ABSTRACT
Merkel cell carcinoma (MCC) is a rare and aggressive skin cancer that responds to PD-1/PD-L1 immune checkpoint inhibitors. CD200 is another checkpoint modulator whose receptor is found on tumor-promoting myeloid cells, including M2 macrophages. We found high CD200 mRNA expression in MCC tumors, and CD200 immunostaining was demonstrated on 95.5% of MCC tumors. CD200R-expressing myeloid cells were present in the MCC tumor microenvironment. MCC-associated macrophages had a higher average CD163:CD68 staining ratio (2.67) than controls (1.13), indicating an immunosuppressive M2 phenotype. Accordingly, MCC tumors contained increased densities of FOXP3+ regulatory T-cells. Intravenous administration of blocking anti-CD200 antibody to MCC xenograft mice revealed specific targeting of drug to tumor. In conclusion, MCC are highly CD200 positive and associated with immunosuppressive M2 macrophages and regulatory T-cells. As anti-CD200 antibody effectively targets CD200 on MCC tumor cells in vivo, this treatment may provide a novel immunotherapy for MCC independent of PD-1/PD-L1 blockade.
KEYWORDS: CD200, Merkel cell carcinoma, immune checkpoint inhibitor, M2 macrophage, regulatory T cell
Introduction
Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine skin cancer. MCC is highly immunogenic, and immune evasion is necessary for tumor progression.1 Recently, targeting T-cell inhibition with anti-PD-L1 immunotherapy has become the standard of care for metastatic MCC. CD200 is a cell surface ligand that confers immune privilege to the thymus, B cells, activated T cells, certain vascular endothelia, kidney glomeruli, placental cells, hair follicles, neurons, and various malignancies including neuroendocrine tumors,2-7 Its receptor (CD200R) is expressed on cells of the monocyte/macrophage lineage and subsets of B and T cells.8 Signaling by CD200 prevents normal activation of CD200R bearing myeloid cells,9,10 eventuating in an immunosuppressive cascade that includes the induction of regulatory T cells (Tregs).11 For example, CD200 signaling inhibits classic macrophages activation (M1 polarization) and supports an immunosuppressive M2 polarized state that secretes high levels of IL-10, thereby inducing Tregs and promoting tumor growth.12 Thus, CD200 signaling to tumor associated macrophages (TAM) represents a cancer immunotherapy target independent of PD-1/PD-L1 signaling.13,14
Several lines of evidence suggest that the immunosuppressive capacity of CD200 promotes progression of cancers including chronic lymphocytic leukemia,15 multiple myeloma,16 and acute myeloid leukemia.17 In multiple myeloma, CD200 expression correlates with patient outcomes. CD200-expressing melanoma and ovarian cancer cells downregulate Th1 cytokine production in vitro in co-culture with mixed leukocytes,18,19 suggesting that CD200-mediated immune suppression may also impact solid tumors. In a mouse model of cutaneous squamous cell carcinoma (SCC), CD200 does not influence primary tumor development, but promotes the survival of metastatic SCC cells and their ability to seed secondary tumors.20 CD200R+ myeloid-derived suppressor cells (MDSC) and TAM are implicated in supporting the survival of metastatic SCC tumors.20
Blockade of CD200-CD200R interaction by an anti-CD200 antibody has been shown to enhance anti-tumor immune activity against CD200-expressing tumor cells in a mouse model of leukemia,15,21 These effects motivated the clinical development of a CD200 inhibitory antibody. A phase I clinical trial studying an anti-CD200 monoclonal antibody (ALXN6000) in patients with CD200-expressing B-cell chronic lymphocytic leukemia or multiple myeloma showed good tolerability and initial evidence of anti-tumor activity (Alexion Pharmaceuticals, NCT00648739).22
Here, we investigate CD200 expression on MCC and characterize the immune suppressive microenvironment of MCC tumors, directing our attention to TAM and Tregs. We demonstrated that CD200 is highly expressed on MCC and is associated with immune infiltrates containing suppressive M2 macrophages and Tregs. Moreover, we found that intravenous administration of anti-CD200 antibody effectively targeted CD200 on MCC tumor cells in vivo. Taken together, these results suggest that CD200 has potential to be developed as a novel immune target in MCC that is independent of T-cell directed immune checkpoints.
Results
Patient characteristics
A total of 71 patients with histopathologically confirmed MCC diagnosed between 1998 and 2013 were identified by a retrospective search of patient records. The patients were 57.7% male with a mean age at diagnosis of 71.1 years-old. Of the 71 patients, 61 had complete data sets including a total of 88 analyzable tumor samples. Characteristics of all patients and their tumor samples are listed in Table S1.
Merkel cell carcinoma expresses CD200
To assess expression of CD200 on MCC we used public microarray data (NCBI GEO GSE50451),23 to examine CD200 mRNA levels in 23 MCC tumors. All MCC tumors showed CD200 transcription (Figure 1A). MCC tumors had higher CD200 expression than nine samples of small cell lung cancer (SCLC), another neuroendocrine tumor.
Figure 1.
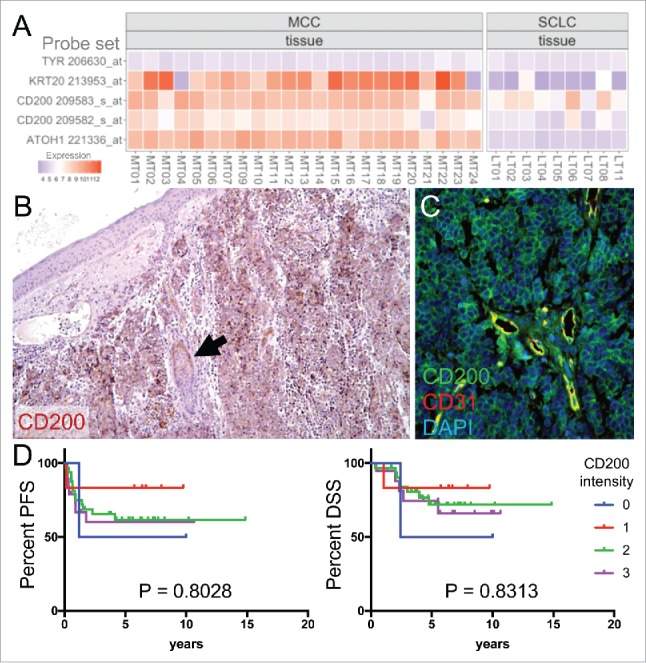
CD200 is expressed in MCC tumors. (A) microarray gene expression using probe sets for CD200, MCC markers KRT20 and ATOH1, and negative control TYR. (B) CD200 staining in MCC tumor and adjacent hair follicle (arrow). Original magnification 100x. (C) CD200 and endothelium marker CD31 immunostaining in MCC tumor. Original magnification 200x. (D) Patient outcomes fail to correlate with CD200 staining level (0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining). MCC, Merkel cell carcinoma; SCLC, small cell lung cancer; PFS, progression free survival; DSS, disease specific survival.
Next, we evaluated CD200 expression by immunohistochemistry (IHC) in 53 primary tumors and 35 MCC metastases (local recurrence, lymph node metastases, in-transit metastases, and distant metastases). Overall, 84 of 88 analyzable MCC specimens (95.5%) showed CD200 staining (Figure 1B, C), including 51 of 53 primary tumors (96.2%) and 33 of 35 metastases (94.3%). Overall, 59 of 61 patients (96.7%) had CD200 staining in their tumors (Table S1). Analogous to the CD200 expression observed in blood vessels of cutaneous SCC,20 we found that CD31-positive endothelial cells in MCC tumors also expressed CD200 (Figure 1C). Consistent with prior reports,24 we also detected CD200 staining in hair follicles (Figure 1B). To assess if frequent CD200 expression is a common feature among neuroendocrine tumors, we also performed CD200 immunostaining on 102 SCLC samples. Consistent with their lower mRNA expression, only 56 SCLC tumors were positive for CD200 staining (55%), 41 were negative (40%) and 5 were not analyzable (5%) (data not shown). Thus MCC has higher CD200 expression relative to SCLC.
CD200 expression levels do not correlate with survival in MCC
Among our patients, there were 59 cases with analyzable MCC tumor tissue from the date of diagnosis (Table S1). These patients were followed for a total of 305.0 person years (median follow-up = 5.3 years). Semi-quantitative CD200 expression levels were scored by relative staining intensity (0 = no staining, 1 = weak staining, 2 = moderate staining, 3 = strong staining). Only 2 cases had no staining. There was no correlation between CD200 expression levels and MCC tumor stage at diagnosis (P = 0.2499). Based on Kaplan-Meier estimates, neither progression free survival (P = 0.8028) nor MCC specific survival (P = 0.8313) differed based on CD200 expression levels (Figure 1D).
M2 tumor associated macrophages and Tregs are present in MCC
Because MCC frequently expresses CD200, we hypothesized that MCC tumors would associate with CD200R+ immunosuppressive myeloid cells,8,20 To confirm the presence of CD200R in the tumor microenvironment, we stained 5 fresh-frozen MCC biopsy specimens with antibodies against CD200R and the myeloid marker CD11b. Monocytes expressing CD11b and CD200R were present within tumor nests and around the tumor periphery (Figure 2A).
Figure 2.
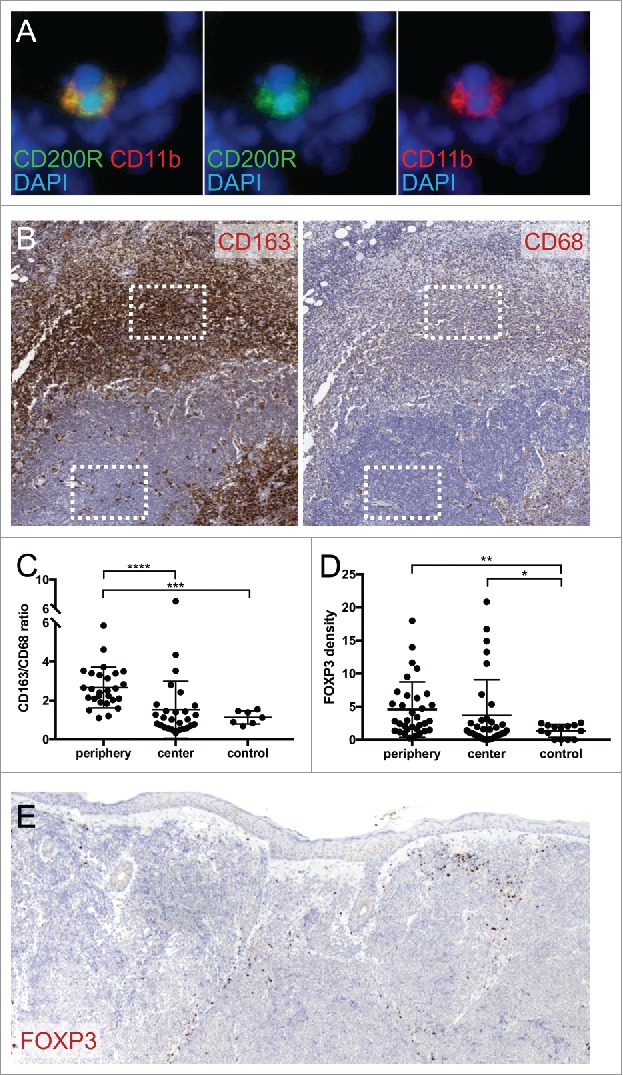
MCC is associated with immunosuppressive immune infiltrate. (A) CD200R and myeloid marker CD11b immunostaining in fresh-frozen MCC tumor. Original magnification 400x. (B) M2 marker CD163 and macrophage marker CD68 staining in MCC tumor. Representative sampling areas for tumor periphery and tumor center used for staining quantifications (boxes). Original magnification 50x. (C) Mean CD163/CD68 staining density ratio for MCC tumor periphery, tumor center, and normal skin control. ****, P<0.0001 by paired t-test; ***, P<0.0001 by Welch's unpaired t-test. (D) Mean Treg marker FOXP3 staining density for MCC tumor periphery, tumor center, and normal skin control. **, P<0.0005; *,P<0.05. (E) FOXP3 staining in MCC tumor. Original magnification 100x.
Atypically activated M2 macrophages can promote tumor immune evasion. Staining 27 primary MCC tumors with the macrophage marker CD68 and the M2 marker CD163 identified CD163+ CD63+ M2 TAM concentrating around the tumor periphery and sparsely infiltrating tumor nests (Figure 2B). Image analysis was used to quantify staining. The mean CD163/CD68 staining density ratio,25 of the tumor periphery (2.67) was significantly higher than the ratio in the tumor center (1.53, P<0.0001), and that of the dermis in 7 normal skin samples (1.13, P<0.0001) (Figure 2C).
M2 macrophages suppress immune responses through many mechanisms including the induction of FOXP3-expressing Tregs. Consistent with M2 macrophages being present, we observed abundant FOXP3-expressing lymphocytes in 33 of 33 tested primary MCC tumors. Although in many samples Tregs concentrated at the tumor periphery, digital image analysis failed to detect a significant difference between mean FOXP3 staining density in the peritumoral infiltrate (4.55) versus the tumor centers (3.67, P = 0.11). However, compared to the mean FOXP3 density in the dermis of 14 normal skin samples (1.36), Treg staining was elevated in both the tumor periphery and center (P = 0.0002 and P = 0.023, respectively) (Figure 2D, E).
In vivo administration of anti-CD200 antibody targets MCC tumor cells
As inhibiting CD200 could potentially reverse immune evasion, blocking antibodies have been developed as anti-cancer drugs.21 To test if systemic administration of anti-CD200 antibody can target CD200 expressed on MCC in vivo, we generated subcutaneous mouse xenograft tumors and performed tail vein infusions with a human IgG4 antibody specific for human CD200. In contrast to mouse epidermis and necrotic MCC tumor cells harvested 6 hours after drug administration, all viable MCC tumor cells showed strong membranous deposition of anti-CD200 antibody (Figure 3). Consistent with its intravascular delivery, drug was also detected within vessels in the mouse dermis and subcutaneous tissue. This demonstrates that CD200 within solid tumors can be targeted by antagonist antibody administration. As no immune-competent mouse models of MCC exist, the functional impact of anti-CD200 therapy could not be tested.
Figure 3.
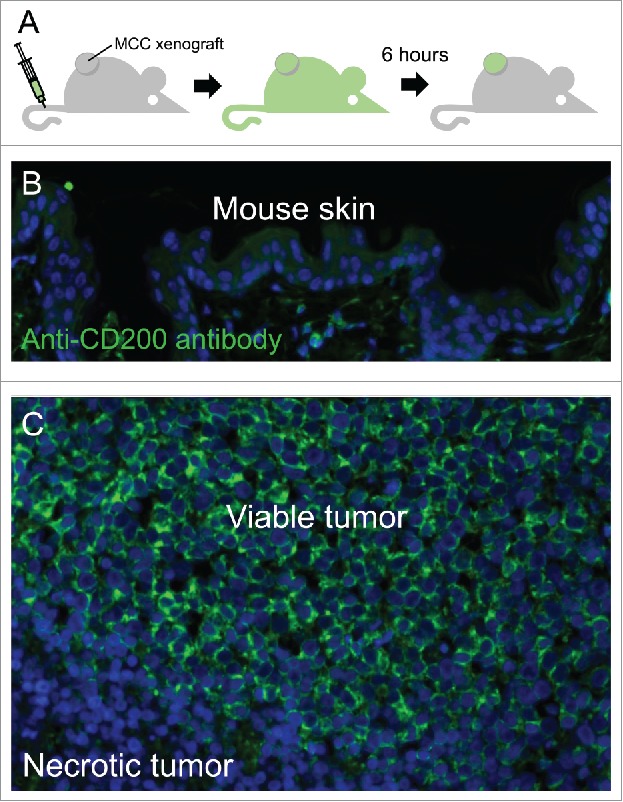
Anti-CD200 antibody effectively targets MCC tumor cells in vivo. (A) schematic of experiment illustrating the intravenous infusion of human IgG4 anti-CD200 antibody followed by 6-hour washout period. The MCC xenograft tumor was then collected and tissues were stained with an anti-human-IgG secondary antibody. (B) Anti-CD200 antibody was not detected in overlying mouse epidermis, (C) but heavily deposited on viable MCC tumor cells.
Discussion
We found that the immunoregulatory ligand CD200 is frequently and highly expressed on MCC tumor cells and on tumor vasculature. There was no correlation between CD200 expression levels and disease outcomes, however there were too few CD200 negative cases to make definitive conclusions about MCC prognosis in the absence of CD200 expression. Consistent with CD200's role in promoting immunosuppressive M2 macrophages, we observed an elevated CD163:CD68 staining ratio in MCC tumor infiltrates and elevated staining for the Treg marker FOXP3 around and within tumors. Whereas these findings are very suggestive, further work will be necessary to evaluate if CD200 signaling is required for the induction and maintenance of M2 TAM and Tregs in MCC. As disrupting CD200 signaling has the potential to reverse MCC immune evasion, we demonstrated that intravenous administration of human anti-CD200 antibody effectively targeted CD200 on MCC tumor cells in vivo. We were unable to test if pharmacological inhibition of CD200 had a biological effect on reversing tumor immune suppression as the xenograft MCC model requires the use of immunodeficient mice.
Detection of CD200R in the tumor microenvironment was technically challenging. Nonetheless, we were able to identify CD200R expressing cells that co-expressed CD11b. We suspect these were the cells with the highest levels of CD200R expression, and likely include myeloid-derived suppressor cells (MDSC) based on their size and morphology. We propose a model of CD200 produced by MCC tumors signaling to MDSC and M2 TAM to promote an immunosuppressive tumor environment, including the induction of Tregs (Figure S1). Consistent with this model, we observed CD200R myeloid cells, M2 TAM, and Tregs in association with MCC tumors. Our finding of M2 TAM is consistent with a prior report of CD163+ VEGF-C+ macrophages in the peritumoral infiltrate of MCC tumors that were associated with lymphangiogenesis.26 Thus, induction of M2 TAM theoretically promotes MCC progression by a combination of immunosuppression and induction of lymphatics.
Demonstrating effective in vivo delivery of anti-CD200 antibody to cells in MCC tumors is an important proof of concept in developing a drug to target CD200 in solid tumors. Importantly, CD200 is an immunotherapy target that is independent of T-cell directed immune checkpoints. Because the macrophage immune synapse is functionally and spatially distinct from the lymphocyte immune synapse, targeting CD200 signaling to immunosuppressive myeloid cells has the potential to synergize with checkpoint inhibitors targeting PD-1 or CTLA4 signaling to T lymphocytes. Clinical trials will be needed to assess the efficacy and safety of CD200 blockade as a single agent and in combination with PD-1/PD-L1 inhibition to treat MCC.
Material & methods
Patients
Paraffin-embedded MCC and SCLC samples were obtained from the Departments of Dermatology and the tissue bank of the National Center for Tumor Diseases (NCT, Heidelberg, Germany) in accordance with the regulations of the tissue bank and the approval of the ethics committee of the Heidelberg University. Clinical data sets for the MCC samples included patient age and sex, immunohistological features, tumor stage, disease course, disease specific survival (DSS), and progression-free survival (PFS). Tumor stages were classified according to AJCC 7th edition Cancer Staging Manual. Fresh-frozen MCC tumor samples were collected as bisected biopsy specimens from patients treated at the National Institutes of Health (NIH) Clinical Center (Bethesda, MD, USA) between 2015 and 2016. Diagnoses were verified histopathologically and immunohistochemically on paraffin-embedded samples from all tumors. All procedures were performed according to the principle of the Declaration of Helsinki and approved by the local medical ethics committee (S570/2013, Heidelberg) and (15-C-0012, National Cancer Institute, NIH).
Gene expression data
Global mRNA expression was examined in 23 MCC and 9 SCLC tumor samples using the GeneChip U133 A 2.0 Array (Affymetrix) and analyzed using R as previously reported.23 All mRNA expression data is available on NCBI Gene Expression Omnibus (GEO) https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE50451.
Tumor specimens and tissue microarray construction
Prior to tissue microarray (TMA) construction, an H&E-stained slide of each block was analyzed in order to select tumor-containing regions. A TMA machine (AlphaMetrix Biotech, Rödermark, Germany) was used to extract a tandem 1.0-mm cylindrical core sample from each tissue donor block. To best mimic the biopsy process, large areas were selected on the H&E-stained slide by a pathologist, and a technician randomly punched core samples from these regions.
Immunohistochemistry (IHC) staining
In brief, after heat induced antigen retrieval and incubation with primary antibodies (anti-CD200: R&D Systems, AF2724, polyclonal, 1:250; anti-EpCAM: Dako, berEP4, 1:100; anti-CD56: Leica, 1B6, 1:100; anti-CK20: Dako, Ks20.8, 1:200; anti-NSE: Dako, BBS/NC/VI-H14, 1:200; anti-ChrA: Linaris, LK2H10, prediluted; anti-MCV large T-antigen, Santa Cruz, CM2B4, 1:100; anti-Synaptophysin: Menarini, 27G12, 35026, 1:50; anti-Ki67: Dako, MIB1, 1:800; anti-CD68: Dako, PG-M1, 1:300; anti-CD163: Novocastra, 10D6, 1:50) binding reaction was visualized by using EnVision+ Kit (Dako Cytomation, Glostrup, Denmark). Human placenta and thymus served as positive controls. All immunostained slides were evaluated by two pathologists (TG, CW) and were scored as following: -, no specific staining; +, weak staining; ++, moderate staining; +++, strong staining.
Immunofluorescent Staining
Standard immunostaining procedures were performed on formalin-fixed, paraffin-embedded MCC tissue sections on glass slides. Sections were deparaffinized with xylene and rehydrated though an ethanol gradient in PBS. Antigen retrieval was performed with 10 mM sodium citrate buffer, pH 6.0, by boiling in a microwave for 1 minute followed by steam heat for 35 minutes. After cooling, slides were incubated in buffer with 5% normal donkey serum for 30 minutes at room temperature prior to overnight incubation at 4°C with goat anti-CD200 (R&D Systems, AF2747, 1:250) and rabbit anti-CD31 (Abcam, ab28364, 1:200). Alexa Fluor conjugated secondary antibodies (Invitrogen, 1:2000) were used to detect the signals with DAPI mount counterstaining.
Tissue sections of fresh-frozen MCC tumor on glass slides were fixed in 4% paraformaldehyde for 15 minutes, followed by block in 1% BSA in 0.1% PBT (0.1% Triton X-100 in PBS) and incubation with fluorophore-conjugated antibodies overnight at 4 °C. The antibodies were: FITC-conjugated mouse anti-CD200R (Bio-Rad, MCA2282 F, 1:50) and Alexa Fluor (AF) 594-conjugated rat anti-CD11b (BioLegend, 101254, 1:50). After washing, coverslips were affixed with DAPI mount for counterstaining.
Quantification of IHC staining and CD163:CD68 ratios
Adjacent tumor sections stained for CD68 and CD163 were fully digitalized using an Aperio ScanScope with a 20x objective (Aperio/Leica biosystems). On each image, three representative regions (1712 × 952 pixels at 5x magnification) were selected at the tumor periphery and within the tumor center for semi-automatic processing to quantify brown (DAB staining) and blue (hematoxylin) pixels using Fiji (http://fiji.sc/). A custom-made macro performed the following: colour deconvolution to detect blue (negatively stained) and brown (positively stained) particles; morphological opening to disconnect overlapping particles; watershed to segment single particles; counting of segmented particles. Segmentation quality was assessed visually by a pathologist. The ratio of brown to blue was then averaged over the three regions.
Mouse experiments
A total of 107 cells of the WaGa MCC cell line was injected per adult athymic nude mouse to generate subcutaneous xenograft tumors as previously described.23 Once tumors exceeded 1.5 cm in the maximum diameter, the mice underwent tail vein injection with 100 ul of human IgG4 anti-CD200 antibody (Trillium Therapeutics Inc., CD200.7-G4P). After 6 hours, the animals were euthanized and tumors were excised and fixed in 10% neutral buffered formalin. The tissue was paraffin embedded and sectioned. Tissue sections were then incubated in goat anti-human IgG (H+L) antibody conjugated with Alexa Fluor 488 (Thermofisher, A-11013), washed, and counterstained with DAPI.
Statistics
Associations between ordered categorical CD200 staining intensity (0/1 vs 2 vs 3) and disease stage (I, II, or III) were determined using a Chi-square test. Disease specific survival (DSS) and progression free survival (PFS) were calculated from the date of diagnosis until the date of death or last follow-up, the date first noted to have progressed, or the date through which patients were known to have not progressed, as appropriate. The probability of DSS or PFS as a function of time was determined by the Kaplan-Meier method. The statistical significance of the difference among a set of Kaplan-Meier curves was determined by the Mantel-Cox log-rank test. Comparisons of mean IHC staining intensities or ratios were performed using a paired t-test for populations with dependent samples, and Welch's t-test for populations with independent samples. P-values of <0.05 were considered statistically significant.
Supplementary Material
Funding Statement
This research was supported by the Olympia-Morata-Program of the University of Heidelberg (grant number F.206814 to MRG), and the NIH Intramural Research Program, Center of Cancer Research, National Cancer Institute (ZIA BC 011394 to IB).
Disclosure of interest
The authors report no conflict of interest. This research was supported by the Olympia-Morata-Program of the University of Heidelberg (grant number F.206814 to MRG), and the NIH Intramural Research Program, Center of Cancer Research, National Cancer Institute (ZIA BC 011394 to IB). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Acknowledgments
We thank Trillium Therapeutics Inc. for providing the human anti-CD200 antibody. We thank Veronica Geissler and Katrin Wolk for their excellent technical help.
References
- 1.Paulson KG, Iyer JG, Blom A, Warton EM, Sokil M, Yelistratova L, Schuman L, Nagase K, Bhatia S, Asgari MM, et al.. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J Invest Dermatol. 2013;133:642–6. doi: 10.1038/jid.2012.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barclay AN, Clark MJ, McCaughan GW. Neuronal/lymphoid membrane glycoprotein MRC OX-2 is a member of the immunoglobulin superfamily with a light-chain-like structure. Biochem Soc Symp. 1986;51:149–57. [PubMed] [Google Scholar]
- 3.Wright GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 2001;102:173–9. doi: 10.1046/j.1365-2567.2001.01163.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garza LA, Yang CC, Zhao T, Blatt HB, Lee M, He H, Stanton DC, Carrasco L, Spiegel JH, Tobias JW, et al.. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 2011;121:613–22. doi: 10.1172/JCI44478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenblum MD, Yancey KB, Olasz EB, Truitt RL. CD200, a “no danger” signal for hair follicles. J Dermatol Sci. 2006;41:165–74. doi: 10.1016/j.jdermsci.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 6.Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, Brady JN, Udey MC, Vogel JC. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116:249–60. doi: 10.1172/JCI26043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Love JE, Thompson K, Kilgore MR, Westerhoff M, Murphy CE, Papanicolau-Sengos A, McCormick KA, Shankaran V, Vandeven N, Miller F, et al.. CD200 Expression in Neuroendocrine Neoplasms. Am J Clin Pathol. 2017;148:236–42. doi: 10.1093/ajcp/aqx071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farre D, Martinez-Vicente P, Engel P, Angulo A. Immunoglobulin superfamily members encoded by viruses and their multiple roles in immune evasion. Eur J Immunol. 2017;47:780–96. doi: 10.1002/eji.201746984 [DOI] [PubMed] [Google Scholar]
- 9.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, et al.. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science. 2000;290:1768–71. doi: 10.1126/science.290.5497.1768 [DOI] [PubMed] [Google Scholar]
- 10.Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD. Regulation of myeloid cell function through the CD200 receptor. J Immunol. 2006;176:191–9. doi: 10.4049/jimmunol.176.1.191 [DOI] [PubMed] [Google Scholar]
- 11.Gorczynski RM, Lee L, Boudakov I. Augmented Induction of CD4+CD25+ Treg using monoclonal antibodies to CD200R. Transplantation. 2005;79:1180–3. doi: 10.1097/01.TP.0000152118.51622.F9 [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Cherwinski H, Sedgwick JD, Phillips JH. Molecular mechanisms of CD200 inhibition of mast cell activation. J Immunol. 2004;173:6786–93. doi: 10.4049/jimmunol.173.11.6786 [DOI] [PubMed] [Google Scholar]
- 13.Kretz-Rommel A, Bowdish KS. Rationale for anti-CD200 immunotherapy in B-CLL and other hematologic malignancies: new concepts in blocking immune suppression. Expert Opin Biol Ther. 2008;8:5–15. doi: 10.1517/14712598.8.1.5 [DOI] [PubMed] [Google Scholar]
- 14.Rygiel TP, Meyaard L. CD200R signaling in tumor tolerance and inflammation: A tricky balance. Curr Opin Immunol. 2012;24:233–8. doi: 10.1016/j.coi.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 15.Kretz-Rommel A, Qin F, Dakappagari N, Ravey EP, McWhirter J, Oltean D, Frederickson S, Maruyama T, Wild MA, Nolan MJ, et al.. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J Immunol. 2007;178:5595–605. doi: 10.4049/jimmunol.178.9.5595 [DOI] [PubMed] [Google Scholar]
- 16.Moreaux J, Hose D, Reme T, Jourdan E, Hundemer M, Legouffe E, Moine P, Bourin P, Moos M, Corre J, et al.. CD200 is a new prognostic factor in multiple myeloma. Blood. 2006;108:4194–7. doi: 10.1182/blood-2006-06-029355 [DOI] [PubMed] [Google Scholar]
- 17.Tonks A, Hills R, White P, Rosie B, Mills KI, Burnett AK, Darley RL. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia. 2007;21:566–8. doi: 10.1038/sj.leu.2404559 [DOI] [PubMed] [Google Scholar]
- 18.Petermann KB, Rozenberg GI, Zedek D, Groben P, McKinnon K, Buehler C, Kim WY, Shields JM, Penland S, Bear JE, et al.. CD200 is induced by ERK and is a potential therapeutic target in melanoma. J Clin Invest. 2007;117:3922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siva A, Xin H, Qin F, Oltean D, Bowdish KS, Kretz-Rommel A. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol Immunother. 2008;57:987–96. doi: 10.1007/s00262-007-0429-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belkin DA, Mitsui H, Wang CQ, Gonzalez J, Zhang S, Shah KR, Coats I, Suarez-Farinas M, Krueger JG, Felsen D, et al.. CD200 upregulation in vascular endothelium surrounding cutaneous squamous cell carcinoma. JAMA dermatology. 2013;149:178–86. doi: 10.1001/jamadermatol.2013.1609 [DOI] [PubMed] [Google Scholar]
- 21.Kretz-Rommel A, Qin F, Dakappagari N, Cofiell R, Faas SJ, Bowdish KS. Blockade of CD200 in the presence or absence of antibody effector function: implications for anti-CD200 therapy. J Immunol. 2008;180:699–705. doi: 10.4049/jimmunol.180.2.699 [DOI] [PubMed] [Google Scholar]
- 22.Mahadevan D, Lanasa M, Whelden M, Faas S, Ulery T, Kukreja A. First-In-Human Phase I Dose Escalation Study of a Humanized Anti-CD200 Antibody (Samalizumab) in Patients with Advanced Stage B Cell Chronic Lymphocytic Leukemia (B-CLL) or Multiple Myeloma (MM). ASH Annual Meeting. 2010;Abstract 2465. [Google Scholar]
- 23.Daily K, Coxon A, Williams JS, Lee CC, Coit DG, Busam KJ, Brownell I. Assessment of cancer cell line representativeness using microarrays for Merkel cell carcinoma. J Invest Dermatol. 2015;135:1138–46. doi: 10.1038/jid.2014.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colmont CS, Benketah A, Reed SH, Hawk NV, Telford WG, Ohyama M, Udey MC, Yee CL, Vogel JC, Patel GK. CD200-expressing human basal cell carcinoma cells initiate tumor growth. PNAS. 2013;110:1434–9. doi: 10.1073/pnas.1211655110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber M, Moebius P, Buttner-Herold M, Amann K, Preidl R, Neukam FW, Wehrhan F. Macrophage polarisation changes within the time between diagnostic biopsy and tumour resection in oral squamous cell carcinomas–an immunohistochemical study. Br J Cancer. 2015;113:510–9. doi: 10.1038/bjc.2015.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werchau S, Toberer F, Enk A, Dammann R, Helmbold P. Merkel cell carcinoma induces lymphatic microvessel formation. J Am Acad Dermatol. 2012;67:215–25. doi: 10.1016/j.jaad.2011.09.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


