., Buchbinder, R., Buckle, G., Budke, C., Burch, M., Burney, P., Burstein, R., Calabria, B., Campbell, B., Canter, C., Carabin, H., Carapetis, J., Carmona, L., Cella, C., Charlson, F., Chen, H., Cheng, A., Chou, D., Chugh, S., Coffeng, L., Colan, S., Colquhoun, S., Colson, K. E., Condon, J., Connor, M., Cooper, L., Corriere, M., Cortinovis, M., Courville de Vaccaro, K., Couser, W., Cowie, B., Criqui, M., Cross, M., Dabhadkar, K., Dahiya, M., Dahodwala, N., Damsere-Derry, J., Danaei, G., Davis, A., De Leo, D., Degenhardt, L., Dellavalle, R., Delossantos, A., Denenberg, J., Derrett, S., Des Jarlais, D., Dharmaratne, S., Dherani, M., Diaz-Torne, C., Dolk, H., Dorsey, E. R., Driscoll, T., Duber, H., Ebel, B., Edmond, K., Elbaz, A., Eltahir Ali, S., Erskine, H., Erwin, P., Espindola, P., Ewoigbokhan, S., Farzadfar, F.,Feigin, V., Felson, D., Ferrari, A., Ferri, C., Févre, E., Finucane, M., Flaxman, S., Flood, L., Foreman, K., Forouzanfar, M., Fowkes, F., Fransen, M., Freeman, M., Gabbe, B., Gabriel, S., Gakidou, E., Ganatra, H., Garcia, B., Gaspari, F., Gillum, R., Gmel, G., Gonzalez-Medina, D., Gosselin, R., Grainger, R., Grant, B., Groeger, J., Guillemin, F., Gunnell, D., Gupta, R., Haagsma, J., Hagan, H., Halasa, Y., Hall, W., Haring, D., Haro, J., Harrison, J., Havmoeller, R., Hay, R., Higashi, H., Hill, C., Hoen, B., Hoffman, H., Hotez, P., Hoy, D., Huang, J., Ibeanusi, S., Jacobsen, K., James, S., Jarvis, D., Jasrasaria, R., Jayaraman, S., Johns, N., Jonas, J., Karthikeyan, G., Kassebaum, N.*, Kawakami, N., Keren, A., Khoo, J., King, C., Knowlton, L., Kobusingye, O., Koranteng, A., Krishnamurthi, R., Laden, F., Lalloo, R., Laslett, L., Lathlean, T., Leasher, J., Lee, Y., Leigh, J., Levinson, D., Lim, S. S., Limb, E., Lin, J., Lipnick, M., Lipshultz, S., Liu, W., Loane, M., Lockett Ohno, S., Lyons, R., Mabweijano, J., MacIntyre, M., Malekzadeh, R., Mallinger, L., Manivannan, S., Marcenes, W., March, L., Margolis, D., Marks, G., Marks, R., Matsumori, A., Matzopoulos, R., Mayosi, B., McAnulty, J., McDermott, M., McGill, N., McGrath, J., Medina-Mora, M., Meltzer, M., Memish, Z., Mensah, G., Merriman, T., Meyer, A., Miglioli, V., Miller, M., Miller, T., Mitchell, P., Mock, C., Mocumbi, A., Moffitt, T., Mokdad, A., Monasta, L., Montico, M., Moradi-Lakeh, M., Moran, A., Morawska, L., Mori, R., Murdoch, M., Mwaniki, M., Naidoo, K., Nair, M. N., Naldi, L., Narayan, K. M. V., Nelson, P., Nelson, R., Nevitt, M., Newton, C., Nolte, S., Norman, P., Norman, R., O'Donnell, M., O'Hanlon, S., Olives, C., Omer, S., Ortblad, K., Osborne, R., Ozgediz, D., Page, A., Pahari, B., Pandian, J., Panozo Rivero, A., Patten, S., Pearce, N., Perez Padilla, R., Perez-Ruiz, F., Perico, N., Pesudovs, K., Phillips, D., Phillips, M., Pierce, K., Pion, S., Polanczyk, G., Polinder, S., Pope III, C. A., Popova, S., Porrini, E., Pourmalek, F., Prince, M., Pullan, R., Ramaiah, K., Ranganathan, D., Razavi, H., Regan, M., Rehm, J., Rein, D., Remuzzi, G., Richardson, K., Rivara, F., Roberts, T., Robinson, C., Rodriguez De Leòn, F., Ronfani, L., Room, R., Rosenfeld, L., Rushton, L., Sacco, R., Saha, S., Sampson, U., Sanchez-Riera, L., Sanman, E., Schwebel, D., Scott, J., Segui-Gomez, M., Shahraz, S., Shepard, D., Shin, H., Shivakoti, R., Silberberg, D., Singh, D., Singh, G., Singh, J., Singleton, J., Sleet, D., Sliwa, K., Smith, E., Smith, J., Stapelberg, N., Steer, A., Steiner, T., Stolk, W., Stovner, L., Sudfeld, C., Syed, S., Tamburlini, G., Tavakkoli, M., Taylor, H., Taylor, J., Taylor, W.*, Thomas, B., Thomson, W. M., Thurston, G., Tleyjeh, I., Tonelli, M., Towbin, J., Truelsen, T., Tsilimbaris, M., Ubeda, C., Undurraga, E., van der Werf, M., van Os, J., Vavilala, M., Venketasubramanian, N., Wang, M., Wang, W., Watt, K., Weatherall, D., Weinstock, M., Weintraub, R., Weisskopf, M., Weissman, M., White, R., Whiteford, H., Wiebe, N., Wiersma, S., Wilkinson, J., Williams, H., Williams, S., Witt, E., Wolfe, F., Woolf, A., Wulf, S., Yeh, P., Zaidi, A., Zheng, Z., Zonies, D., Lopez, A. (2012c). Disability-Adjusted Life Years (DALYs) for 291 Diseases and Injuries in 21 Regions, 1990-2010: A Systematic Analysis for the Global Burden of Disease Study 2010. The Lancet, 380, 2197–2223. [DOI] [PubMed] [Google Scholar]
- Nord E. (2002) My Goodness—and Yours: A History, and Some Possible Futures, of DALY Meanings and Valuation Procedures In Mathers C., Lopez A. (eds), Summary Measures of Population Health. Geneva: World Health Organization, pp. 139–146. [Google Scholar]
- Nord E. (2013). Disability Weights in the Global Burden of Disease 2010: Unclear Meaning and Overstatement of International Agreement. Health Policy, 111, 99–104. [DOI] [PubMed] [Google Scholar]
- Nord E. (2015). Uncertainties About Disability Weights for the Global Burden of Disease study. The Lancet Global Health, 3, e661–e662. [DOI] [PubMed] [Google Scholar]
- Salomon J. A. (2010). New Disability Weights for the Global Burden of Disease. Bulletin of the World Health Organization, 88, 879–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon J. A., Haagsma J. A. H., Davis A., de Noordhout C. M., Polinder S., Havelaar A. H., Cassini A., Devleesschauwer B., Kretzschmar M., Speybroeck N., Murray C. J., Vos T. (2015). Disability Weights for the Global Burden of Disease 2013 Study. The Lancet. Global Health, 3, e712–e723. [DOI] [PubMed] [Google Scholar]
- Schroeder S. A. (2017). Value Choices in Summary Measures of Population Health. Public Health Ethics, 10, 176–187. [Google Scholar]
- Sharp D., Millum J. (2015a). Prioritarianism for Global Health Investments: Identifying the Worst Off. Journal of Applied Philosophy, online first. DOI: 10.1111/japp.12142. [Google Scholar]
- Sharp D., Millum J. (2015b). The Post-2015 Development Agenda: Keeping Our Focus on the Worst Off. The American Journal of Tropical Medicine and Hygiene, 92, 1087–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman J. (2006). Donor Funding Priorities for Communicable Disease Control in the Developing World. Health Policy and Planning, 21, 411–420. [DOI] [PubMed] [Google Scholar]
- Stuckler D., King L., Robinson H., McKee M. (2008). WHO's Budgetary Allocations and Burden of Disease: A Comparative Analysis. The Lancet, 372, 1563–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt K. (2012) Measuring Global Health In Lenard P., Straehle C. (eds), Health Inequalities and Global Justice. Edinburgh: Edinburgh University Press, pp. 139–156. [Google Scholar]
- Voigt K., King N. B. (2014). Disability Weights in the Global Burden of Disease 2010 Study: Two Steps Forward, One Step Back? Bulletin of the World Health Organization, 92, 226–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J., de-Shalit A. (2007) Disadvantage. Oxford: Oxford University Press. [Google Scholar]
- Yach D., Hawkes C., Gould C. L., Hofman K. J. (2004). The Global Burden of Chronic Diseases: Overcoming Impediments to Prevention and Control. Journal of the American Medical Association, 291, 2616–2622. [DOI] [PubMed] [Google Scholar]