Abstract
Transgenic potato (Solanum tuberosum) plants expressing Arabidopsis phytochrome B were characterized morphologically and physiologically under white light in a greenhouse to explore their potential for improved photosynthesis and higher tuber yields. As expected, overexpression of functional phytochrome B caused pleiotropic effects such as semidwarfism, decreased apical dominance, a higher number of smaller but thicker leaves, and increased pigmentation. Because of increased numbers of chloroplasts in elongated palisade cells, photosynthesis per leaf area and in each individual plant increased. In addition, photosynthesis was less sensitive to photoinactivation under prolonged light stress. The beginning of senescence was not delayed, but deceleration of chlorophyll degradation extended the lifetime of photosynthetically active plants. Both the higher photosynthetic performance and the longer lifespan of the transgenic plants allowed greater biomass production, resulting in extended underground organs with increased tuber yields.
Plant growth, development, and metabolic activities are regulated by a range of environmental factors, including light, which is of central importance. Light is perceived by a variety of photoreceptors that control developmental processes such as germination, photomorphogenesis, flowering, and senescence, as well as metabolic processes such as photosynthesis and assimilate allocation. It has been pointed out before that the agricultural productivity of crop plants might be enhanced by overexpressing one of these central regulators (Smith, 1992), and phytochromes are promising candidates for such an improvement (Robson et al., 1996).
Phytochromes represent a family of red-light-absorbing photoreceptors that can exist in the physiologically inactive Pr form and the active Pfr form (for reviews, see Quail et al., 1995; Smith, 1995; Casal et al., 1998). Pr and Pfr are interconvertible by red or FR light, respectively. This absorption profile is extremely useful for the detection of shade or the presence of neighboring plants. At high relative proportions of FR radiation, which occur under shade conditions or in dense plant populations, the photoequilibrium is shifted toward the inactive Pr form. Under these conditions, green plants exhibit various symptoms of the shade-avoidance response, such as promotion of stem and petiole elongation, reduced leaf thickness, reduced chlorophyll synthesis, and increased apical dominance (Smith and Whitelam, 1997). The shade-avoidance response reduces the availability of resources for storage and reproduction.
Five PHY genes have been identified in Arabidopsis that have 50% to 80% identity at the amino acid level (Sharrock and Quail, 1989; Clack et al., 1994). The best-characterized members are PHYA and PHYB. phyA accumulates in the dark and is rapidly degraded upon conversion to the labile Pfr form (Pratt et al., 1997). It is responsible for detecting continuous FR light and dampens the shade-avoidance response under high relative proportions of FR light (McCormac et al., 1992; Heyer et al., 1995). In contrast, light-stable phyB is expressed at low but relatively constant levels in light- and dark-grown plants. Being sensitive for the detection of red light, phyB suppresses the shade-avoidance response under high relative proportions of red light.
Tobacco plants strongly overexpressing oat phyA (Keller et al., 1989) show a light-exaggerated phenotype even under white light: internodes are shorter, leaves are darker green, smaller in size, and slightly thicker, and the axillary buds are under less apical control. Because many genes involved in photosynthesis are coordinately regulated by phytochrome, phyA overexpressors consistently express higher amounts of several enzymes of carbon metabolism (Sharkey et al., 1991). The latter result seemed to be promising with respect to increasing the photosynthetic performance. However, unexpected physical modifications of the chloroplasts worsened the productivity of these plants (Sharkey et al., 1991). Lower-expressing lines do not show the light-exaggerated phenotype under white light. Robson et al. (1996) nevertheless demonstrated that the harvest index of these low phyA overexpressors was positively affected when they were planted at high densities. Under these conditions the shade-avoidance response was suppressed because of the higher sensitivity of these plants to FR light reflected by neighboring plants. Thus, assimilates were allocated to leaves rather than to stems. These data indicate that the performance of crop plants might be improved by altering phytochrome levels. Whether increased harvest indices can also be obtained for other organs, such as tubers or seeds, remains to be determined.
In this paper we addressed the question of whether improvement of photosynthetic performance by overexpressing phytochrome can be realized without causing the adverse effects described above. For this purpose, Arabidopsis phyB was used. Arabidopsis plants overexpressing either Arabidopsis or rice phyB have been reported to show a light-exaggerated phenotype, with reduced hypocotyl length and increased chlorophyll content (Wagner et al., 1991; McCormac et al., 1992; Wester et al., 1994). Tobacco plants overexpressing Arabidopsis phyB also show semidwarfism (Halliday et al., 1997) but, to our knowledge, detailed physiological studies have not yet been reported. Here we describe the morphological and physiological characterization of transgenic potato (Solanum tuberosum) plants grown under white light in the greenhouse. By using potato as a host, we were able to test the hypothesis that enhancing the responsiveness to white light results in higher yields of storage organs.
MATERIALS AND METHODS
Plant Material
Plasmid pMAB316 (Wagner et al., 1991) encodes the Arabidopsis PHYB cDNA under the control of the CaMV 35S promoter. Transformation of potato (Solanum tuberosum cv Désirée) plants with pMAB316 was performed according to the method of Rocha-Sosa et al. (1989). We selected two lines with different expression levels on the basis of reduced internode elongation in white light. These lines were named “Dara” after the host cultivar (Désirée) and the donor species (Arabidopsis).
Growth Conditions
Plants cultivated in a sterile culture (Murashige and Skoog, 1962) in a controlled-environment chamber (CU 32-L, Percival Scientific, Boone, IA) at photon flux densities of approximately 0.1 mmol m−2 s−1 white light and at 24°C (day)/22°C (night) were transferred to soil (maximum pot diameter, 20 cm) and grown in a greenhouse at 0.15 to 0.5 mmol m−2 s−1 white light, 15°C to 35°C, and a RH of approximately 55%. After 2 weeks, all but the two strongest shoots were cut to obtain plants of comparable strength.
RNA Extraction and Northern Analysis
Preparation of poly(A+) RNA from leaves and subsequent northern analysis were performed as described by Heyer and Gatz (1992a). Arabidopsis phyB transcripts were probed with a 3.9-kb KpnI fragment generated from pMAB316 and potato phyB transcripts with a 2.7-kb HpaI/KpnI fragment of a plasmid encoding the full-length cDNA (Ruddat et al., 1997). Probes against the potato ribosomal protein S4 (Devi et al., 1989) served to normalize for equal loading of poly(A+) RNA. Radioactive signals were detected with a bio-imaging analyzer (BAS 1000, Fuji, Tokyo) and quantified with TINA 2.0 software (Raytest, Straubenhardt, Germany).
Protein Extraction and Immunoblot Analysis
Protein extraction and immunoblotting were performed as described by van Tuinen et al. (1995) with slight modifications. The protein pellet (corresponding to 0.3 g of leaf material) resulting from the ammonium sulfate precipitation was resuspended in 15 μL of 0.5 m Tris buffer, pH 6.8. The protein concentration of the extract was determined (Bradford, 1976) and the extract was dissolved at 100°C for 2 min in 4× sample buffer (Laemmli, 1970). Equal amounts of protein (100 μg) were separated on a 7% SDS-PAGE gel and electroblotted onto a PVDF membrane (Millipore). Poinceau staining confirmed uniform protein loading and homogenous blotting. The membrane was incubated with either a mixture of three monoclonal antibodies raised against Arabidopsis phyB in a mouse (1:5000 dilution; Somers et al., 1991) or a polyclonal serum raised against potato phyB in a rabbit (1:400 dilution). After treatment with peroxidase-conjugated secondary antibody (1:1000 dilution, anti-mouse or anti-rabbit; Amersham), the enhanced chemiluminescence kit (Amersham) visualized phyB and Aida 2.0 software (Raytest) quantified it densitometrically. Separate tests confirmed that the phyB signal was proportional to the amount of protein loaded.
Phenotypic Analysis
Stem height was measured as the distance from the apex to the soil surface. For determination of the leaf-to-stem-weight ratio, representative plants were cut above the soil and the leaf laminas were separated from the petioles and stems. Stem circumferences were measured 1 cm above the soil. Weighing leaf discs randomly cut from mature leaves determined the specific leaf fresh weight (milligrams per centimeter). We estimated total leaf area (centimeters square) per plant from the mean specific leaf weight and the total leaf weight per plant. We determined tuber yield by measuring tuber number, total tuber fresh weight per plant, and fresh weight per single tuber, considering only tubers of at least 1 g. The tubers were harvested after growth for approximately 5 months in two consecutive winters (until February 1998 and April 1997). In the summer plants aged much earlier, living only to a maximum age of 3 months, due to higher temperatures and stronger pathogen exposure. In addition, developing tubers that had grown during summer and winter were harvested from a few green plants at 3 months.
Chlorophyll Determination
Leaf discs (1.3 cm2) were ground in a mortar with liquid nitrogen; the chlorophyll was quantitatively extracted with 80% acetone in the presence of approximately 1 mg of NaCO3. After the sample was centrifuged for 2 min at maximum speed, we determined the total chlorophyll content (chlorophyll a and b) of the supernatant photometrically (Uvicon 932, Kontron, Neuzahrn, Germany), according to the method of Lichtenthaler (1987). Chlorophyll contents were in terms of leaf area or leaf fresh weight.
Photosynthesis Measurements
We determined the CO2 uptake per leaf area using IR spectroscopy with a transportable gas-exchange porometer (ADC, Hoddesdon, UK), consisting of a central LCA-3 analyzer and a PLC-3 leaf chamber for simultaneous recording of photon fluxes and endogenous chamber temperature. An integrated personal computer stored and processed the data. The terminal leaflets of leaves 6 to 8 (leaf 1 was the first leaf larger than 1 cm) of 32- to 37-d-old plants were used. Before measurement, the leaflet was fixed in the chamber and exposed to 50 to 500 μmol m−2 s−1 white light provided by a 150-lux lamp (Flexilux, Schölly Fiberoptik, Denzlingen, Germany) at 22°C to 25°C until CO2 assimilation reached a maximum steady-state level (10–15 min).
High-Light Studies
To study light-stress sensitivity, we exposed attached leaves (leaves 6–8 of 32- to 37-d-old plants) for 5 h to 1.8 mmol m−2 s−1 white light (Power Star HQIT N/E, 2000 W, Osram, Munich, Germany) at a maximum (fan cooled) leaf temperature of 38°C. We again determined CO2-assimilation rates as described above. Additionally, leaf discs from three different plants were floated on water and exposed to the same light source at 25°C to 30°C for 5 h. The ratio of variable to maximum chlorophyll a fluorescence recorded with a fluorometer (PAM 101, Walz, Effeltrich, Germany; kindly provided by Dr. K. Raschke, Göttingen), after 10 min of dark adaptation, served as a measure of photosynthetic efficiency (Krause and Weis, 1991). High-light phenotypes were investigated by cultivating plants under the same light source between 1.5 and 1.8 mmol m−2 s−1 white light (continuously fan cooled).
Preparation and Documentation of Light Micrographs
Cross-sections were cut from the center of mature, nonsenescent leaf laminas of 6-week-old plants. The preparation procedure followed the paraffin method (Gerlach, 1969). A microscope (DNLS, Leica) magnified and a camera (NPS48, Leica) photographed the cross-sections.
RESULTS
Determination of phyB Transcript and Protein Levels
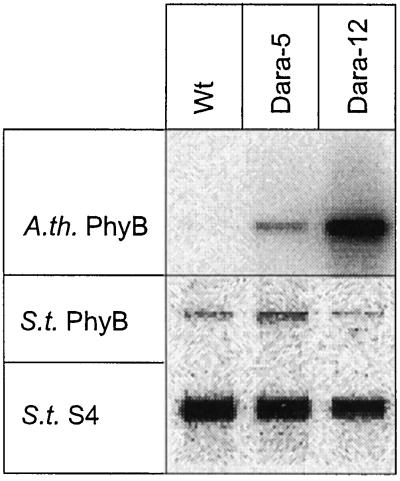
Potato plants were transformed with the Arabidopsis phyB cDNA under the control of the CaMV 35S promoter. After regeneration of 12 independent transformants, we selected two plants exhibiting different degrees of stem elongation suppression in white light for further characterization. Northern analysis of poly(A+) RNA from leaves (Fig. 1) showed that the potato line Dara-5 expressed 0.2 relative units (the phyB signal divided by the S4 signal), whereas Dara-12 expressed 3.9 relative units of phyB mRNA. No signal was obtained in wild-type plants. Transcription of the endogenous PHYB was not significantly affected in the transgenic lines.
Figure 1.
Northern analysis showing Arabidopsis phyB (A.th. PhyB) and potato PhyB (S.t. PhyB) transcript levels in leaves of wild-type (Wt), Dara-5, and Dara-12 potato plants. Hybridization with a potato S4 probe was done to correct the signal for loading differences; 1.5 μg of poly(A+) RNA was loaded per lane.
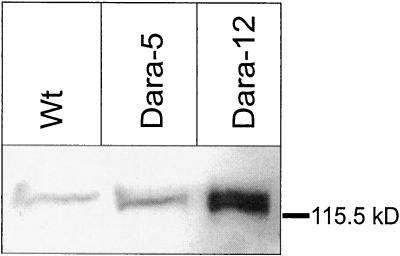
For determination of phyB protein levels, we used monoclonal antibodies raised against Arabidopsis phyB (Fig. 2; Somers et al., 1991) to perform immunoblots with protein extracts from leaves. Because the applied antibodies recognized equally Arabidopsis and potato phyB, the signal obtained with wild-type extracts represented the amount of endogenous potato phyB. Densitometric quantification of three blots indicated approximately 4-fold (Dara-5) and 20-fold (Dara-12) overexpression of phyB in the transgenic lines compared with the wild type. Low-temperature fluorescence measurements (Sineshchekov et al., 1996) on etiolated sprouts served to prove spectral activity of the transgenic phytochrome. Dark-grown sprouts (lower stem part) yielded 1.80 ± 0.49 relative fluorescence units in the wild type and 2.46 ± 0.44 relative fluorescence units in the transgenic Dara-12 plants. Irradiation for 3.5 h with red light depleted the phytochrome pool in wild-type plants to levels below 0.1 relative units, due to selective degradation of phyA. In Dara-12 plants, 30% of the total phytochrome survived this light treatment, yielding relative fluorescence units of 0.82 ± 0.08 and indicating at least an 8-fold overexpression of phyB in the sprouts of this transgenic line.
Figure 2.
Immunoblot showing expression levels of phyB in leaves of wild-type (Wt), Dara-5, and Dara-12 potato plants using a monoclonal antibody raised against Arabidopsis phyB. Both Arabidopsis phyB and endogenous potato phyB were equally recognized by this antibody; 100 μg of protein was loaded per lane.
Shoot Phenotype
Phenotypic differences between the shoots of wild-type and transgenic plants were not evident when plants were grown in sterile culture, but strong differences in internode length and leaf pigmentation developed about 10 d after transfer to soil and greenhouse conditions. Soil-grown transgenic plants exhibited several features also observed in other phyA- or phyB-overexpressing plants: Arabidopsis, tomato, and tobacco grown in white light (Fig. 3, a and b; Boylan and Quail, 1989, 1991; Keller et al., 1989; Wagner et al., 1991; Halliday et al., 1997). Internodes of the phyB overexpressors were much shorter but thicker, leading to a semidwarf phenotype (37% of wild-type height) in the moderately expressing Dara-5 and to a stronger dwarf phenotype (29% of wild-type height) in the forcefully expressing Dara-12 (Table I). Toward the end of the vegetative period (after flowering), these differences decreased substantially. Dara-12, however, never reached the height of wild-type plants. Apical dominance was reduced, leading to increased stem branching (Fig. 3, a and b). Biomass allocation was directed toward leaves, resulting in an increased leaf-to-stem ratio and an increased amount of fresh leaf material per plant (Table I).
Figure 3.
a, Phenotype of a representative wild-type (Wt) potato. b, Phenotype of a 5-week-old Dara-12 potato plant showing shortened internodes and increased branching. Dara-5, which moderately expresses phyB, exhibits an intermediate phenotype (not shown; see data in Table I). c, Phenotype of leaves of wild-type (Wt), Dara-5, and Dara-12 plants grown under moderate light conditions (0.15–0.5 mmol m−2 s−1) in a greenhouse. Leaves 5 (counted from the apex) from 10-week-old plants are shown. Bar = 6 cm.
Table I.
Anatomical characteristics of Dara-5 and Dara-12 shoots compared with wild-type potato plants
| Parameter | Wild Type | Dara-5 | Dara-12 | n |
|---|---|---|---|---|
| Stem height (cm) | 82 ± 6 | 30 ± 11.1 | 24 ± 4 | 10 |
| Stem circumference (cm)a | 2.5 ± 0.4 | 2.9 ± 0.4 | 4.8 ± 1.0 | 10 |
| Leaf-to-stem wt ratiob | 0.34 ± 0.03 | 0.48 ± 0.07 | 0.65 ± 0.04 | 5 |
| Leaf fresh wt/plant (g)b | 51.8 ± 3.2 | 62.0 ± 9.6 | 63.9 ± 11.5 | 5 |
| Leaf area/plant (m2)b | 0.21 ± 0.02 | 0.23 ± 0.02 | 0.23 ± 0.04 | 5 |
All measurements were done on 6- to 8-week-old plants.
Measured 1 cm above the soil.
Data are from representative plants; effects were reproduced with five to eight different plants at other developmental stages; sd as indicated.
Leaf Characteristics
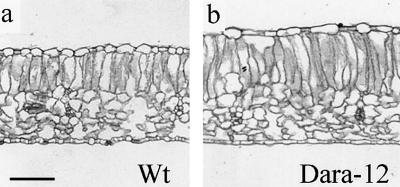
As shown in Figure 3c, leaves of the transgenic plants were considerably smaller than wild-type leaves. However, the number of leaves was higher, resulting in the same total leaf area per individual plant (Table I). Increased total fresh weight per leaf area (14% for Dara-5 and 23% for Dara-12, Table II) resulted from the increased thickness of the leaves. Light micrographs of leaf cross-sections (Fig. 4) revealed that the increased thickness was basically due to the increased length of palisade cells in the leaf mesophyll (19% more for Dara-5 and 30% more for Dara-12), whereas the dimensions of the spongy tissues remained largely unaffected (Fig. 4; Table II). The enlarged palisade cells contained more chloroplasts, resulting in strongly elevated chlorophyll contents on a leaf-area basis (Fig. 5) but not on the basis of leaf fresh weight (Table II). Despite the differences in chlorophyll content per leaf area, fractional red-light absorbance (Dr. G.H. Krause, Düsseldorf, Germany, personal communication) was very similar for all three lines (0.79 relative unit for wild type and 0.80 relative unit for the two transgenic plants). The impression of a darker pigmentation was intensified by slightly higher amounts of anthocyanins in the leaves (Fig. 3c), which increased considerably when the plants grew under higher light intensities (see Fig. 7).
Table II.
Characteristics of Dara-5 and Dara-12 leaves compared with wild-type potato plants
| Parameter | Wild Type | Dara-5 | Dara-12 | n/na |
|---|---|---|---|---|
| Fresh wt/leaf area (mg/cm2)b | 24.3 ± 1.8 | 27.7 ± 2.5 | 29.9 ± 2.1 | 90 /10 |
| Chl content/fresh wt (mg/g)c | 1.4 ± 0.3 | 1.5 ± 0.3 | 1.4 ± 0.4 | 22 /4 |
| Palisade cell length (μm)c | 130 ± 21 | 155 ± 16 | 170 ± 25 | 50 /6 |
| Total width (μm)c | 274 ± 28 | 307 ± 25 | 341 ± 35 | 50 /6 |
No. of samples/no. of individual plants.
Random samples from apical to basal leaves of 4- to 10-week-old plants.
Data are from leaves 8 (leaf 1 is the first leaf >1 cm) of 6-week-old plants; effects were reproduced at other nonsenescent stages; sd as indicated.
Figure 4.
Light micrographs of leaf cross-sections of wild-type (a) and transgenic Dara-12 leaves (b). Representative sections were taken from leaves 8 of 6-week-old plants. Bar = 150 μm.
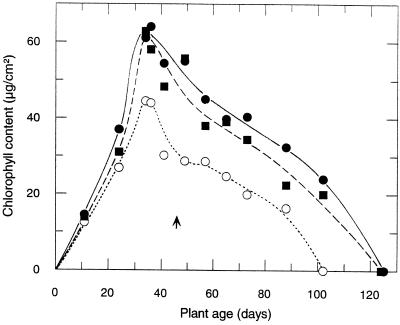
Figure 5.
Mean chlorophyll content during the course of leaf maturation and senescence of wild-type (○), Dara-5 (▪), and Dara-12 (•) potato plants. The arrow indicates onset of flowering, which was at about the same time for all lines. Day 1 was October 26, 1997. Each point represents the mean chlorophyll content of 10 segments cut (two were taken at d 11) in regular distances from leaflets of apical to basal leaves. Data from two representative plants per line are depicted. Because of the sampling, aging was slightly (about 3 weeks) accelerated in all plants.
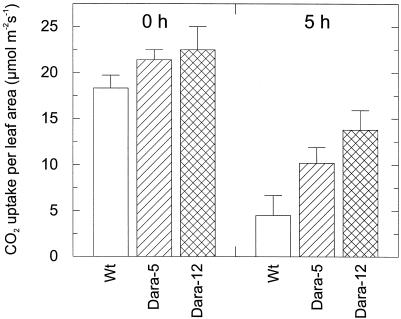
Figure 7.
Photosynthesis rates at 1.8 mmol photons m−2 s−1 of wild-type, Dara-5, and Dara-12 before (0 h) and after high-light stress (under 1.8 mmol m−2 s−1) for 5 h. Leaf material was the same as for Figure 6. Data are from nine leaves of three plants from each line (sd as indicated).
Chlorophyll Pigmentation during the Course of Leaf Maturation and Senescence
To study the course of maturation and senescence of the transgenic plants, the mean chlorophyll contents were analyzed at different stages of development. Leaf segments sampled at temporal intervals and at regular distances from apical to basal leaflets were pooled, and chlorophyll was extracted and analyzed photometrically. The results are presented in Figure 5. The chlorophyll content per leaf area in the transgenic leaves began to rise within the first 20 d after transfer from sterile to soil culture. Soon after the chlorophyll accumulation per leaf area reached a maximum, senescence started simultaneously in both the transgenic and the wild-type leaves. Flowering, which initiates senescence, started at approximately the same time (d 47). The time needed for complete chlorophyll degradation, however, extended the lifetime of photosynthetically active transgenic plants by 3 to 4 weeks.
Photosynthetic Activity
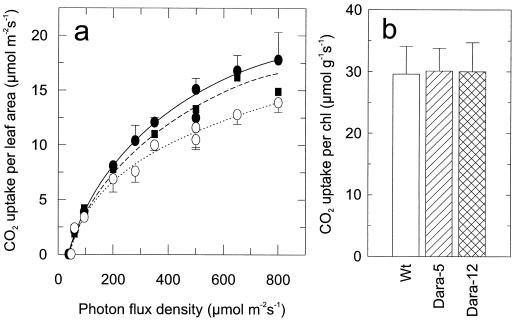
Figure 6 presents the photosynthetic activity of mature wild-type and transgenic leaves. Young leaves from nonsenescent plants (32–37 d old, compare with Fig. 5) were used. Photon fluxes of more than approximately 250 μmol m−2 s−1 in the transgenic plants showed higher rates of leaf-area photosynthesis than did those in the wild-type plants (Fig. 6a). As the senescence-related breakdown of chlorophylls proceeded (Fig. 5), the advantages in the photosynthetic performance of the transgenic plants became even more pronounced (data not shown). We observed no difference in photosynthetic activity (Fig. 6b) when the chlorophyll content was normalized. The increased rates per unit leaf area can thus be attributed to thicker leaf cross-sections and elevated chlorophyll contents. Because the total amount of leaf area was unaffected, increases in photosynthetic rates per individual plant (23% and 30% for Dara-5 and Dara-12, respectively) were extrapolated from the mean rates of leaves 5 to 12.
Figure 6.
Photosynthesis rates of mature, nonsenescent leaves (leaves 6–8) of 32- to 37-d-old wild-type, Dara-5, and Dara-12 plants. a, Rates of wild-type (○), Dara-5 (▪), and Dara-12 (•) at different photon fluxes (50–500 μmol m−2 s−1) normalized to leaf area (μmol CO2 m−2 s−1). Data points are means of measurements of 4 to 15 leaves from eight plants of each line. b, Rates at 500 μmol photons m−2 s−1 normalized to chlorophyll contents at site of the investigated leaf (μmol CO2 g−1 s−1). In a data points are for 4 to 15 leaves from eight plants of each line; in b data are from nine leaves of three plants of each line (sd = 0.5–2.6 μmol m−2 s−1 for Dara-5 or as indicated). chl, Chlorophyll.
No significant structural changes in the photosynthetic apparatus of the transgenic leaves were detected. HPLC analysis of ethanolic leaf extracts of 30- to 40-d-old plants (device kindly provided by Dr. P. Jahns, Düsseldorf; method modified from that of Gilmore and Yamamoto, 1991) revealed no changes in the carotenoid composition (data not shown). Also, the ratios of chlorophyll a to b were almost identical in wild-type and transgenic leaves (2.6 ± 0.3 for wild type and Dara-5 and 2.7 ± 0.4 for Dara-12). Finally, electron microscopy (kindly performed by Dr. S. Hillmer, Göttingen; data not shown) did not indicate substantial changes or deformations in the structure of the chloroplasts of the transgenic plants, as previously observed in oat phyA-overexpressing tobacco plants (Sharkey et al., 1991).
Light-Stress Experiments
Because the transgenic leaves revealed a sun leaf phenotype, we analyzed the susceptibility of the photosynthetic apparatus to strong irradiance using the nonsenescent leaves of plants grown under moderate white light (0.15–0.5 mmol m−2 s−1). After 5 h at 1.8 mmol m−2 s−1 white light and 25°C to 30°C, maximum CO2 assimilation had dropped to about 25% of that in the plants maintained under moderate light in the wild type and to only 50% (Dara-5) and 60% (Dara-12) in the transgenics (Fig. 7). During that period, the ratio of variable to maximum chlorophyll a fluorescence, used as a measure of photosynthetic efficiency, declined from approximately 0.8 in the dark controls to approximately 0.2 in the wild-type leaves to 0.3 in the Dara-5 leaves and to 0.35 in the Dara-12 leaves. Both studies indicated a significantly lower sensitivity of the transgenic leaves to photoinhibition.
To study phenotypes under high irradiation, five plants per line were cultivated under artificial white light at 1.5 to 1.8 mmol m−2 s−1 in a greenhouse. Differences in internode length and leaf size were less pronounced under these conditions. However, the differences in leaf thickness and chlorophyll contents remained. The formation of anthocyanins in leaves (primarily in the lower epidermis, near veins, and in the petiole and rachis) and, to a smaller degree, also in stems (outer cortex cells) of the transgenic plants was strongly enhanced compared with plants grown under moderate light (compare Fig. 3c with Fig. 8).
Figure 8.
Leaf phenotypes of wild-type, Dara-5, and Dara-12 plants grown under high intensities of white light (1.5–1.8 mmol m−2 s−1) in a greenhouse, demonstrating increased anthocyanin contents. Leaves 5 of 8-week-old plants are shown. Bar = 3 cm.
Tuber Yields and Underground Organ Growth
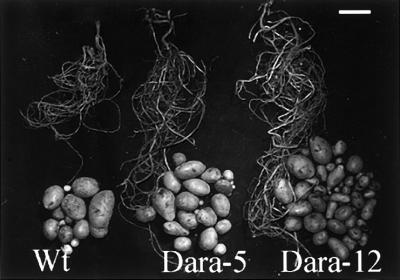
The phenotypes of the underground organs were determined when the plants were totally senescent after approximately 5 months of growth in a greenhouse. Table III summarizes the yield data and Figure 9 shows representative underground organs of each line. Biomass allocation to underground organs (tubers and the root and underground shoot systems) increased in the transgenic lines. The number of tubers per plant was approximately 2-fold higher in Dara-5 and 3-fold higher in Dara-12 compared with the wild type. The tuber weight per plant in Dara-5 and Dara-12 increased by 56% and 30%, respectively, of the tuber weight of the wild type; and the average tuber size decreased to 65% and 43%, respectively, of the tuber size of the wild type. Relative dry weight and starch contents of the tubers (Dr. B. Marty, Golm, Germany, personal communication) were constant in the wild type, Dara-5, and Dara-12 (22 ± 1 and 15 ± 1, respectively; n = 15). However, when harvested before 3 months (repeated several times on a small scale), no significant increases in organ expansion appeared and depressed tuber yields were even found in the transgenic lines. Table III shows the tuber weights of three representative plants per line, harvested at 3 months. At that age, yields were about 14% (Dara-5) and 23% (Dara-12) lower in the transgenics compared with the wild type.
Table III.
Tuber yield of Dara-5, Dara-12, and wild-type potato plants after decay of the shoots following growth for 5 months and after only 3 months
| Parameter | Wild Type | Dara-5 | Dara-12 |
|---|---|---|---|
| Tuber wt (g)/plant | 122 ± 35 | 190 ± 52 | 158 ± 25 |
| Tuber no./plant | 9 ± 3 | 22 ± 7 | 27 ± 6 |
| Average wt (g)/single tuber | 14 ± 12 | 9 ± 8 | 6 ± 6 |
| Tuber wt (g)/plant after 3 monthsa | 104 ± 36 | 92 ± 21 | 86 ± 29 |
Only tubers >1 g were considered.
Data are from 3 representative plants; all others are from 12 plants; sd as indicated.
Figure 9.
Expansion of underground organs and tuber yields of the wild type (Wt), Dara-5, and Dara-12 harvested upon decay of the aerial parts after 5 months of cultivation in a greenhouse. The transgenic lines showed enlarged underground organs and more but smaller tubers, resulting in increased total tuber weight per plant (Table III). Bar = 5 cm.
DISCUSSION
Transgenic plants overexpressing different members of the phytochrome family have been extremely valuable for elucidating the specific functions of individual phytochromes (McCormac et al., 1992, 1993). Moreover, they have also been used to map functional domains important for photosensory specificity, dimerization, signal transduction, and light-dependent degradation (for review, see Quail, 1997). Because of suppression of the shade-avoidance response, transgenic tobacco plants overexpressing oat phyA revealed an increased leaf-harvest index, opening new avenues for biotechnological applications of transgene technology (Robson et al., 1996). Being particularly interested in the latter application, we generated transgenic potato plants overexpressing phyB. Potato was chosen as a host to explore the possibility that improved harvest index could also be applied to tubers.
By choosing phyB we tried to circumvent the adverse effects of phyA on chloroplast structure (Sharkey et al., 1991). We preferred Arabidopsis phyB over potato phyB because it encodes an N-terminal hydrophobic extension that is missing in potato phyB (Heyer and Gatz, 1992b). Deletion of this extension decreased the sensitivity of the protein to red light (Wagner et al., 1996). Thus, we speculated that expression of Arabidopsis phyB might be more effective than expression of potato phyB. Transgenic potato plants transformed with the Arabidopsis phyB cDNA under the control of the CaMV 35S promoter supported the notion that phytochrome-overexpressing plants may have agricultural importance. We discuss the data presented here in the context of results previously obtained with other phyB- or phyA-overexpressing plants.
Transgenic potato plants overexpressing Arabidopsis phyB exhibited a semidwarf phenotype, with shorter and thicker stems, reduced apical dominance, smaller but thicker leaves, a higher leaf-to-stem weight ratio, increased chlorophyll content, higher rates of photosynthesis, prolonged timespan for photosynthesis, and, when harvested after the natural decay of the shoots, higher tuber numbers and yields. Physiological data from other Arabidopsis phyB-overexpressing plants under white light are limited. Transgenic Arabidopsis plants also revealed an increased chlorophyll content. In addition, a slightly reduced ratio of chlorophyll a to b was detected (Wester et al., 1994), a phenomenon not observed in transgenic potato plants. Like the transgenic potato plants described here, transgenic tobacco plants overexpressing phyB showed reduced stem extension (Halliday et al., 1997).
Transgenic tobacco plants overexpressing oat phyA have been studied in more detail. Like phyB-overexpressing potato plants, phyA-overexpressing tobacco plants are characterized by semidwarfism, reduced apical dominance, smaller but thicker leaves, increased chlorophyll content, and delayed senescence (Keller et al., 1989; Cherry et al., 1991). The elicitation of this “light-exaggerated” phenotype by overexpression of either phyA or phyB is consistent with the notion that a variety of developmental processes can be controlled by either phytochrome, with phyA being activated by FR light and phyB by red light (Quail et al., 1995). As white light contains red and FR light, both types of overexpressors contain elevated levels of Pfr under white light. The increased sensitivity of phyA-overexpressing plants to FR light has been well documented (McCormac et al., 1992; Robson et al., 1996). However, it remains to be shown that it is the redlight component of white light that is responsible for the light-exaggerated phenotype in the transgenic plants described here.
In terms of photosynthetic performance, phyB-overexpressing potato plants were superior to phyA overexpressors (Sharkey et al., 1991). Both types of phytochrome overexpressors had thicker leaves and higher chlorophyll content per leaf area. Light micrographs illustrate that leaf thickening was due to general enlargement of mesophyll cells in phyA-expressing tobacco (Sharkey et al., 1991), whereas only palisade cells were elongated in phyB-overexpressing potato (Fig. 4; Table II). Electron micrographs from transgenic phyA overexpressors showed that many of the chloroplasts were cup-shaped, with the middle portions of the chloroplasts bowing away from the plasmalemma (Sharkey et al., 1991). These abnormalities worsened the photosynthetic performance of the plants at normal CO2 concentrations.
Abnormalities of the chloroplasts were not detectable in transgenic phyB-overexpressing potato plants. In contrast, these plants displayed higher photosynthesis rates (Fig. 6a). This effect was proportional to the increased chlorophyll levels (Fig. 6b). The differences in photosynthetic activity were even more pronounced in senescing leaves as the relative differences in pigmentation increased. In addition, photosynthesis was less sensitive to photoinhibition (Fig. 7), which may have been due to the higher chlorophyll content per unit leaf area resulting in lower excitation of pigments compared with wild-type leaves. Whether the stimulated anthocyanin formation observed under high-light conditions was also involved in protecting the transgenic photosynthesis apparatus (especially under high UV radiation in the field) remains to be elucidated.
The enhanced responsiveness of the transgenics to white light led not only to increased thickening of leaves, and thus to higher photosynthetic performance and higher biomass production of the aerial parts of the plants, but also to a preferential allocation of assimilates to the leaves at the expense of the stems (Table I). This is consistent with the phenotype of transgenic tobacco plants overexpressing phyA (Robson et al., 1996). phyA-overexpressing plants also displayed this altered assimilate allocation but, in contrast to plants overexpressing phyB, only in response to low relative ratios of red to FR light.
Both phyA-overexpressing tobacco plants and phyB-overexpressing potato plants stayed green longer. To analyze whether this apparently delayed senescence was due to a delayed beginning or to a slower senescing process, we monitored the chlorophyll contents over the life cycle of the phyB-overexpressing potato plants (Fig. 5). Chlorophyll levels of wild-type and transgenic plants increased at different rates, resulting in 35% more chlorophyll in the mature leaves of the transgenic lines. Chlorophyll contents started to decrease at approximately d 40 in all of the plant lines, indicating no difference in the start of senescence. Also, the slope of the decline was almost identical. However, assuming a similar amount of chlorophyll per chloroplast in the nonsenescent state (deduced from the concomitant increases in chloroplast number per cell and chlorophyll level per leaf area), the rate of chlorophyll loss per individual chloroplast decelerated; the time needed for complete chlorophyll degradation was several weeks longer in the transgenic plants. This result is consistent with findings of Cherry et al. (1991), who also observed a simultaneous start but a decelerated senescence process in phyA-overexpressing plants.
Tuber yield is the essential parameter for estimating the agricultural potential of transgenic potato plants. Keiller and Smith (1989) have shown that increasing the ratio of red to FR light for radish results in preferential assimilate allocation to the storage organ at the expense of leaf petiole extension. To our knowledge, similar effects have not been reported in potato. By increasing phyB levels, and thus presumably the sensitivity to red light, we explored the possibility of stimulating photosynthesis and assimilating allocation to tubers using one central regulator. When harvested after 3 months, tuber yields were lower than in wild-type plants. phyB apparently delays tuber formation, although we observed no effect on flowering. The delayed tuber formation in phyB overexpressors corresponds well with accelerated tuber formation in phyB antisense plants (Jackson et al., 1996). In these plants tuber formation was induced even under long-day conditions using the short-day potato cv Andigena. Because the work presented here was done with the potato cv Désirée, which is under less-stringent photoperiodic control, these experiments are not directly comparable. Once tuber induction was initiated, the high photosynthetic performance of even senescing plants led to storage of elevated amounts of biomass into tubers, resulting in higher yields. In spite of higher yields, average tuber size was smaller than in wild-type plants. It may be speculated that decreased apical dominance of the underground shoot leads to increased stolon formation. Increased biomass allocation was not confined to tubers but was also present in other underground organs such as roots and shoots.
The results of this study support the idea that improving agricultural performance by altering phyB levels is feasible. Higher photosynthetic rates led to more biomass, which is allocated into leaves but also into underground organs such as tubers. Whether these features are maintained under field conditions remains to be determined. Judging from our experiments here, we believe that these plants are potentially more productive, especially in areas with high irradiation; therefore, reduced photoinhibition becomes advantageous. As plants are able to assimilate CO2 for a longer time because of decelerated chlorophyll breakdown, they might be preferentially valuable in areas with long growing seasons.
ACKNOWLEDGMENTS
We are grateful to Dr. V. Sineshchekov (Moscow State University), who provided the data concerning low-temperature fluorescence. We also wish to thank Dr. E. Reiner-Drehwald and G. Weis for support with light microscopy, Dr. S. Hillmer for carrying out the electron microscopy, and Dr. H.W. Heldt for providing his gas-exchange porometer. We give special thanks to F. Glasenapp and U. Wedemeyer, the greenhouse staff in Göttingen, for skillfully cultivating the plants.
Abbreviations:
- CaMV
cauliflower mosaic virus
- FR
far-red
- phyA and phyB
phytochromes A and B, respectively
Footnotes
This work was supported by the European Communities BIOTECH program (grant no. BIO2 CT-930400), by a Department of Energy grant (no. DE-FG03-87ER13742) to P.H.Q., and by a U.S. Department of Agriculture grant (no. CRIS 5335-21000-010-00D) to P.H.Q.
LITERATURE CITED
- Boylan MT, Quail PH. Oat phytochrome is biologically active in transgenic tomatoes. Plant Cell. 1989;1:765–773. doi: 10.1105/tpc.1.8.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan MT, Quail PH. Phytochrome A overexpression inhibits hypocotyl elongation in transgenic Arabidopsis. Proc Natl Acad Sci USA. 1991;88:10806–10810. doi: 10.1073/pnas.88.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Rodolfo AS, Botto JF. Modes of action of phytochromes. J Exp Bot. 1998;49:127–138. [Google Scholar]
- Cherry JR, Hershey HP, Vierstra RD. Characterization of tobacco expressing functional oat phytochrome. Plant Physiol. 1991;96:775–785. doi: 10.1104/pp.96.3.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Devi KRG, Chan Y-L, Wool IG. The primary structure of rat ribosomal protein S4. Biochim Biophys Acta. 1989;1008:258–262. doi: 10.1016/0167-4781(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Gerlach D (1969) Botanische Mikrotechnik. G. Thieme-Verlag, Stuttgart, Germany
- Gilmore AM, Yamamoto HY. Resolution of lutein and zeaxanthin using a nonencapped, lightly carbon-loaded C18 high-performance liquid chromatographic column. J Chromatogr. 1991;543:137–145. [Google Scholar]
- Halliday KJ, Thomas B, Whitelam GC. Expression of heterologous phytochromes A, B, or C in transgenic tobacco plants alters vegetative development and flowering time. Plant J. 1997;12:1079–1090. doi: 10.1046/j.1365-313x.1997.12051079.x. [DOI] [PubMed] [Google Scholar]
- Heyer AG, Gatz CG. Isolation and characterization of a cDNA clone coding for potato type A phytochrome. Plant Mol Biol. 1992a;18:535–544. doi: 10.1007/BF00040669. [DOI] [PubMed] [Google Scholar]
- Heyer AG, Gatz CG. Isolation and characterization of a cDNA clone coding for potato type B phytochrome. Plant Mol Biol. 1992b;20:589–600. doi: 10.1007/BF00046444. [DOI] [PubMed] [Google Scholar]
- Heyer AG, Mozley D, Landschütze V, Thomas B, Gatz C. Function of phytochrome A in Solanum tuberosum as revealed through the study of transgenic plants. Plant Physiol. 1995;105:941–948. doi: 10.1104/pp.109.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SD, Heyer A, Dietze J, Prat S. Phytochrome-B mediates the photoperiodic control of tuber formation in potato. Plant J. 1996;9:159–166. [Google Scholar]
- Keiller D, Smith H. Control of carbon partitioning by light quality mediated by phytochrome. Plant Sci. 1989;63:25–29. [Google Scholar]
- Keller JM, Shanklin J, Vierstra RD, Hershey HP. Expression of a functional monocotyledonous phytochrome in transgenic tobacco. EMBO J. 1989;8:1005–1012. doi: 10.1002/j.1460-2075.1989.tb03467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol. 1991;42:313–349. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- McCormac AC, Wagner D, Boylan MT, Quail PH, Smith H, Whitelam GC. Photoresponses of transgenic Arabidopsis seedlings expressing introduced phytochrome B-encoding cDNAs: evidence that phytochrome A and phytochrome B have distinct photoregulatory functions. Plant J. 1993;4:19–27. [Google Scholar]
- McCormac AC, Whitelam GC, Smith H. Light-grown plants of transgenic tobacco expressing an introduced oat phytochrome A gene under the control of a constitutive viral promoter exhibit persistent growth inhibition by far-red light. Planta. 1992;188:173–181. doi: 10.1007/BF00216811. [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Pratt LH, Cordonnier-Pratt MM, Kelmenson PM, Lazarova GI, Kubota T, Alba RM. The phytochrome family in tomato (Solanum lycopersicum L.) Plant Cell Environ. 1997;20:672–677. [Google Scholar]
- Quail PH. An emerging molecular map of the phytochromes. Plant Cell Environ. 1997;20:657–665. [Google Scholar]
- Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D. Phytochromes: photosensory perception and signal transduction. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- Robson PRH, McCormac AC, Irvine AS, Smith H. Genetic engineering of harvest index in tobacco through overexpression of a phytochrome gene. Nat Biotechnol. 1996;14:995–998. doi: 10.1038/nbt0896-995. [DOI] [PubMed] [Google Scholar]
- Rocha-Sosa M, Sonnewald U, Frommer W, Stratmann M, Schell J, Willmitzer L. Both developmental and metabolic signals activate the promoter of a class I palatin gene. EMBO J. 1989;8:23–29. doi: 10.1002/j.1460-2075.1989.tb03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddat A, Schmidt P, Gatz C, Braslavsky SE, Gärtner W, Schäffner K. Recombinant type A and B phytochromes from potato transient absorption spectroscopy. Biochemistry. 1997;36:103–111. doi: 10.1021/bi962012w. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Vassey TL, Vanderveer PJ, Vierstra RD. Carbon metabolism and photosynthesis in transgenic tobacco (Nicotiana tabacum L.) having excess phytochrome. Planta. 1991;185:287–296. doi: 10.1007/BF00201046. [DOI] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis: structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Sineshchekov VA, Heyer GA, Gatz C. Phytochrome states in transgenic potato plants with altered phytochrome A levels. Photochem Photobiol. 1996;34:137–142. [Google Scholar]
- Smith H. The ecological functions of the phytochrome family: clues to a transgenic programme of crop improvement. Photochem Photobiol. 1992;56:815–822. [Google Scholar]
- Smith H. Physiological and ecological functions within the phytochrome family. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:289–315. [Google Scholar]
- Smith H, Whitelam GC. The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant Cell Environ. 1997;20:840–844. [Google Scholar]
- Somers DE, Sharrock RA, Teppermann JM, Quail PH. The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell. 1991;3:1263–1274. doi: 10.1105/tpc.3.12.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tuinen A, Kerckhoffs LHJ, Nagatani A, Kendrick RE, Koornneef M. A temporarily red-light-insensitive mutant of tomato lacks a light-stable, B-like phytochrome. Plant Physiol. 1995;108:939–947. doi: 10.1104/pp.108.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Koloszvari M, Quail PH. Two small spatially distinct regions of phytochrome B are required for efficient signaling rates. Plant Cell. 1996;8:859–871. doi: 10.1105/tpc.8.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D, Teppermann JM, Quail PH. Overexpression of phytochrome B induces a short hypocotyl phenotype in transgenic Arabidopsis. Plant Cell. 1991;3:1275–1288. doi: 10.1105/tpc.3.12.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester L, Somers DE, Clack T, Sharrock RA. Transgenic complementation of the hy3 phytochrome B mutation and response to PHYB gene copy number in Arabidopsis. Plant J. 1994;5:261–272. doi: 10.1046/j.1365-313x.1994.05020261.x. [DOI] [PubMed] [Google Scholar]