Abstract
Objectives
The study examined the typical diurnal cortisol trajectory and its differential associations with an intervention, the adult day services (ADS) use, among a sample of family caregivers who experienced high levels of daily stress.
Method
On hundred and sixty-five caregivers of individuals with dementia completed an 8-day diary on daily stressors, positive events, sleep quality, and ADS use. The caregivers also provided five saliva samples on each diary day. Daily cortisol trajectories were modeled as a function of time elapsed since awakening, and three spline growth curve models were fit to the cortisol data. Based on the best-fitting linear spline model, the effect of daily ADS use was examined at both daily and person levels. Covariates included daily experiences and other caregiving characteristics.
Results
On ADS days, caregivers had a steeper cortisol awakening response (CAR) slope and a steeper morning decline. ADS use remained significant after controlling for covariates at both daily and person levels.
Discussion
The findings suggested potential biophysiological benefits of daily ADS use for a sample that was under chronic stress and high levels of daily stress.
Keywords: Adult day services (ADS), Daily diary, Daily stress, Diurnal cortisol slope, Family caregivers, Salivary cortisol
Prior studies have shown that the chronic stress of assisting loved ones with disabilities may place caregivers at heightened risk for compromised health and well-being (Liu, Kim, & Zarit, 2015; Vitaliano, Zhang, & Scanlan, 2003; Zarit, Kim, Femia, Almeida, & Klein, 2014). Caregiving stress manifests itself physiologically, with some caregivers showing biological dysregulation, including cortisol responses (Klein et al., 2014). Cortisol has many regulatory functions; it plays an important role in the central nervous system for learning, memory, and emotion, in the metabolic system for glucose reserve and utilization, in the immune system for regulating inflammatory responses and lymphocytes activity and for organs such as the liver and kidney (Miller, Chen, & Zhou, 2007; Weiner, 1992). These cortisol responses may provide a link between daily experiences and health and are one of the hypothesized biological mechanisms by which stress brings about malfunction and disease in the body (Almeida, Piazza, Stawski, & Klein, 2011). Evidence for the cortisol-health link has been found for psychiatric disorders of depression, medical conditions of cancer and diabetes, and lifestyle problems such as obesity (Abercrombie et al., 2004; Abraham, Rubino, Sinaii, Ramsey, & Nieman, 2013; Bremmer et al., 2007).
Given these links of cortisol to stressful situations and health, the present study examines how daily caregiving is associated with diurnal rhythms of cortisol. Using daily diaries, we focused on family caregivers of individuals with dementia (IWDs) who utilized adult day services (ADS) at varying amounts of days during the week. Caregivers’ cortisol samples were collected five times a day for eight consecutive days, along with daily interviews on their daily stressor experiences. The study expands prior research in three ways. First, it explores the best-fitting model for characterizing the diurnal cortisol curve. Second, using the best-fitting model, we examine impact of an intervention, ADS use, which lowers care-related stressor exposure (Zarit et al., 2011), on cortisol profiles by comparing caregivers on days they use ADS and days they do not. Third, ADS effects are evaluated in the context of daily experiences by adding naturally occurring daily stressors in the model to evaluate their effects on cortisol responses.
Measures of Diurnal Cortisol Rhythm
There are different analytical approaches to cortisol levels, depending on the sampling frequencies. The first and second approaches are using individual cortisol samples and daily total output measured by the area under the curve (AUC) as outcomes (e.g., Klein et al., 2014). The other perspective is using multiple daily cortisol samples across days as the outcome, which generates cortisol diurnal curves or slope trajectories. These perspectives are complementary to each other. They are capable of testing different hypotheses on cortisol reactivity. All together, they offer a comprehensive assessment on the association between cortisol reactivity to daily stressors in the context of chronic stress. A research interest in cortisol diurnal curves can only be tested with a more integrated modeling approach such as growth curves, using multiple cortisol levels within a day and across days as outcomes. Furthermore, the growth curve models can accommodate variations in sampling time, which can be substantial in studies examining daily cortisol and stress.
One parameter of diurnal cortisol regulation is the level change as a function of time elapsed, or cortisol slopes. Some commonly studied slopes include cortisol awakening response (CAR) and diurnal declining slopes (Stalder et al., 2015). Salivary cortisol typically has a rapid rise upon awakening, the CAR, which has been utilized as an index of hypothalamic–pituitary–adrenal (HPA) activity. Although perceived chronic stress and anticipated acute stressors were associated with CAR and cortisol declining slopes (Clow, Thorn, Evans, & Hucklebridge, 2004; Stawski, Cichy, Piazza, & Almeida, 2013), the nature of association varied with some studies showing cortisol elevation and others showing lower cortisol levels and attenuated slopes (Chida & Steptoe, 2009). Flattened cortisol slopes were typically expected in samples that were experiencing chronic stress and with an older age (Strahler, Berndt, Kirschbaum, & Rohleder, 2010).
To better evaluate within- and between-person variations in factors associated with differential cortisol diurnal trajectories, Ranjit, Young, Raghunathan, and Kaplan (2005) suggested using a piecewise linear regression model with random effects, which is essentially a zero-degree spline-based model, also known as the linear spline model. Specifically, diurnal cortisol levels were modeled using multiple joined pieces of linear components as a function of time elapsed since wake-up. The joining locations of the linear components are defined as knots (Wold, 1974). Advantages of this modeling approach include cortisol samples not needing to be evenly distributed over the course of the day, and accommodating participants’ unequal number of samples during the study period (Ranjit, Young, Raghunathan, et al., 2005). A more frequent daily cortisol sampling scheme can usually generate smoother curves, which may offer better model fit. Such growth curve approaches utilize the spline function, defined as the piecewise polynomial; the polynomials join in the knots, generating a high degree of smoothness (Wold, 1974). Additionally, these models can best accommodate variations in the sampling time, which are typical in daily cortisol studies using a diary design. This model and its variations have been applied to population-based samples, but they have not been tested in a sample experiencing relatively high levels of daily stressors such as family caregivers of IWDs (Karlamangla, Friedman, Seeman, Stawksi, & Almeida, 2013; Ranjit, Young, & Kaplan, 2005).
There can be more than one growth curve model for typical diurnal cortisol slopes (Karlamangla et al., 2013). Using linear spline growth curves, the nuances in the declining slopes during different time windows across the day can be modeled with fixed inflection time points (Ranjit, Young, Raghunathan, et al., 2005); using linear-quadratic and linear-cubic growth curves, smoother declining slopes can be modeled (Karlamangla et al., 2013). It is unclear, however, which piecewise growth curve model can best capture dementia caregivers’ cortisol diurnal shape and account for its natural variability in the context of specific daily experiences of caregiving. The current study explores the best model of diurnal cortisol slopes using three different piecewise growth curves. Describing the best-fitting typical diurnal curve serves as the first aim for the study.
The ADS Hypothesis
A unique feature of this study is to examine the best-fitting model in a chronic stress context when caregivers are experiencing high and low levels of daily stressors. This is accomplished by studying caregivers of IWD who are using ADS. ADS programs offer daily care and activities for individuals with dementia and other disabilities and time away for their caregivers. Typical programs provide cognitive, social, and physical stimulation (e.g., Woodhead, Zarit, Braungart, Rovine, & Femia, 2005). In the current study, we focused on the effects of the “time-away” feature on family caregivers associated with ADS use, which was similar across all ADS programs. Prior work has found that ADS use reduces time of exposure to care-related stressors by 43% (Zarit et al., 2011). Furthermore, because caregivers use ADS for their relative on some days a week and provide care themselves on other days, it is possible to examine the effects of variation in caregivers’ daily stressor exposures on cortisol. Prior work has shown that caregivers have better regulated cortisol (i.e., more robust morning rise) on ADS compared to non-ADS days (Klein et al., 2014). We extend this prior work using piecewise growth curves to determine if this approach might pinpoint more effectively within- or between-person associations with ADS use on caregivers’ diurnal cortisol slopes. This is the second aim of the study.
Daily Stressors and Diurnal Cortisol Rhythms
Prior research has examined both acute and chronic stressors and cortisol diurnal rhythms using the piecewise growth curve approach. Following this line of research, Stawski and colleagues (2013) used a piecewise linear-quadratic model and found that people who experienced acute daily stressors more frequently exhibited a steeper diurnal cortisol slope that decelerated more rapidly. Using a quadratic growth curve model, Savla and colleagues (2013) examined daily stressors in spouses of persons with mild cognitive impairment. They found that on days caregivers reported any mood disturbances of their spouse, they showed a flatter cortisol rhythm with elevated daily cortisol output. Using three alternative piecewise growth curves, Karlamangla and colleagues (2013) found that daily cortisol rhythm was flatter and more blunted in individuals with less privileged social status characterized by lower education and ethnic minority background. The third aim of the current study is to evaluate the effects of distinctive types of care stressors on cortisol diurnal curves, in addition to the effects of daily ADS use. Additionally, daily variables shown to covary with cortisol outcomes in prior studies such as daily wake-up time, sleep duration, and quality are considered as covariates of cortisol diurnal slopes (Stawski et al., 2013).
Using the best-fitting model of diurnal cortisol rhythms, two hypotheses were tested. First, ADS use will be related to cortisol regulation at both within- and between-person levels. Specifically, daily ADS use and total number of ADS days across the study period are expected to associate with a more prominent CAR and steeper decline across the day. Second, the associations between ADS use and diurnal cortisol slopes are expected to remain significant in the context of daily stressor exposures of family caregivers.
Method
Participants
Participants were 176 family caregivers from the Daily Stress and Health (DaSH) study (Zarit et al., 2014). To be eligible, caregivers had to be (a) providing primary care to an IWD that lived in the same household and (b) using ADS programs at least 2 days a week. In addition, the person they were caring for had to have a diagnosis of a dementing illness made by a physician.
As part of the study, participants were asked to complete 8 days of daily interviews, which were gathered each evening by telephone, and to provide five saliva samples each day. From the sample of 176 caregivers, 11 participants (6.3%) were excluded from the analysis. Three had mixed up or missed salivettes (1.7%) and two (1.1%) were sisters who shared care responsibilities equally. An additional six caregivers (3.4%) were excluded who only provided a homogeneous set of interviews (i.e., all ADS or all non-ADS days).
In total, the working sample consisted of 165 caregivers, who provided 6,234 cortisol samples (94.5% compliance) on 1,299 valid diary days (98.4% compliance) that were available for analysis. Samples with missing values or collection times (n = 261) were excluded. Of the 6,234 useable samples, 6,132 were valid (98.4%). A cortisol sample was not valid if (a) the participant was awake for less than 12 hr or greater than 20 hr (n = 14), or (b) there was a greater than 10 nmol/l rise between the second (30 min after getting out of bed) and third samples (before lunch; n = 11), or (c) the recorded collection time between the first (upon wake-up) and second samples is either less than 15 min or greater than 60 min (n = 99). Among these 6,132 valid cortisol samples, 3,189 (52%) in total were collected on ADS days (mean = 4.16, SD = 1.44) and 2,943 were collected on days (mean = 3.78, SD = 1.43) when IWDs were at home with their family caregivers.
Procedures
ADS programs were identified through regional and state associations. Programs that agreed to participate were provided with detailed information about the study, recruitment brochures, and announcements that could be included in the newsletters. Fifty-seven ADS programs expressed interest in study participation. Caregivers were phoned by the research coordinator, given additional information about the study, and screened for eligibility. Subsequently, an initial face-to-face interview was conducted at the caregiver’s home, during which they signed consent forms and completed a set of questionnaires. After the initial meeting and baseline assessment, caregivers participated in daily interviews for eight consecutive days via evening phone calls (conducted by the staff at the Penn State Survey Research Center); they also provided saliva samples five times each day. Caregivers received $100 for completing the daily and biomarker study protocol.
To collect saliva samples, participants were instructed to (a) take saliva samples at specified times during the day by chewing on a cotton swab for 2 min, (b) record their saliva collection times, (c) avoid taking samples within 30 min of eating, drinking, brushing teeth, using tobacco, or caffeinated products, and (d) refrigerate saliva samples until the end of the 8 days. Additionally, participants recorded medications taken over the past 48 hr, tobacco smoking status, and, for females, information on the menstrual cycles. Instructions for saliva collection were also reviewed during the first phone interview. At the end of the saliva collection period, salivettes were couriered to the lab at the Pennsylvania State University where they were frozen at –80°C until assayed.
Measures
Saliva samples
On each of the diary study days, participants provided five saliva samples: upon wake-up, 30 min after getting out of bed, before lunch, late afternoon, and before bed. They recorded the exact sample collection time, which was also confirmed during the evening interview. Salivary cortisol levels were assayed at the Pennsylvania State University’s General Clinical Research Center using commercially available enzyme immunoassay kits (DSL, Webster, TX). The sample test volume was 25 µl. The assay had a lower limit of sensitivity of 0.03 μg/dl, with an average inter- and intra-assay covariance of less than 7% and 4%, respectively. Samples from each participant were tested in duplicate in a single assay batch. Duplicate test values that varied by more than 5% were tested repeatedly. Values used in data analyses are the averages of duplicate tests. Cortisol measurement units were converted to nmol/l (μg/dl × 27.6). Raw cortisol distribution was examined to see if log transformation is necessary for the analysis.
ADS use
In each daily diary interview, the caregivers indicated whether they had made use of ADS that day. From these reports, both time-varying and time-invariant variables were derived. Daily ADS use (time-varying and within-person) was a binary variable indicating use (= 1) or nonuse (= 0) that day. Time-invariant ADS use (between-person) was computed as the sum of total ADS days across the daily interview period.
Daily stressors
Two types of daily stressors were assessed: care-related stressors and noncare stressors. Care-related stressors reflect the IWD’s daily behavior problems and were measured using the Daily Record of Behavior (DRB). The DRB, designed specifically for use in daily diaries, assesses the frequency with which 19 behaviors occurred over a 24-hr time frame (α = .78, see Femia, Zarit, Stephens, & Greene, 2007 for detailed psychometric properties). To assist caregivers in reporting behavior problems, the day is broken up into four time-blocks that correspond to the modal periods during which caregivers use ADS: (a) waking to 9:00 a.m., (b) 9:00 a.m. to 4:00 p.m. (typical ADS attendance hours), (c) 4:00 p.m. to bedtime, and (d) overnight. For each period of the day, caregivers were asked whether each behavior had occurred (yes/no). From these reports, both time-varying and time-invariant variables were derived. The time-varying care-related stressors were the sum of total behavior occurrences that were reported that day, including the overnight period of the previous day; the time-invariant care-related stressors were the average level of daily behavior occurrences reported across the interview period.
Noncare stressors were measured using the Daily Inventory of Stressful Events (DISE; Almeida, Wethington, & Kessler, 2002). Each evening, caregivers reported on the occurrence (yes/no) of eight events over the previous 24-hr period (α = .59): arguments with other people, whether they avoided an argument with someone, incidents concerning their friends or family, health-related issues, money or finance-related issues, work-related issues, and other stressful issues or incidents. In order to separate care-related from noncare stressors, caregivers were specifically instructed to report events they found stressful other than those encountered when assisting their relative. Both time-varying and time-invariant variables were derived based on the daily reports. The time-varying noncare stressors were the sum of stressors reported across all eight categories on each of the diary interview days; the time-invariant noncare stressors were the average level of daily stressors reported across the interview period.
Daily positive events
Using five items drawn from the DISE (Sin, Graham, & Almeida, 2014), caregivers reported occurrences of positive experiences during the past 24 hr (α = .63): sharing a laugh with someone, having an experience at home, with a close friend or relative, or at work that others would consider positive, and any other positive experience. Both time-varying and time-invariant variables based on the daily reports were derived. The time-varying variable was the sum of positive events reported based on all five categories on each of the diary interview days; the time-invariant variable was the average level of daily positive events across the interview period.
Covariates
Additional variables that are often associated with caregivers’ cortisol levels were considered as covariates. These covariates were as follows: caregivers’ chronological age, gender (1 = female and 0 = male), duration of care provision (months), the IWD’s ADL dependency (mean of 13 items; coded on a 4-point scale; 1 = does not need help to 4 = cannot do without help; higher scores indicated greater dependency; α = .83), caregiver sleep quality assessed each day as the response to the item: “Rate the quality of your sleep last night” (5-point scale; 1 = poor to 5 = excellent), caregiver self-reported wake-up time, and sleep duration calculated as the time difference between self-reported wake-up and bedtime. Caregivers’ daily sleep quality, wake-up time, and sleep duration were used as time-varying covariates, and caregivers’ average sleep quality, wake-up time, and sleep duration across days were used as time-invariant covariates.
Analytical Strategy
Prior studies have shown that cortisol level is driven primarily by time elapsed since wake-up and less by the clock time. This study, therefore, modeled daily cortisol trajectories as a function of time elapsed since wake-up. Although caregivers were instructed to collect the second saliva sample 30 min after getting out of bed, significant variation in actual sample collection time was expected, with the second sample collected between 15 and 45 min after wake-up in 94.1% of all valid cortisol samples. Similarly, greater variation was expected in the collection time for the other saliva samples throughout the day due to vastly varying daily routines and experiences. Thus, it was possible to model caregivers’ cortisol levels at different sampling times and to examine the factors associated with different trajectories of the typical daytime cortisol trajectory.
Based on prior studies on daily cortisol (Karlamangla et al., 2013), a typical trajectory across the day can be represented by a linearly increasing segment during the 30 min of awakening, and a gradual decline throughout the day, with a slight uptick slope on some days with a very long sampling duration. This study adopted the piecewise growth curve approach and compared model fit among three fitted growth curves of diurnal cortisol using (a) a four-part piecewise linear spline model, (b) a linear-quadratic spline model, and (c) a linear-cubic spline model. The linear spline model had four linear components, joining in the fixed knots at 0.5, 6, and 10.5 hr after wake-up, which allowed the timing of the cortisol peak to vary across individuals. The linear-quadratic and linear-cubic spline models had a linear and a higher order polynomial component (i.e., a second order for quadratic function vs. a third order for cubic function), which joined in the only fixed knot at 0.5 hr after wake-up. As there can be more than one way to determine the location of knots, these fixed knots (i.e., inflection time points) were determined based on the observed average cortisol sampling time as suggested by Ranjit, Young, Raghunathan, and colleagues (2005). A three-level unconditional model, with time as the independent variable, was used to model the cortisol diurnal curves to account for within-day and within-person correlations in cortisol levels. Then, these three unconditional models were compared for the best model fit for the current caregiver sample. Finally, to test hypotheses on ADS use and the daily context of stressors, the best-fitting unconditional model was expanded to include key predictors and covariates in the appropriate level of equations. Equations were detailed in the Supplemental Material.
Results
Caregiver characteristics at baseline are presented in Table 1. The mean and standard deviation on salivary cortisol sampling times and levels are presented in Table 2. Substantial variation in cortisol sampling time and levels was observed within caregivers across days and between caregivers. To evaluate associations between cortisol levels, daily experiences, and caregiving characteristics, preliminary models were run first using cortisol levels at each sampling occasion as outcomes. Parameter estimates of these preliminary models are presented in Table 3. In general, shorter sleep durations tended to be associated with higher cortisol levels across all sampling occasions. Additionally, higher cortisol levels upon awakening (β = 0.07, p = .04) and in the late afternoon (β = 0.04, p = .04) were associated with older caregiver age. Higher cortisol levels at 30 min after waking was associated with daily ADS use (β = 1.21, p = .002) and more positive daily experiences (β = 0.96, p = .02). Higher before-lunch cortisol levels were associated with shorter previous night’s sleep duration (β = −0.25, p = .02), later daily wake-up time (β = 0.34, p = .008), and being male (β = −1.03, p = .03). Higher late-afternoon cortisol levels were associated with a poorer previous night’s sleep (β = −0.41, p = .0009), older age (β = 0.04, p = .04), and IWD’s ADL dependency (β = 0.98, p = .03). Higher bedtime cortisol levels were associated with more daily noncare stressors (β = 0.30, p = .03) and early wake-up time at the between-person levels (β = −0.50, p = .04).
Table 1.
Caregiver (N = 165) Characteristics at Baseline
| Variable | M or frequency | SD or % | Minimum | Maximum |
|---|---|---|---|---|
| Age | 61.99 | 10.70 | 39 | 89 |
| Female | 119 | 87.50 | 0 | 1 |
| Educationa | 4.41 | 1.21 | 1 | 6 |
| Married with a partner | 89 | 65.44 | 0 | 1 |
| Spouse caregiver | 64 | 38.79 | 0 | 1 |
| Adult child caregiver | 80 | 58.82 | 0 | 1 |
| Depressive symptomsb | 1.53 | 0.62 | 1 | 5 |
| Duration of carec | 64.42 | 46.76 | 3 | 216 |
| IWD’s ADLs dependencyd | 3.01 | 0.50 | 2 | 4 |
| Number of ADS days per week | 3.86 | 1.17 | 2 | 5 |
Note: ADL = activities of daily living; ADS = adult day services; IWD = individual with dementia.
aMeasured on a 6-point scale: 1 (less than high school) to 6 (postcollege degree). bMeasured as the mean of seven items on a 5-point scale: 1 (none of the day) to 5 (all day). cMeasured in months. dMeasured as the mean of 13 ADL items on a 4-point scale: 1 (does not need help) to 4 (cannot do without help).
Table 2.
Descriptive Statistics for Salivary Cortisol
| Sample collection times | Mean | SD (BP), in minutes | SD (WP), in minutes |
|---|---|---|---|
| Waking | 06:45 hr | 62 | 43 |
| 30 min after waking | 07:19 hr | 62 | 43 |
| Before lunch | 12:49 hr | 51 | 51 |
| Late afternoon | 17:27 hr | 54 | 42 |
| Before bed | 22:41 hr | 62 | 36 |
| Sample levels (nmol/l) | Mean | SD (BP) | SD (WP) |
| Waking | 9.19 | 4.02 | 3.93 |
| 30 min after waking | 12.32 | 4.74 | 4.78 |
| Before lunch | 4.02 | 1.97 | 1.81 |
| Late afternoon | 3.04 | 2.65 | 1.89 |
| Before bed | 2.77 | 2.87 | 2.17 |
Note: SD (BP) = standard deviation between person; SD (WP) = standard deviation within person across days.
Table 3.
Salivary Cortisol Levels and Associations With Daily Experiences and Caregiving Characteristics
| Parameter | Waking | 30 min after waking | Before lunch | Late afternoon | Before bed |
|---|---|---|---|---|---|
| Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) | Estimate (SE) | |
| Intercept | 9.17 (0.35) | 11.67 (0.42) | 3.97 (0.18) | 3.11 (0.23) | 2.89 (0.26) |
| Within-person covariates | |||||
| Daily ADS use | 0.01 (0.33) | 1.21 (0.39)** | 0.11 (0.18) | −0.15 (0.22) | −0.26 (0.29) |
| Daily positive experience | 0.13 (0.15) | −0.14 (0.18) | 0.01 (0.08) | −0.05 (0.10) | −0.06 (0.13) |
| Daily noncare stressors | 0.03 (0.16) | 0.12 (0.18) | −0.06 (0.08) | −0.02 (0.10) | 0.30 (0.14)* |
| Daily care-related stressors | −0.01 (0.04) | −0.02 (0.05) | −0.03 (0.02) | 0.01 (0.03) | −0.04 (0.04) |
| Daily sleep quality | 0.08 (0.19) | −0.02 (0.22) | −0.09 (0.10) | −0.41 (0.12)*** | 0.13 (0.16) |
| Daily sleep duration | −0.17 (0.20) | −0.26 (0.23) | −0.25 (0.10)* | 0.02 (0.13) | −0.15 (0.17) |
| Daily wake-up time | 0.39 (0.25) | −0.24 (0.29) | 0.34 (0.13)** | 0.10 (0.16) | 0.25 (0.22) |
| Between-person covariates | |||||
| Total ADS days | −0.12 (0.23) | −0.48 (0.27) | −0.03 (0.11) | −0.02 (0.15) | 0.21 (0.16) |
| Caregiver age | 0.07 (0.03)* | 0.06 (0.04) | 0.02 (0.02) | 0.04 (0.02)* | 0.03 (0.02) |
| Caregiver gender | −1.09 (0.96) | 0.38 (1.13) | −1.03 (0.47)* | −0.71 (0.62) | −0.76 (0.66) |
| Duration of care | 0.00 (0.01) | 0.01 (0.01) | 0.00 (0.00) | 0.00 (0.00) | −0.01 (0.00) |
| IWD’s ADL dependency | 0.79 (0.66) | 0.91 (0.79) | 0.38 (0.32) | 0.92 (0.43)* | 0.62 (0.46) |
| Average positive experience | 0.66 (0.35) | 0.96 (0.42)* | 0.21 (0.17) | 0.32 (0.23) | 0.17 (0.24) |
| Average noncare stressors | −0.28 (0.41) | −0.44 (0.48) | −0.29 (0.20) | −0.23 (0.26) | −0.05 (0.28) |
| Average care-related stressors | −0.02 (0.05) | −0.03 (0.06) | 0.03 (0.02) | 0.00 (0.03) | −0.01 (0.04) |
| Average sleep quality | −0.02 (0.46) | 0.25 (0.54) | 0.28 (0.22) | 0.23 (0.30) | 0.36 (0.32) |
| Average sleep duration | −0.90 (0.37)* | −0.96 (0.44)* | −0.44 (0.18)* | −0.60 (0.24)* | −0.50 (0.26)* |
| Average wake-up time | −0.05 (0.34) | −0.45 (0.41) | 0.04 (0.17) | 0.03 (0.22) | −0.50 (0.24)* |
Note: ADS = adult day services; IWD = individual with dementia; ADL = activities of daily living. All daily (within-person) covariates were person-mean centered. All between-person covariates were grand-mean centered.
*p ≤ .05, **p ≤ .01, ***p ≤ .001.
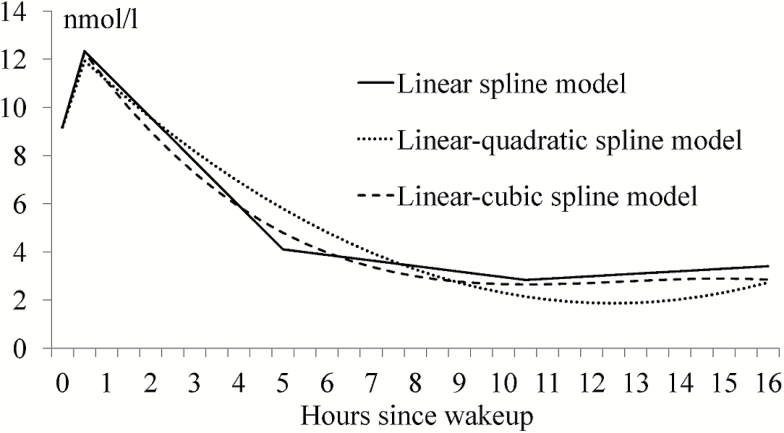
To answer the research question of best-fitting model on diurnal cortisol rhythms among alternative piecewise growth curves, the following three-level unconditional models were run: a four-part linear spline, a linear-quadratic spline, and a linear-cubic spline. Parameter estimates and overall model fit indices are presented in Table 4. Diurnal cortisol curves based on these three unconditional models are presented in Figure 1. For each of the alternative models, all cortisol diurnal slopes were significant. However, the four-part linear spline model had the lowest Bayesian information criterion (Table 4), suggesting best model fit for this dementia caregiver sample. It was therefore chosen as the base model for all ensuing analyses.
Table 4.
Model Comparison Among Three Unconditional Piecewise Growth Curves on Cortisol Diurnal Trajectory
| Parameter | Linear spline model | Linear-quadratic spline model | Linear-cubic spline model |
|---|---|---|---|
| Estimate (SE) | Estimate (SE) | Estimate (SE) | |
| Intercept | 9.18 (0.32)*** | 9.18 (0.32)*** | 9.18 (0.32)*** |
| 4-Part piecewise linear model | |||
| CAR slope | 6.32 (0.59)*** | 5.52 (0.49)*** | 6.22 (0.50)*** |
| 1st linear decline slope from 30 min after waking to before lunch | −1.83 (0.08)*** | ||
| 2nd linear decline slope from before lunch to late afternoon | −0.25 (0.04)*** | ||
| 3rd linear decline slope from late afternoon to before bed | 0.07 (0.03)* | ||
| Quadratic and cubic spline model | |||
| Linear decline slope from 30 min after waking throughout the day | −1.68 (0.04)*** | −2.50 (0.08)*** | |
| Quadratic decline slope from 30 min after waking throughout the day | 0.07 (0.00)*** | 0.21 (0.01)*** | |
| Cubic decline slope from 30 min after waking throughout the day | −0.01 (0.00)*** | ||
| REML deviance | 32,959.3 | 33,378.1 | 33,245.7 |
| AIC | 32,993.3 | 33,402.1 | 33,271.7 |
| BIC | 33,045.4 | 33,438.9 | 33,311.5 |
Note: AIC = Akaike information criterion; BIC = Bayesian information criterion; CAR = cortisol awakening response; REML = restricted maximum likelihood. All daily (within-person) covariates were person-mean centered. All between-person covariates were grand-mean centered.
*p ≤ .05, ***p ≤ .001.
Figure 1.
Diurnal salivary cortisol (measured in nmol/l) curves based on three unconditional models.
Based on the unconditional linear spline model, the sample average level of waking cortisol was 9.18 nmol/l and CAR was 6.32 nmol/l per hour. Cortisol declined initially at 1.83 nmol/l per hour from the second to the third sampling occasion (i.e., from 30 min after awakening to before lunch), and the rate of decline decelerated at 0.25 nmol/l per hour from before lunch to late afternoon. There was a slight uptick in cortisol levels between late afternoon and bedtime at 0.07 nmol/l per hour.
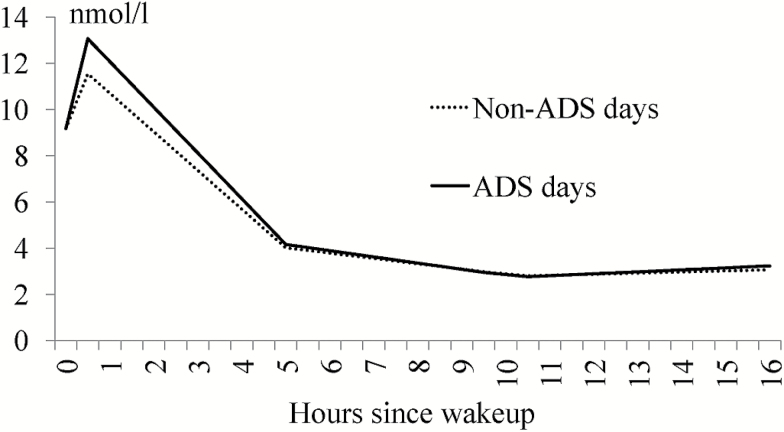
Next, to test the hypothesis on ADS use and associations with diurnal cortisol slopes, two models were run with ADS use as the covariate at the within-person (i.e., the daily ADS effect, Model 1) and between-person (i.e., the total ADS days effect, Model 2) levels. Parameter estimates are presented in Table 5. Model 1 showed that the daily ADS effect was primarily in the morning. Specifically, daily ADS use was significantly associated with a more prominent CAR (β = 3.26, p < .001), and a steeper decline starting from 30 min after waking and leading to before lunch (β = −0.22, p = .001). On non-ADS days, however, caregivers tended to have a flatter cortisol diurnal pattern (Figure 2). Daily ADS use did not have any effect on the other two declining slopes starting from before lunch and thereafter. Model 2 showed that total ADS days across the 8-day period also had some effect on the cortisol slope between late afternoon and before bed. Specifically, more ADS days were associated with a slight but significant cortisol uptick later in the day.
Table 5.
Effect of ADS Use on Diurnal Cortisol Slopes Covarying for Daily Experiences and Caregiving Characteristics
| Parameter | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Estimate (SE) | Estimate (SE) | Estimate (SE) | |
| Intercept | 9.25 (0.34)*** | 9.18 (0.32)*** | 9.31 (0.33) |
| CAR | 4.40 (0.67)*** | 6.11 (0.58)*** | 4.35 (0.67) |
| AD1, the first linear decline slope | −1.52 (0.07)*** | −1.64 (0.07)*** | −1.51 (0.08) |
| AD2, the second linear decline slope | −0.01 (0.07) | −0.07 (0.05) | −0.03 (0.07) |
| AD3, the third linear decline slope | −0.08 (0.05) | −0.06 (0.03) | −0.05 (0.05) |
| Daily ADS use (1 = ADS day) | −0.13 (0.24) | −0.26 (0.25) | |
| CAR × ADS | 3.26 (0.65)*** | 3.43 (0.66)*** | |
| AD1 × ADS | −0.22 (0.07)*** | −0.24 (0.07)*** | |
| AD2 × ADS | −0.13 (0.08) | −0.09 (0.10) | |
| AD3 × ADS | 0.05 (0.06) | 0.00 (0.07) | |
| Total ADS days | −0.01 (0.22) | −0.04 (0.22) | |
| CAR × ADS days | −0.11 (0.41) | −0.52 (0.42) | |
| AD1 × ADS days | 0.04 (0.05) | 0.07 (0.05) | |
| AD2 × ADS days | −0.06 (0.03) | −0.05 (0.03) | |
| AD3 × ADS days | 0.07 (0.02)** | 0.07 (0.02)** | |
| Within-person covariate | |||
| Daily positive experience | −0.04 (0.07) | ||
| Daily noncare stressors | 0.09 (0.07) | ||
| Daily care-related stressors | −0.01 (0.02) | ||
| Daily sleep quality | −0.01 (0.09) | ||
| Daily sleep duration | −0.15 (0.09) | ||
| Daily wake-up time | 0.02 (0.11) | ||
| Between-person covariate | |||
| Average positive experience | 0.17 (0.17) | ||
| Average noncare stressors | −0.11 (0.19) | ||
| Average care-related stressors | 0.00 (0.02) | ||
| Average sleep quality | 0.26 (0.22) | ||
| Average sleep duration | −0.44 (0.18)* | ||
| Average wake-up time | −0.29 (0.16) | ||
| Caregiver age | 0.03 (0.02)* | ||
| Caregiver gender | −0.65 (0.45) | ||
| Duration of care | 0.00 (0.00) | ||
| IWD’s ADL dependency | 0.56 (0.32) | ||
| REML deviance | 33,000.5 | 33,039.8 | 33,049.6 |
| AIC | 33,024.5 | 33,063.8 | 33,065.6 |
| BIC | 33,061.2 | 33,100.6 | 33,090.1 |
Note: ADL = activities of daily living; ADS = adult day services; AIC = Akaike information criterion; BIC = Bayesian information criterion; CAR = cortisol awakening response; IWD = individual with dementia; REML = restricted maximum likelihood. All daily (within-person) covariates were person-mean centered. All between-person covariates were grand-mean centered. The base model was the best-fitting linear spline model. Model 1 tested the daily ADS effect; Model 2 tested the total ADS days effect; Model 3 was the full model, controlling for covariates of daily experiences and caregiving characteristics. Nonsignificant interactions between daily experiences and cortisol slopes were trimmed from Model 3.
*p ≤ .05, **p ≤ .01, ***p ≤ .001.
Figure 2.
The effect of daily adult day services (ADS) use on salivary cortisol (measured in nmol/l) diurnal slopes.
To test the hypothesis on ADS use and associations with diurnal cortisol slopes in the context of daily stressor exposures, a full model as specified earlier was run with ADS effects controlling for daily experiences and caregiving characteristics such as caregiver age, gender, and duration of care (Model 3). All significant ADS effects on diurnal cortisol slopes remained. However, none of the daily stressor and positive experience interactions with cortisol slopes was significant at either the within- or between-person level. All nonsignificant interactions were trimmed from the final model. Parameter estimates are presented for Model 3 in Table 5.
Discussion
This study is among the first to examine dementia caregivers’ salivary cortisol diurnal rhythms in relation to an intervention, in the context of daily experiences. Prior studies using the same data set found some physiological benefits of ADS use on better cortisol regulation (Klein et al., 2014). Also, daily stressor exposures were found to be predictive of emotional responses to stressors, such as depression and anger, whereas ADS use, in turn, may alleviate some of these emotional responses (Zarit et al., 2014). The current study complements this work by demonstrating at a daily level that stressor exposures among dementia caregivers are associated with increased cortisol levels at certain times of the day (i.e., before bed). Daily ADS use was associated with a more robust CAR, which could benefit the chronically stressed caregivers physiologically.
The study had a number of notable contributions to the literature. First, it explored three alternative spline models to see which had the best fit to the naturally occurring cortisol. Although the linear-quadratic spline and linear-cubic spline models produced smoother declining curves, the linear spline model was the best representation of the naturally occurring cortisol pattern among dementia caregivers given the data. The sample of dementia caregivers in this study was different from a general population sample in two ways. Family caregivers were under chronic stress (Aneshensel, Pearlin, Mullan, Zarit, & Whitlatch, 1995); meanwhile, they also tended to experience high levels of daily stressors such as behavior problems of IWDs as well as other noncare stressors (Zarit et al., 2014). Although prior studies have typically utilized some form of quadratic functions to model cortisol diurnal trajectories (Savla et al., 2013; Stawski et al., 2013), Ranjit, Young, Raghunathan, and colleagues (2005) recommended the piecewise linear spline as an effective way to model naturally occurring cortisol profile. Furthermore, Karlamangla and colleagues (2013) found the piecewise linear spline model fit better than other alternative piecewise growth curve models for a nationally representative sample. Thus, the daily stress context of the specific sample may have played a key role in determining what would be the best cortisol model.
Second, the associations between ADS use and cortisol in the context of daily experiences were complex and differed depending on whether the cortisol outcomes were the levels or diurnal slopes and whether the associations were between-person or within-person over time. ADS use manipulated caregivers’ daily exposures to primary stressors of caregiving, the behavior problems of IWDs. On some diary days, caregivers used ADS; on the other days, they actively provided care for the IWD. The findings that, at the within-person level, ADS use was robustly associated with higher levels of cortisol 30 min after waking and a more prominent CAR were consistent with prior studies on the negative associations between CAR and burnout, and the physiological benefits of ADS (Chida & Steptoe, 2009). ADS use can improve HPA functioning (Klein et al., 2014) among chronically stressed caregivers; the current study showed further that ADS can help restore a healthier CAR and a more prominent declining cortisol profile. One explanation is that the anticipation effect of an ADS day may have elevated the hypoactivated cortisol pattern among dementia caregivers (Klein et al., 2014; Leggett, Liu, Klein, & Zarit, 2015). The finding that more daily noncare stressors were associated with higher cortisol levels before bed and probably greater daily total cortisol output was also consistent with previous research on the positive association between daily stressor exposures and naturally occurring cortisol levels (Stawski et al., 2013). However, significant within-person associations between daily stressors and cortisol levels, and slopes were not observed at other sampling occasions, or over the course of the day. One explanation could be the time-dependent nature of HPA axis reactions to daily stressors. Laboratory-induced cortisol levels typically increase 20–40 min after stressor exposures (Dickerson & Kemeny, 2004). The retrospective stressor report at the end of the day and the five-sample cortisol assessment within-a-day design utilized in the current study may be less ideal to capture the time-dependent association between stressor exposures and cortisol levels.
Third, the associations between ADS use and cortisol levels and slopes were also between-person, in the context of other daily experiences. More positive experiences were associated with higher cortisol levels 30 min after waking. Additionally, more total ADS days during the study period were associated with an increasing cortisol slope starting from late afternoon to before bed. We included that time point specifically because we thought there might be an uptick related to when the IWD comes home. A prior study on this sample found that on ADS days, noncare stressors tended to increase; total ADS days were also associated with significantly higher cortisol daily total output measured by AUC (Klein et al., 2014). The current findings confirmed these results. Although ADS use is able to provide some caregiving respite and decrease care-related stressor exposures (Zarit et al., 2014), it also opens up opportunities for caregivers to engage in a full life, which may have increased the exposures to other noncare stressors such as work demands as well as positive experiences. The heightened cortisol levels 30 min after waking and the increasing cortisol evening slope reflected in part the increased total stressor exposures associated with greater ADS use.
The study had some limitations. The current daily diary design relied on retrospective self-report on stressor exposures at the end of the day. The salivary cortisol samplings were also intended at relatively fixed time windows. Thus, it was impossible to precisely align the timing of stressors to cortisol samples to accurately track the cortisol diurnal rhythms in association of stressor exposures. This general limitation on naturally occurring cortisol studies can be partially addressed by using a more intense ecological momentary assessment design, where events sampling may be used (Smyth et al., 1998). Also, considering the relatively demanding nature of participating in a daily study, findings based on this sample of dementia caregivers may not be generalizable to a broader population of family caregivers. Furthermore, the study provided some evidence at the daily level that ADS use may be associated with better HPA functioning among caregivers. Future studies need to consider the associations between daily stressor exposures and more than one kind of biomarkers. Additionally, the associations between daily biomarkers and long-term health and well-being need to be explored in the future to better understand the effects of daily stressor exposures on health outcomes.
The study suggested that the four-part linear spline model could best represent the naturally occurring cortisol diurnal trajectory among dementia caregivers who tended to experience a relatively high level of daily stressors. ADS use as a respite had some physiological benefits for dementia caregivers. One pathway through which dementia caregiving compromises health and well-being is through chronic exposures to daily stressors. ADS use provides partial relief from primary stressors of behavior problems on a daily basis, which makes family caregiving more manageable and may offer caregivers some actual health benefits. Given the health benefits for caregivers associated with respite, findings from this study could be used to argue for increasing affordability of ADS and other respite programs. This could be accomplished through expansion of coverage of lower income people under “dual eligible” programs (Medicare and Medicaid) and more broadly by inclusion of ADS under Medicare. Future studies need to focus on the consequences of CAR and the initial morning decline over time. Specifically, testing if these effects are buffered by both daily ADS use and total ADS days used across a period can help fully understand the health effects of respite care on dementia caregivers.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This work was supported by the National Institute on Aging (NIA) at the National Institute of Health (grant number RO1 AG031758, “Daily Stress and Health of Family Caregivers”).
Supplementary Material
References
- Abercrombie H. C. Giese-Davis J. Sephton S. Epel E. S. Turner-Cobb J. M., & Spiegel D (2004). Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology, 29, 1082–1092. doi:10.1016/j.psyneuen.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Abraham S. B. Rubino D. Sinaii N. Ramsey S., & Nieman L. K (2013). Cortisol, obesity, and the metabolic syndrome: A cross-sectional study of obese subjects and review of the literature. Obesity, 21, E105–E117. doi:10.1002/oby.20083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida D. M. Piazza J. R. Stawski R. S., & Klein L. C (2011). The speedometer of life: Stress, health and aging. In Schaie K. W., Willis S. L. (Eds.), Handbook of the psychology of aging (7th ed, pp. 191–206). San Diego, CA: Academic Press. [Google Scholar]
- Almeida D. M. Wethington E., & Kessler R. C (2002). The Daily Inventory of Stressful Events: An interview-based approach for measuring daily stressors. Assessment, 9, 41–55. doi:10.1177/1073191102091006 [DOI] [PubMed] [Google Scholar]
- Aneshensel C. S. Pearlin L. I. Mullan J. T. Zarit S. H., & Whitlatch C. J (1995). Profiles in caregiving: The unexpected career. San Diego, CA: Academic Press. [Google Scholar]
- Bremmer M. A. Deeg D. J. H. Beekman A. T. F. Penninx B. W. J. H. Lips P., & Hoogendijk W. J. G (2007). Major depression in late life is associated with both hypo- and hypercortisolemia. Biological Psychiatry, 62, 479–486. doi:10.1016/j.biopsych.2006.11.033 [DOI] [PubMed] [Google Scholar]
- Chida Y., & Steptoe A (2009). Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology, 80, 265–278. doi:10.1016/j.biopsycho.2008.10.004 [DOI] [PubMed] [Google Scholar]
- Clow A. Thorn L. Evans P., & Hucklebridge F (2004). The awakening cortisol response: Methodological issues and significance. Stress, 7, 29–37. doi:10.1080/10253890410001667205 [DOI] [PubMed] [Google Scholar]
- Dickerson S. S., & Kemeny M. E (2004). Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin, 130, 355–391. doi:10.1037/0033-2909.130.3.355 [DOI] [PubMed] [Google Scholar]
- Femia E. E. Zarit S. H. Stephens M. A. P., & Greene R (2007). Impact of adult day services on behavioral and psychological symptoms of dementia. The Gerontologist, 47, 775–788. doi:10.1093/geront/47.6.775 [DOI] [PubMed] [Google Scholar]
- Karlamangla A. S. Friedman E. M. Seeman T. E. Stawksi R. S., & Almeida D. M (2013). Daytime trajectories of cortisol: Demographic and socioeconomic differences—Findings from the National Study of Daily Experiences. Psychoneuroendocrinology, 38, 2585–2597. doi:10.1016/j.psyneuen.2013.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L. C. Kim K. Almeida D. M. Femia E. E. Rovine M. J., & Zarit S. H (2014). Anticipating an easier day: Effects of adult day services on daily cortisol and stress. The Gerontologist. Advance online publication. doi:10.1093/geront/gnu060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggett A. N. Liu Y. Klein L. C., & Zarit S. H. V. P (2015). Sleep duration and the cortisol awakening response in dementia caregivers utilizing adult day services. Health Psychology. Advance online publication. doi:10.1037/hea0000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Kim K., & Zarit S. H (2015). Health trajectories of family caregivers: Associations with care transitions and adult day service use. Journal of Aging and Health, 27, 686–710. doi:10.1177/0898264314555319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G. E. Chen E., & Zhou E. S (2007). If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin, 133, 25–45. doi:10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Ranjit N. Young E. A., & Kaplan G. A (2005). Material hardship alters the diurnal rhythm of salivary cortisol. International Journal of Epidemiology, 34, 1138–1143. doi:10.1093/ije/dyi120 [DOI] [PubMed] [Google Scholar]
- Ranjit N. Young E. A. Raghunathan T. E., & Kaplan G. A (2005). Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology, 30, 615–624. doi:10.1016/j.psyneuen.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Savla J. Granger D. A. Roberto K. A. Davey A. Blieszner R., & Gwazdauskas F (2013). Cortisol, alpha amylase, and daily stressors in spouses of persons with mild cognitive impairment. Psychology and Aging, 28, 666–679. doi:10.1037/a0032654 [DOI] [PubMed] [Google Scholar]
- Sin N. L. Graham J. E., & Almeida D. M (2014). Daily positive events and inflammation: Findings from the National Study of Daily Experiences. Brain, Behavior, and Immunity, 43, 130–138. doi:10.1016/j.bbi.2014.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth J. Ockenfels M. C. Porter L. Kirschbaum C. Hellhammer D. H., & Stone A. A (1998). Stressors and mood measured on a momentary basis are associated with salivary cortisol secretion. Psychoneuroendocrinology, 23, 353–370. doi:10.1016/S0306-4530(98)00008-0 [DOI] [PubMed] [Google Scholar]
- Strahler J. Berndt C. Kirschbaum C., & Rohleder N (2010). Aging diurnal rhythms and chronic stress: Distinct alteration of diurnal rhythmicity of salivary α-amylase and cortisol. Biological Psychology, 84, 248–256. doi:10.1016/j.biopsycho.2010.01.019 [DOI] [PubMed] [Google Scholar]
- Stalder T. Kirschbaum C. Kudielka B. M. Adam E. K. Pruessner J. C. Wüst S., … Clow A (2015). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology. Advance online publication. doi:10.1016/j.psyneuen.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Stawski R. S. Cichy K. E. Piazza J. R., & Almeida D. M (2013). Associations among daily stressors and salivary cortisol: Findings from the National Study of Daily Experiences. Psychoneuroendocrinology, 38, 2654–2665. doi:10.1016/j.psyneuen.2013.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaliano P. P. Zhang J., & Scanlan J. M (2003). Is caregiving hazardous to one’s physical health? A meta-analysis. Psychological Bulletin, 129, 946–972. doi:10.1037/0033-2909.129.6.946 [DOI] [PubMed] [Google Scholar]
- Weiner H. (1992). Perturbing the organism: The biology of stressful experience. Chicago, IL: University of Chicago press. [Google Scholar]
- Wold S. (1974). Spline functions in data analysis. Technometrics, 16, 1–11. doi:10.2307/1267485 [Google Scholar]
- Woodhead E. L. Zarit S. H. Braungart E. R. Rovine M. R., & Femia E. E (2005). Behavioral and psychological symptoms of dementia: The effects of physical activity at adult day service centers. American Journal of Alzheimer’s Disease and Other Dementias, 20, 171–179. doi:10.1177/153331750502000314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarit S. H. Kim K. Femia E. E. Almeida D. M., & Klein L. C (2014). The effects of adult day services on family caregivers’ daily stress, affect, and health: Outcomes from the Daily Stress and Health (DaSH) study. The Gerontologist, 54, 570–579. doi:10.1093/geront/gnt045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarit S. H. Kim K. Femia E. E. Almeida D. M. Savla J., & Molenaar P. C. M (2011). Effects of adult day care on daily stress of caregivers: A within-person approach. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66, 538–546. doi:10.1093/geronb/gbr030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.