Abstract
Background
Fecal carriage of multidrug-resistant and extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae is one of the important risk factors for infection with antibiotic-resistant bacteria. In this report, we examined the prevalence of multidrug-resistant and ESBL-producing common enterobacterial strains colonizing the intestinal tract of apparently healthy adults in Kathmandu, Nepal.
Methods
During a 6-month period (February–July 2016), a total of 510 stool specimens were obtained from apparently healthy students of Manmohan Memorial Institute of Health Sciences, Kathmandu, Nepal. Stool specimens were cultured, and the most common enterobacterial isolates (Escherichia coli and Klebsiella species) were subjected to antimicrobial susceptibility tests according to the standard microbiologic guidelines. Multidrug-resistant isolates were selected for ESBL confirmation by combined disk test and E-test methods. Molecular characterization of plasmid-borne ESBL genes was performed by using specific primers of cefotaximase Munich (CTX-M), sulfhydryl variant (SHV), and temoniera (TEM) by polymerase chain reaction.
Results
Among 510 bacterial strains, E. coli (432, 84.71%) was the predominant organism followed by Klebsiella oxytoca (48, 9.41%) and K. pneumoniae (30, 5.88%). ESBLs were isolated in 9.8% of the total isolates including K. oxytoca (29.17%), E. coli (7.87%), and K. pneumoniae (6.67%). Among ESBLs, bla-TEM was the predominant type (92%) followed by bla-CTX-M (60%) and bla-SHV (4%).
Conclusion
Multidrug-resistant and ESBL-producing enterobacterial commensal strains among healthy individuals are of serious concern. Persistent carriage of ESBL organisms in healthy individuals suggests the possibility of sustained ESBL carriage among the diseased and hospitalized patients. We recommend similar types of epidemiologic surveys in larger communities and in hospital settings to ascertain the extent of ESBL resistance.
Keywords: healthy adults, intestinal carriage, ESBL, CTX-M, TEM, SHV, Nepal
Background
The advent of antibiotics for the treatment of severe infections was one of the most significant achievements of the last century.1 With industrialization, improved sanitation, housing, and nutrition, as well as the revolutionary development of disease-fighting antimicrobials, infectious diseases became a scourge of the past.2 However, within a short time span, their efficacy has been increasingly discomfited by ubiquitous dissemination of acquired resistance determinants among pathogenic bacteria.3 In today’s health care environment, almost every single species of pathogenic bacteria has developed resistance to at least one antimicrobial agent. Furthermore, infections caused by such multidrug-resistant (MDR) bacteria impose an adverse impact worldwide on public health and economy as well as increasing global morbidity and mortality.4
Increased rate of antibiotic resistance, initially common among severely ill patients with hospital-acquired infections, has now spread into the community.5 Of great concern is the emergence of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae because these organisms are resistant to penicillins, extended-spectrum cephalosporins, and monobactams.6,7 Furthermore, many ESBL-producing strains are concurrently resistant to other non-beta lactam antibiotics including fluoroquinolones and aminoglycosides, further compounding the difficulties in treatment and control of their spread.8 Since their first report in 1983, >300 variants of ESBLs have been documented. Among the several genotypes, CTX-M (cefotaximase Munich), SHV (sulfhydryl variant), and TEM (temoniera) are the most common in different Gram-negative clinical strains as well as intestinal commensals.9,10
MDR intestinal commensals, including Escherichia coli, from healthy individuals, have been demonstrated several years ago, and there is a continuous rise in the incidence of antibiotic resistance in commensals in recent years.11 E. coli, an important part of fecal flora, is associated with varying human infections and has been shown to be the main carrier of antimicrobial resistance genes among the fecal flora.12 Intestinal carriage of MDR bacteria, as well as ESBL-producing Enterobacteriaceae has increased significantly globally, with developing countries being the most affected.13 However, the majority of these studies were investigating the commensal flora linked to nosocomial infections in hospitalized patients and were intended for outbreak investigations.12 Despite a large magnitude of antimicrobial resistance problem in hospital and community settings in Nepal, the extent of intestinal carriage of MDR in healthy adults is largely undefined. Hence, to acquire the updated knowledge on commensal bacteria colonizing the healthy human gut, this study was carried out to determine the intestinal carriage of MDR ESBL-producing bacteria among apparently healthy adults of Kathmandu, Nepal.
Methods
This descriptive cross-sectional study was carried out among the healthy adult volunteers of a health science college in Kathmandu, Nepal. Specimens were obtained from the college students (before clinical posting), and micro-biologic procedures were carried out at the Department of Microbiology, Manmohan Memorial Institute of Health Sciences, Kathmandu, Nepal.
Ethical approval
This research was approved (approval no: MMIHS/18/072) by the Institutional Review Committee of Manmohan Memorial Institute of Health Sciences (IRC-MMIHS), Kathmandu, Nepal. Written informed consent was obtained from every participant after describing the procedure and implications of the research. The participants were recruited on the basis of their voluntary enrollment.
Inclusion and exclusion criteria
Individuals (aged between 16 and 30 years) without antibiotic exposure for at least 3 months prior to study enrollment and those without a known active infection were included in this study. However, individuals with an active infection who were under antimicrobial chemotherapy and those who refused to give consent were excluded.
Laboratory methods
During a 6-month period (February–July 2016), a total of 510 stool samples from 510 participants (one from each) were collected in a sterile plastic container. A questionnaire was completed for each participant regarding the name, age, gender, and medical history (previous antibiotic or antacid intake, previous hospital admission, surgical procedure). Stool specimens were inoculated onto a single MacConkey agar plate (HiMedia Laboratories, Mumbai, Maharashtra, India) and incubated aerobically for 24 hours at 37°C. Each morphotype of bacterial colonies grown on the plate was selected and identified by standard micro-biologic techniques including the morphologic appearance of the colony, Gram’s staining, catalase test, oxidase test along with other biochemical tests (carbohydrate utilization, indole production, urea hydrolysis, citrate utilization, and amino acid decarboxylation).14 E. coli and Klebsiella species were further selected for antimicrobial susceptibility and detection of ESBL production.
Antimicrobial susceptibility testing
The antimicrobial susceptibility of the bacterial isolates was tested with various antibiotics using Mueller Hinton agar (MHA) by modified Kirby–Bauer disk diffusion technique following the recommendations of Clinical and Laboratory Standards Institute (CLSI).15 The battery of antibiotics and their specific concentration included in the study were ampicillin (10 μg), aztreonam (30 μg), trimethoprim-sulfamethoxazole/cotrimoxazole (25 μg), ciprofloxacin (5 μg), cefotaxime (30 μg), cefixime (5 μg), ceftazidime (30 μg), ceftriaxone (30 μg), chloramphenicol (30 μg), gentamycin (10 μg), imipenem (10 μg), tetracycline (30 μg), and piperacillin-tazobactam (100/10 μg) (HiMedia Laboratories). The results of the antimicrobial susceptibility tests were interpreted as per the zone size interpretative guidelines of CLSI in terms of “sensitive”, “resistant”, and “intermediate sensitive”.15 E. coli ATCC 25922 and Klebsiella pneumoniae ATCC 13883 were tested in every set of experiments, in parallel, as part of quality control.
Detection of MDR and potential ESBL producers
MDR isolates were identified according to the combined guidelines of the European Centre for Disease Prevention and Control and the Centers for Disease Control and Prevention.16 The isolate resistant to at least one antimicrobial from three different groups of first-line drugs tested was regarded as MDR. For ESBL screening, bacterial isolates were examined for their susceptibility to third-generation cephalosporins by using ceftriaxone, ceftazidime (30 μg), and cefotaxime (30 μg) disks. If the zone of inhibition (ZOI) was ≤25 mm for ceftriaxone, ≤22 mm for ceftazidime, and/or ≤27 mm for cefotaxime, the isolate was considered a potential ESBL producer. Phenotypic confirmation of ESBL producers was carried out by combination disk test (CDT) and ESBL-E test as recommended by the CLSI.15 Ceftazidime (30 μg) disk alone and in combination with clavulanic acid (ceftazidime+clavulanic acid 30/10 μg) for CDT and a strip containing cefotaxime (minimum inhibitory concentration [MIC] test range 0.25–16 μg/mL) at one end and cefotaxime plus a constant concentration of clavulanate (CA; 4 μg/mL) on the other end (Triple ESBL detection Ezy MIC™ Strip [MIX+/MIX]; HiMedia Laboratories) for ESBL-E test were applied onto a plate of MHA previously inoculated with the test strain and incubated in ambient air for 16–18 hours of incubation at 35±2°C. Isolates showing an increase of ≥5 mm in the ZOI of the combination disks in comparison with that of the ceftazidime disk alone and that showed ≥8-fold concentration decrease in MIC of cefotaxime combined with CA versus its MIC of cefotaxime alone were considered as ESBL producers.15,17 As a quality control, K. pneumoniae ATCC 700603 and E. coli ATCC 25922 were used as positive and negative controls for ESBL production, respectively.
Molecular assays for detection of ESBL genes
The isolates that were phenotypically confirmed as ESBL producers were then selected for the detection of β-lactamase-encoding genes. Molecular detection of isolates harboring ESBL genes (bla-CTX-M, bla-TEM, and bla-SHV) were carried out by conventional polymerase chain reaction (PCR) at Kathmandu Center for Genomics and Research Laboratory (KCGRL).
Plasmid DNA extraction and amplification
The bacterial plasmid DNA was extracted using SureSpin Plasmid Mini kit Catalogue no.NP-37105 (Genetix Biotech, New Delhi, India) following the manufacturer’s instructions. Briefly, a single colony from each phenotypic ESBL-producing isolate was transferred to Luria–Bertani broth and incubated to obtain logarithmic bacterial growth. The extraction procedure includes preparation and clearing of bacterial lysate using alkaline lysis method, adsorption of DNA onto the SureSpin column membrane, and elution of plasmid DNA. The extracted DNA from bacterial isolates was used as a template to detect the ESBL genotypes bla-CTX-M, bla-TEM, and bla-SHV. Primers of ESBL genotypes were purchased from GeNei™, Bangalore, India, and their sequence is listed in Table 1.
Table 1.
Primer for bla-CTX-M, bla-TEM, and bla-SHV genes
| Gene | Primer (5′–3′) | Amplicon size (bp) |
|---|---|---|
| SHV | FP: 5′-GTCAGCGAAAAACACCTTGCC-3′ | 383 |
| RP: 5′-GTCTTATCGGCGATAAACCAG-3′ | ||
| TEM | FP: 5′-GAGACAATAACCCTGGTAAAT-3′ | 459 |
| RP: 5′-AGAAGTAAGTTGGCAGCAGTG-3′ | ||
| CTX-M | FP: 5′-GAAGGTCATCAAGAAGGTGCG-3′ | 560 |
| RP: 5′-GCATTGCCACGCTTTTCATAG-3′ |
Abbreviations: CTX-M, cefotaximase Munich; FP, forward primer; RP, reverse primer; SHV, sulfhydryl variant; TEM, temoniera.
For amplification reaction, a multiplex PCR was carried out to detect the plasmid genes for SHV and CTX-M, whereas conventional linear PCR was used for TEM-type ESBL genes. PCRs reactions were carried out in 25 μL volume with the master mix containing 200 μM of dNTP (dATP, dCTP, dGTP, and dTTP), 120 nM of each primer, 0.5 U/μL of Taq polymerase in 1× PCR buffer, 25 mM MgCl2, and 1μL of DNA in nuclease-free water. Amplification reactions were carried out in a DNA thermal cycler (CG) under the following thermal and cycling conditions: initial denaturation at 94°C for 3 minutes, denaturation at 94°C for 45 seconds of 35 cycles, annealing at 60°C for 30 seconds of 35 cycles (for bla-SHV and bla-CTX-M) and 55°C for 30 seconds of 35 cycles (for bla-TEM), extension at 72°C for 3 minutes of 35 cycles, and final extension at 72°C for 2 minutes. As a quality control, previously confirmed K. pneumoniae clinical isolates possessing bla-TEM, bla-SHV, and bla-CTX-M genes were used as a positive control, and nuclease-free water was used as negative in each run of PCR.
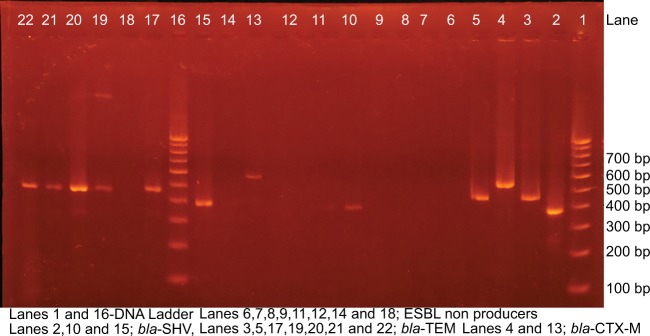
After PCR amplification, 2.5 μL of each reaction was separated by electrophoresis on 1.5% agarose gel for 30 minutes at 100 V in 0.5× TBE buffer. DNA was stained with ethidium bromide (1 μg/mL), and the bands were detected using UV-transilluminator (Major Science UVDI) (Figure 1).
Figure 1.
Gel electrophoresis with bands of bla-CTX-M, bla-TEM, and bla-SHV.
Notes: Figure 1 illustrates the electrophoretic bands of ESBL genotypes where lanes 1 and 17 are DNA ladder; lane 2 (SHV, 383 bp), lane 3 (TEM, 459 bp), and lane 4 (CTX-M, 560 bp) are ESBL-positive controls, while lanes 5 (bla-TEM), 10, and 16 (bla-SHV) and lanes 18, 20, 21, 22, and 23 (bla-CTX-M) are test strains.
Abbreviations: CTX-M, cefotaximase Munich; ESBL, extended-spectrum β-lactamases; SHV, sulfhydryl variant; TEM, temoniera.
Data analysis
Data analysis was carried out using the SPSS version 20.0 (IBM, Armonk, NY, USA) and Microsoft Excel 2007 and interpreted according to frequency distribution and percentage. Variables were compared using the Pearson’s χ2 test and data with the p-value <0.05 (95% CI) were regarded as significant.
Results
Five hundred ten healthy volunteers within the age range of 16–30 years participated in this study, and of that, 110 (21.6%) were male and 400 (78.4%) were female. From these specimens, 432 (84.7%) E. coli, 48 (9.4%) Klebsiella oxytoca, and 30 (5.9%) K. pneumoniae were isolated.
Antimicrobial susceptibilities
We observed variable susceptibilities among bacterial isolates toward the tested antimicrobials. Considerably high resistance among E. coli isolates was observed against ampicillin (46.76%), tetracycline (28.71%), and cotrimoxazole (24.07%), and a smaller proportion was resistant to cefixime (10.19%), cefotaxime (10.19%), aztreonam (8.33%), and ciprofloxacin (6.02%). Among the K. oxytoca isolates, the highest resistance was observed toward cotrimoxazole (45.83%) followed by cefotaxime (37.5%), cefixime (37.5%), tetracycline (33.33%), and aztreonam (29.17%), while K. pneumoniae isolates were resistant to tetracycline and piperacillin-tazobactam (33.33% each) and cefotaxime, cefixime, and aztreonam (6.67% each). All of the K. pneumoniae isolates were found to be sensitive to imipenem and gentamycin, whereas two isolates each of E. coli and K. oxytoca were imipenem and gentamycin resistant (Table 2).
Table 2.
Antimicrobial susceptibility pattern of isolated strains
| Antibiotics |
Escherichia coli (n=432)
|
Klebsiella oxytoca (n=48)
|
Klebsiella pneumoniae (n=30)
|
|||
|---|---|---|---|---|---|---|
| Sensitive (%) | Resistant (%) | Sensitive (%) | Resistant (%) | Sensitive (%) | Resistant (%) | |
| Ampicillin | 53.24 | 46.76 | 0 | 100 | 0 | 100 |
| Aztreonam | 91.67 | 8.33 | 70.83 | 29.17 | 93.33 | 6.67 |
| Cotrimoxazole | 75.93 | 24.07 | 54.17 | 45.83 | 100 | 0 |
| Ciprofloxacin | 93.98 | 6.02 | 83.34 | 16.66 | 100 | 0 |
| Chloramphenicol | 95.83 | 4.17 | 100 | 0 | 100 | 0 |
| Cefotaxime | 89.81 | 10.19 | 62.5 | 37.5 | 93.33 | 6.67 |
| Cefixime | 89.81 | 10.19 | 62.5 | 37.5 | 93.33 | 6.67 |
| Ceftriaxone | 89.81 | 10.19 | 62.5 | 37.5 | 93.33 | 6.67 |
| Ceftazidime | 89.81 | 10.19 | 62.5 | 37.5 | 93.33 | 6.67 |
| Gentamycin | 99.54 | 0.46 | 95.83 | 4.17 | 100 | 0 |
| Imipenem | 99.54 | 0.46 | 95.83 | 4.17 | 100 | 0 |
| Piperacillin tazobactam | 97.69 | 2.31 | 87.5 | 12.5 | 86.67 | 13.33 |
| Tetracycline | 71.29 | 28.71 | 66.67 | 33.33 | 86.67 | 13.33 |
MDR and ESBL producers
One hundred sixteen (22.7%) isolates were MDR, including E. coli (92, 21.3%), K. oxytoca (22, 45.8%), and K. pneumoniae (2, 6.7%). The screening test for presumptive ESBL producers detected 74 (14.5%) of the isolates to be presumptive ESBL producers, E. coli (54, 12.5%), K. oxytoca (18, 37.5%), and K. pneumoniae (2, 6.67%). Subsequently, 50 (9.8%) bacterial isolates were confirmed as ESBL positive by phenotypic CDT and ESBL Etest. Regarding ESBL-positive isolates, K. oxytoca was the predominant ESBL producer (14, 29.17%), followed by E. coli (34, 7.87%) and K. pneumoniae (2, 6.67%) (Table 3).
Table 3.
Multidrug resistant and ESBL-producing enterobacterial isolates (phenotypic)
| Bacterial isolates | MDR
|
ESBL producers
|
||||
|---|---|---|---|---|---|---|
| Presumptive
|
Confirmatory
|
|||||
| No. % | No. | % | No. | % | ||
| Escherichia coli (n=432) | 92 | 21.3 | 54 | 12.5 | 34 | 7.87 |
| Klebsiella oxytoca (n=48) | 22 | 45.83 | 18 | 37.5 | 14 | 29.17 |
| Klebsiella pneumoniae (n=30) | 2 | 6.67 | 2 | 6.67 | 2 | 6.67 |
| Total | 116 | 22.7 | 74 | 14.5 | 50 | 9.8 |
Abbreviations: ESBL, extended-spectrum β-lactamases; MDR, multidrug-resistant.
Antimicrobial susceptibilities of ESBL producers and nonproducers
There was a significant variation among the susceptibilities of ampicillin, aztreonam, third-generation cephalosporins, and piperacillin-tazobactam in ESBL producers and nonproducers. Notably, ESBL producers were significantly more resistant to cotrimoxazole, ciprofloxacin, and imipenem than nonproducers of ESBL (Table 4).
Table 4.
Antimicrobial susceptibilities of ESBL-producing and nonproducing isolates
| Antibiotics | ESBL negative (n=460) |
ESBL positive (n=50) |
p-value |
|---|---|---|---|
| Ampicillin | 230 (50%) | 50 (100%) | 0.001 |
| Aztreonam | 2 (0.43%) | 50 (100%) | 0.001 |
| Cotrimoxazole | 100 (21.74%) | 26 (52%) | 0.003 |
| Ciprofloxacin | 22 (4.78%) | 12 (24%) | 0.004 |
| Chloramphenicol | 18 (3.91%) | 2 (4%) | 0.240 |
| Cefotaxime | 14 (3.04%) | 50 (100%) | 0.001 |
| Cefixime | 14 (3.04%) | 50 (100%) | 0.001 |
| Ceftriaxone | 14 (3.04%) | 50 (100%) | 0.001 |
| Ceftazidime | 14 (3.04%) | 50 (100%) | 0.001 |
| Gentamycin | 2 (0.43%) | 2 (4%) | 0.060 |
| Imipenem | 4 (0.87%) | 2 (4%) | 0.05 |
| Piperacillin-Tazobactam | 14 (3.04%) | 6 (12%) | 0.009 |
| Tetracycline | 128 (27.83%) | 16 (32%) | 0.510 |
Note: The bold values represent the statistically significant values.
Abbreviation: ESBL, extended-spectrum β-lactamases.
Beta-lactamase genes encoding ESBL production
bla-TEM (92%) was the most predominant ESBL genotype among the isolates, followed by bla-CTX-M (60%) and bla-SHV (4%). Multiple occurrences of genes was common, and coexistence of bla-CTX-M and bla-TEM was observed in 48% of the isolates, whereas bla-TEM and bla-SHV coexisted in 4% of the isolates. Thirty four (7.87%) strains of E. coli were ESBL positive and 18 (52.9%) strains harbored both bla-TEM and bla-CTX-M genes, whereas 2 (5.8%) strains harbored bla-TEM and bla-SHV genes. Among 14 ESBL-producing K. oxytoca isolates, 6 (42.8%) were harboring both bla-TEM and bla-CTX-M genes. Only two isolates of K. pneumoniae were ESBL positive, and they harbored only bla-TEM gene (Table 5).
Table 5.
Genotypic distribution of ESBL genes among the isolated strains
| Bacterial isolates | bla-CTX-M (%) | bla-TEM (%) | bla-SHV (%) | bla-CTX-M+bla-TEM (%) | bla-TEM+bla-SHV (%) | bla-CTX-M+bla-SHV (%) |
|---|---|---|---|---|---|---|
| Escherichia coli | 20 (58.82) | 34 (100.0) | 2 (5.88) | 18 (52.9) | 2 (5.8) | 0 (0.0) |
| K. oxytoca | 10 (71.43) | 10 (71.43) | 0 (0.0) | 6 (42.8) | 0 (0.0) | 0 (0.0) |
| K. pneumoniae | 0 (0.0) | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Total | 30 (60.0) | 46 (92.0) | 2 (4.0) | 24 (48.0) | 2 (4.0) | 0 (0.0) |
Abbreviations: bla, β-lactamase; CTX-M, cefotaximase Munich; ESBL, extended-spectrum β-lactamases; SHV, sulfhydryl variant; TEM, temoniera.
Discussion
The human gastrointestinal tract has been well described as an epicenter of drug-resistant bacteria.18 Different studies carried out in various geographical areas of the world have shown an increased prevalence and dispersion of ESBL-producing isolates in fecal samples of healthy individuals.19,20 Colonization with MDR bacteria, including ESBL-producing isolates, may induce nosocomial infections, cross-transmission to other individuals, and cause outbreaks in hospitalized patients.18 High rates of intestinal carriage of such resistant bacteria have been described from developing countries.21 Thus, it is becoming a necessity to detect the carriers harboring antimicrobial-resistant bacteria in the community, including the healthy population.
To the best of our knowledge, this study represents the very first report about the carriage of ESBL-producing commensal enterobacterial strains in the intestine of healthy Nepalese adults. Overall, the incidence of ESBL-producing fecal commensals in this study was 9.8%, which is comparable to the previous reports of Luvsansharav et al (6.4%) from Japan,22 Kader et al (13.1%) from Saudi Arabia,12 and Ahmed et al (13.4%) from Libya.9 Fecal commensal Enterobacteriaceae carrying ESBL genes have emerged as a significant proportion of fecal flora among healthy adults. Notably, there was a considerable increase in the rate of ESBL fecal carriage (0.6%–6%) in healthy subjects in France over a 5-year period, and it was indicated that acquisition of ESBL E. coli was associated with common food sources and person-to-person transmission through oral–fecal route.23–25 More recently, extremely higher rates of ESBL-producing commensal enterobacterial strains have been reported by Rahman et al (63.3%) from Egypt,26 Sasaki et al (58.2%) from Thailand,27 and Mathai et al (19%) from India.28 These reports suggest the continuous evolution and dissemination of multiresistant enterobacterial commensals in the healthy guts. The ubiquitous presence of resistant commensals in the bowel was suggested to be a risk factor for infections and dissemination of resistance to other pathogens.29 In developing countries like Nepal, antibiotics are readily available and most people undergo antimicrobial treatment without needing a prescription. This provides selective pressure on enteric commensals to develop drug resistance and leads to a potential source for nosocomial infections with MDR organisms.30
In our study, the majority of ESBL-producing enterobacterial strains were K. oxytoca (29.17%), E. coli (7.87%), and K. pneumoniae (6.67%). Among ESBL genotypes, bla-TEM was the most predominant gene (92%) followed by bla-CTX-M (60%) and bla-SHV (4%). The coexistence of bla-CTX-M and bla-TEM was present in 48% of isolates, whereas both bla-TEM and bla-SHV coexisted in 4% of the isolates. However, there are several reports describing the dominance of bla-CTX-M gene among ESBL-producing enterobacterial strains. Mathai et al from India documented that bla-CTX-M (95.5%) was the most common ESBL genotype, and the coproduction of bla-TEM and bla-CTX-M was observed in 63.6% of strains.28 Another study from Japan revealed 92.9% of the ESBL-positive isolates harboring bla-CTX-M gene and a high proportion (84.6%) of that was E. coli.22 Recently, a French study reported that 86% of the ESBL strains isolated from the human gut were bla-CTX-M type.30 However, Al-Agamy et al from Saudi Arabia observed the presence of bla-TEM and bla-CTX-M genes among all ESBL-producing enterobacterial strains.31 The prevalence and genotypes of ESBL-producing fecal enterobacterial isolates vary widely from country to country, from region to region, and at different time periods. However, high incidence of fecal carriage rate of ESBL-producing E. coli has been observed in Asia, Africa, and South America.9,28,32,33 The higher ESBL rates and diverse genotypes among commensals may result in easy transmission of resistant genes to the pathogenic strains causing health care-associated infections and the cause of large outbreaks in community.18 Moreover, the frequent detection of CTX-M-, SHV-, and TEM-type ESBLs in healthy guts is of particular importance as they are present in mobile plasmids and have been reported from varieties of bacterial infections.10,34 Therefore, we believe that there is a continuous rise in the ESBL-producing isolates in Nepal among healthy individuals as well as in patients with infections.
Multidrug resistance is a common phenomenon among the enterobacterial isolates in our study. About 23% of the isolates were MDR including K. oxytoca (45.83%), E. coli (21.3%), and K. pneumoniae (6.67%). Although the rate of MDR is high, our rate is comparatively lower than the reported rate from India and Nigeria.28,35 Furthermore, E. coli isolates were highly resistant to ampicillin (46.76%), tetracycline (28.71%), and cotrimoxazole (24.07%). Besides β-lactam drugs, ESBL-producing isolates were highly resistant to non-beta-lactam antibiotics including cotrimoxazole (52%), tetracycline (32%), and ciprofloxacin (24%). Mathai et al from India28 and Luvsansharav et al from Japan22 also found that ESBL strains were resistant to quinolones, trimethoprim-sulfamethoxazole, tetracycline, and aminoglycosides. The occurrence of combined resistance among these three antibiotics (ampicillin, tetracyclines, and cotrimoxazole) has become a common finding in ESBL isolates in recent years as the genes encoding resistance to these antimicrobials are located on the same plasmid.36 In addition, Mathai et al28 have observed that the cotransfer of the qnr determinant on ESBL-producing plasmids confers resistance to nalidixic acid with reduced susceptibility to fluoroquinolones.
Limitations
Although this was a cross-sectional study among the healthy adults, we could not evaluate the risk factors associated with ESBL-producing commensal enterobacterial strains. Furthermore, only three common ESBL genotypes prevalent in the strains were investigated. Molecular methods including the sequencing of individual genotypes along with their distribution among various strains would be helpful for understanding their epidemiology. In addition, this report represents only health science students predominantly in one age group residing in the capital city that possibly has greater exposure of MDROs than the general population, and the inclusion of more participants from various parts of the country would facilitate the generation of recommendations.
Conclusion
High incidence of ESBL-producing enterobacterial commensal isolates was detected among healthy adults in Kathmandu, Nepal. Our results emphasize the importance of the intestinal tract as a reservoir for ESBL-producing E. coli and Klebsiella spp. The increased prevalence of these organisms in human fecal flora and their establishment in the community represent an opportunity for these clones to become persistent. We recommend the continuous surveillance of these organisms with the introduction of molecular diagnostic procedures in a clinical or reference laboratory to track their spread in the community and in hospital settings.
Acknowledgments
We are deeply thankful to all the participating students, laboratory staff, management, and officials of Manmohan Memorial Institute of Health Sciences, Kathmandu, for providing the opportunity to carry out this research work. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The abstract of this work was presented in the 4th International Conference on Prevention and Infection Control (June 20–23, 2017), Geneva, Switzerland, and has been published as meeting abstracts in Antimicrobial Resistance and Infection Control 2017, 6(Suppl 3), P264. Data regarding this research will be made available to the interested researchers by the corresponding author upon written request.
Footnotes
Author contributions
NPP and AM conceived the design of the study, reviewed the literature, and involved in the laboratory investigations and data analysis. AB, SS, DS, S Shah, GJ, and PRK recruited the volunteers, performed laboratory tests, reviewed the literature, and helped in manuscript preparation. NPP and AM prepared the manuscript. All authors contributed toward drafting and revising the paper, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
All the co-authors agreed to the publication of the findings of this study and provided the consent for submission into the journal.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Wenzel RP, Edmond MB. Managing antibiotic resistance. N Engl J Med. 2000;343(26):1961–1963. doi: 10.1056/NEJM200012283432610. [DOI] [PubMed] [Google Scholar]
- 2.WHO . Essentials Drug Monitor. Geneva: World Health Organization; 2000. Antimicrobial resistance: a global threat; pp. 28–29. Vols 28 and 29. [Google Scholar]
- 3.Spellberg B, Powers JH, Brass EP, Miller LG, Edwards JJE. Trends in antimicrobial drug development: implications for the future. Clin Infect Dis. 2004;38(9):1279–1286. doi: 10.1086/420937. [DOI] [PubMed] [Google Scholar]
- 4.Colomb-Cotinat M, Lacoste J, Brun-Buisson C, Jarlier V, Coignard B, Vaux S. Estimating the morbidity and mortality associated with infections due to multidrug-resistant bacteria (MDRB), France, 2012. Antimicrob Resist Infect Control. 2016;5(1):56. doi: 10.1186/s13756-016-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res. 2005;36(6):697–705. doi: 10.1016/j.arcmed.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Livermore DM. Current epidemiology and growing resistance of gram-negative pathogens. Korean J Intern Med. 2012;27(2):128–142. doi: 10.3904/kjim.2012.27.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 8.Pilmis B, Delory T, Groh M, et al. Extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE) infections: are carbapenem alternatives achievable in daily practice? Int J Infect Dis. 2015;39:62–67. doi: 10.1016/j.ijid.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed SF, Ali MM, Mohamed ZK, Moussa TA, Klena JD. Fecal carriage of extended-spectrum beta-lactamases and AmpC-producing Escherichia coli in a Libyan community. Ann Clin Microbiol Antimicrob. 2014;13:22. doi: 10.1186/1476-0711-13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jena J, Debata NK, Sahoo RK, Gaur M, Subudhi E. Molecular characterization of extended spectrum beta-lactamase-producing Enterobacteriaceae strains isolated from a tertiary care hospital. Microb Pathog. 2017;115:112–116. doi: 10.1016/j.micpath.2017.12.056. [DOI] [PubMed] [Google Scholar]
- 11.Bailey JK, Pinyon JL, Anantham S, Hall RM. Commensal Escherichia coli of healthy humans: a reservoir for antibiotic-resistance determinants. J Med Microbiol. 2010;59(11):1331–1339. doi: 10.1099/jmm.0.022475-0. [DOI] [PubMed] [Google Scholar]
- 12.Kader AA, Kumar A, Kamath KA. Fecal carriage of extended-spectrum β-lactamase–producing Escherichia coli and Klebsiella pneumoniae in patients and asymptomatic healthy individuals. Infect Control Hosp Epidemiol. 2007;28(09):1114–1116. doi: 10.1086/519865. [DOI] [PubMed] [Google Scholar]
- 13.Boonyasiri A, Tangkoskul T, Seenama C, Saiyarin J, Tiengrim S, Thamlikitkul V. Prevalence of antibiotic resistant bacteria in healthy adults, foods, food animals, and the environment in selected areas in Thailand. Pathog Glob Health. 2014;108(5):235–245. doi: 10.1179/2047773214Y.0000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isenberg HD. Clinical Microbiology Procedure Handbook. 2nd ed. Vol. 1. Washington, DC: American Society for Microbiology; 2004. [Google Scholar]
- 15.CLSI . Performance Standards for Antimicrobial Disk Susceptibility Tests CLSI Supplement M100S. 26 ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2016. [Google Scholar]
- 16.Magiorakos AP, Srinivasan A, Carey R, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 17.Triple ESBL detection Ezy MICTM Strip (MIX+/MIX) Antimicrobial Susceptibility Testing 2015. [Accessed August 19, 2017]. Available from: http://www.himedialabs.com/TD/EM079.pdf.
- 18.Carlet J. The gut is the epicentre of antibiotic resistance. Antimicrob Resist Infect Control. 2012;1(1):39. doi: 10.1186/2047-2994-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantón R, Novais A, Valverde A, et al. Prevalence and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae in Europe. Clin Microbiol Infect. 2008;14(Suppl 1):144–153. doi: 10.1111/j.1469-0691.2007.01850.x. [DOI] [PubMed] [Google Scholar]
- 20.Anil Kumar V, Babu R. Fecal carriage of extended-spectrum β-lactamase producing enterobacteriaceae. J Med Microb Diagn. 2012;1:e119. [Google Scholar]
- 21.Shanahan P, Thomson C, Amyes S. β-Lactam resistance in normal faecal flora from South Africa. Epidemiol Infect. 1995;115(02):243–253. doi: 10.1017/s0950268800058374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luvsansharav UO, Hirai I, Niki M, et al. Prevalence of fecal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae among healthy adult people in Japan. J Infect Chemother. 2011;17(5):722–725. doi: 10.1007/s10156-011-0225-2. [DOI] [PubMed] [Google Scholar]
- 23.Leflon-Guibout V, Blanco J, Amaqdouf K, Mora A, Guize L, Nicolas-Chanoine MH. Absence of CTX-M enzymes but high prevalence of clones, including clone ST131, among fecal Escherichia coli isolates from healthy subjects living in the area of Paris, France. J Clin Micro-biol. 2008;46(12):3900–3905. doi: 10.1128/JCM.00734-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nandre R, Ruan X, Duan Q, Zhang W. Enterotoxigenic Escherichia coli heat-stable toxin and heat-labile toxin toxoid fusion 3xSTaN12S-dmLT induces neutralizing anti-STa antibodies in subcutaneously immunized mice. FEMS Microbiol Lett. 2016;363(21) doi: 10.1093/femsle/fnw246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rausch D, Ruan X, Nandre R, et al. Antibodies derived from a toxoid MEFA (multiepitope fusion antigen) show neutralizing activities against heat-labile toxin (LT), heat-stable toxins (STa, STb), and Shiga toxin 2e (Stx2e) of porcine enterotoxigenic Escherichia coli (ETEC) Vet Microbiol. 2017;202:79–89. doi: 10.1016/j.vetmic.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdul Rahman EM, El-Sherif RH. High rates of intestinal colonization with extended-spectrum lactamase-producing Enterobacteriaceae among healthy individuals. J Investig Med. 2011;59(8):1284–1286. doi: 10.2130/JIM.0b013e318238748e. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki T, Hirai I, Niki M, et al. High prevalence of CTX-M beta-lactamase-producing Enterobacteriaceae in stool specimens obtained from healthy individuals in Thailand. J Antimicrob Chemother. 2010;65(4):666–668. doi: 10.1093/jac/dkq008. [DOI] [PubMed] [Google Scholar]
- 28.Mathai D, Kumar VA, Paul B, et al. Fecal carriage rates of extended-spectrum beta-lactamase-producing Escherichia coli among antibiotic naive healthy human volunteers. Microb Drug Resist. 2015;21(1):59–64. doi: 10.1089/mdr.2014.0031. [DOI] [PubMed] [Google Scholar]
- 29.Rossi F, Baquero F, Hsueh PR, et al. In vitro susceptibilities of aerobic and facultatively anaerobic Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: 2004 results from SMART (Study for Monitoring Antimicrobial Resistance Trends) J Antimicrob Chemother. 2006;58(1):205–210. doi: 10.1093/jac/dkl199. [DOI] [PubMed] [Google Scholar]
- 30.Nicolas-Chanoine MH, Gruson C, Bialek-Davenet S, et al. 10-fold increase (2006–11) in the rate of healthy subjects with extended-spectrum beta-lactamase-producing Escherichia coli faecal carriage in a Parisian check-up centre. J Antimicrob Chemother. 2013;68(3):562–568. doi: 10.1093/jac/dks429. [DOI] [PubMed] [Google Scholar]
- 31.Al-Agamy MH, El Mahdy TS, Shibl AM. Fecal colonization with extended-spectrum beta-lactamase and AmpC-producing Escherichia coli. Biomed Res Int. 2016;2016:3704150. doi: 10.1155/2016/3704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li B, Sun JY, Liu QZ, Han LZ, Huang XH, Ni YX. High prevalence of CTX-M beta-lactamases in faecal Escherichia coli strains from healthy humans in Fuzhou, China. Scand J Infect Dis. 2011;43(3):170–174. doi: 10.3109/00365548.2010.538856. [DOI] [PubMed] [Google Scholar]
- 33.Villar HE, Baserni MN, Jugo MB. Faecal carriage of ESBL-producing Enterobacteriaceae and carbapenem-resistant Gram-negative bacilli in community settings. J Infect Dev Ctries. 2013;7(8):630–634. doi: 10.3855/jidc.2900. [DOI] [PubMed] [Google Scholar]
- 34.Sherchan JB, Hayakawa K, Miyoshi-Akiyama T, et al. Clinical epidemiology and molecular analysis of extended-spectrum-β-lactamase-producing Escherichia coli in Nepal: characteristics of sequence types 131 and 648. Antimicrob Agents Chemother. 2015;59(6):3424–3432. doi: 10.1128/AAC.00270-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newman MJ, Seidu A. Carriage of antimicrobial resistant Escherichia coli in adult intestinal flora. West Afr J Med. 2002;21(1):48–50. [PubMed] [Google Scholar]
- 36.Nys S, Okeke IN, Kariuki S, Dinant G, Driessen C, Stobberingh E. Antibiotic resistance of faecal Escherichia coli from healthy volunteers from eight developing countries. J Antimicrob Chemother. 2004;54(5):952–955. doi: 10.1093/jac/dkh448. [DOI] [PubMed] [Google Scholar]