ABSTRACT
Introduction: Checkpoint inhibitors, including the CTLA-4 blocking antibody ipilimumab, have become the new standard therapy for many metastatic cancers. Development of anti-drug antibodies (ADAs) after treatment with other biopharmaceuticals has been thoroughly described, but the induction of ADAs after treatment with checkpoint inhibitors has been inadequately investigated. In this retrospective study, we relate ipilimumab serum levels and anti-ipilimumab antibody levels to clinical outcomes in patients with metastatic melanoma (MM).
Method: Serum samples from 31 patients with MM were analyzed for serum levels of ipilimumab and ADAs to ipilimumab at baseline, and before the 2nd and 4th infusion using an in-house bead-based assay. The results were correlated with progression-free survival (PFS) and overall survival (OS).
Results: Low serum levels of ipilimumab before the 2nd infusion correlated significantly with a shorter OS (p = 0.01) and PFS (p = 0.02). Eight patients (26%) were ADA-positive at either timepoint. ADA positivity correlated significantly with a shorter OS (p = 0.03) with a hazard ratio (HR) of 3.0 (95% CI: 1.2-7.8). Four of 8 ADA-positive patients (50%) discontinued therapy before the 4th infusion due to disease progression, compared to three of 23 (13%) ADA-negative patients.
Conclusion: We confirm that low serum levels of ipilimumab are associated with a shortened OS, and we show for the first time that ADAs to ipilimumab are associated with shorter OS in patients with MM.
KEYWORDS: Ipilimumab, checkpoint inhibitors, immunogenicity, anti-drug antibodies, cancer, malignant melanoma
Introduction
Ipilimumab (Yervoy®) is a human IgG1/kappa monoclonal antibody that blocks the inhibitory receptor cytotoxic T-lymphocyte antigen 4 (CTLA-4) on T cells.1 It was the first checkpoint-inhibitor approved for the treatment of unresectable or metastatic melanoma (MM),2 and is currently being tested in several other cancer types. Ipilimumab acts primarily by blocking the inhibitory CTLA-4 signaling pathway in effector T cells and regulatory T cells.3 The introduction of ipilimumab in MM patients has improved median survival to more than 10 months with a 2-year survival rate of around 25%.4,5
The clinical response to ipilimumab varies from no benefit to sustained response, and even long-lasting, complete remission (CR).6,7 Serious autoimmune grade 3 or 4 adverse events occur in about 15–25% of patients.4 Several immunologic and disease-related biomarkers, including tumor mutational load, absolute lymphocyte peripheral counts, and the number of tumor-infiltrating CD8+ lymphocytes have been suggested to correlate with clinical response.8–11 In dose-escalation trials, high serum levels of ipilimumab correlate directly with prolonged survival, but also with increased immune-related toxicity.12–14
Anti-drug antibodies (ADAs) may appear after administration of therapeutic monoclonal antibodies, and may neutralize drug target binding or enhance drug clearance, resulting in suboptimal levels of active drug and loss of treatment efficacy.15–20 In addition, ADA development may cause hypersensitivity reactions such as anaphylaxis, infusion reactions, and immune-complex mediated diseases.21,22
Little is currently known about the clinical consequences of ADA development to ipilimumab or other biologic anti-neoplastic drugs. According to the Food and Drug Administration (FDA) in the USA, ADAs are detectable in ∼5% of patients treated with ipilimumab, but a similar proportion of positive samples can be found in individuals receiving placebo.23 The FDA does not report on neutralizing ADAs, or any clinical consequences of ADA positivity. The public assessment report (EPAR) from the European Medicines Agency (EMA) reports that less than 2% of patients develop ADAs to ipilimumab, without occurrence of neutralizing antibodies.24 A recent study showed that 13% of patients with malignant melanoma and lung cancer treated with the anti-programmed-death-1 (anti-PD-1) checkpoint inhibitor nivolumab developed ADAs.25 The ADAs were primarily non-neutralizing and caused only a minor increase in drug clearance. In a subgroup analysis of survival in lung cancer patients, no statistically significant correlation was found, and the ADAs were deemed clinically irrelevant.
In the present study, we investigated the association between circulating levels of ipilimumab and ADAs to ipilimumab and progression-free survival (PFS) and overall survival (OS) in patients with MM.
Materials and methods
Patient population
This was a retrospective study conducted on samples collected from patients in an observational study in stage IV MM treated with ipilimumab monotherapy at Herlev University Hospital between October 2012 and May 2014, published elsewhere.8 The patients received induction therapy of up to 4 treatment series (3 mg/kg) about 3 weeks apart. Blood samples from 31 patients were available for inclusion. PFS and OS data were retrieved from the Danish Melanoma Web Database administered by the Clinical Research Unit (KFE) at Odense University Hospital.
Blood sampling
Blood samples were drawn in dry Vacutainer® tubes (BD Biosciences, Lyngby, Denmark) before the 1st (baseline) and 2nd and 4th ipilimumab infusions. Blood samples were left to coagulate, and serum was then isolated by centrifugation at 1000 g for 10 min and frozen at −80°C until analysis.
Baseline blood samples were drawn a median of 5 days (range: day 0–35) before the 1st infusion. Subsequent blood samples were drawn before the 2nd” infusion at day 20 (range: day 16–24), and before the 4th” infusion at day 62 (range: day 59–68) relative to the 1st infusion. To minimize the influence of physiological drug clearance, blood samples drawn less than 18 days or more than 24 days after the previous infusion were excluded from the analysis of serum drug levels, but not from the ADA analysis.
Measurement of ADAs and serum levels of ipilimumab
Serum levels of ADAs and ipilimumab were measured using in-house bead-based assays.
For measurement of serum levels of ipilimumab, recombinant human CTLA-4 (Sino Biological, Beijing, China) was covalently coupled to xMAP®-beads (Luminex Corporation, Eindhoven, Netherlands) using the xMAP® Antibody Coupling Kit (Luminex Corporation) as described by the manufacturer. The most concentrated assay standard was prepared by diluting ipilimumab stock in neat serum from blood group AB-positive healthy donors (Sigma-Aldrich, Copenhagen, Denmark) to a concentration of 60 µg/ml. A standard curve was then prepared by three-fold serial dilutions with additional serum. Both standard curve and samples were diluted 1:1000 in phosphate buffered saline containing 0.05% Tween-20 (PBST) and incubated with CTLA-4 coupled beads for 2 hours at room temperature. After three PBST washes, samples were incubated for 1.5 hours at room temperature with a biotinylated goat anti-human kappa light-chain detection antibody (Bio-Rad, Copenhagen, Denmark). Following three additional PBST washes, samples were incubated with a streptavidin-R-phycoerythrin-conjugate (SA-R-PE) (ProZyme, Hayward, CA), washed three times with PBST, and measured on a Luminex 100IS instrument (Luminex Corporation). Standard curves used for quantifying samples on the same plate were subsequently constructed using a five-parameter sigmoidal fit algorithm in Bio-Plex Manager v6.0 (Bio-Rad, Copenhagen, Denmark).
For ADA measurement, ipilimumab was covalently coupled to xMAP® beads using the xMAP® Antibody Coupling Kit (Luminex Corporation), as described by the manufacturer. Serum samples were diluted 1:40 in PBST and incubated with the ipilimumab-coupled beads for 2 hours at room temperature. After three PBST washes, samples were incubated for 1.5 hours at room temperature with a biotinylated goat anti-human lambda light-chain detection antibody (Bio-Rad). Following three further PBST washes, samples were incubated with SA-R-PE, washed three times with PBST, and subsequently quantitated on a Luminex 100IS instrument (Luminex Corporation). Samples with an increase in median fluorescence intensity (MFI) by 25% from the crude baseline value in combination with a MFI exceeding 130 (the 75th percentile for baseline samples) were defined as ADA-positive. A positive ADA status was considered as an irreversible risk factor.
Statistics
The Wilcoxon matched-pairs signed-rank test was used with pairwise comparisons to determine the significance of the difference between different timepoints. The Mann-Whitney U test was used to test the difference in serum drug levels between ADA-positive and ADA-negative patients. Cox proportional hazards regression was used to determine hazard ratios with 95% confidence intervals (CIs) and the significance of differences in OS and PFS in relation to ADA status or circulating drug levels. Positive ADA status was treated as a time-dependent covariate to take changes in ADA status over time into account. Cox proportional hazards regression was also used to test the log-transformed MFI values from the ADA measurements for a statistically significant influence on OS and PFS. P-values <0.05 were considered statistically significant. Statistical analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria) and GraphPad Prism v7 (GraphPad Software, La Jolla, CA).
Results
Patient characteristics and survival
Characteristics of the 31 patients included are shown in Table 1. All patients received at least one infusion of ipilimumab; 24 patients (77%) received all four ipilimumab infusions. Clinical data on hypersensitivity, e.g. drug rash or anaphylaxis, were not available.
Table 1.
Demographics of ipilimumab-treated stage IV malignant melanoma patients.
| Demographics | All | ADA-positive | ADA-negative |
|---|---|---|---|
| No. of patients (n) | 31 | 8 | 23 |
| Age | |||
| Median (range) | 67 (40–77) | 67 (49–72) | 67 (40–77) |
| Sex | |||
| Female | 16 | 3 | 13 |
| Male | 15 | 5 | 10 |
| Disease stage at baseline | |||
| M1a | 6 | 2 | 4 |
| M1b | 4 | 0 | 4 |
| M1c | 21 | 6 | 15 |
| Cerebral metastasis | 5 | 2 | 3 |
| Previous treatment | |||
| IL-2 | 13 | 4 | 9 |
| Temozolomide | 8 | 2 | 6 |
| None | 10 | 2 | 8 |
| No. of ipilimumab infusions | |||
| 1 | 1 | 0 | 1 |
| 2 | 2 | 2 | 0 |
| 3 | 4 | 2 | 2 |
| 4 | 24 | 4 | 20 |
ADA: Anti-drug antibodies measured before 2nd and 4th infusion of ipilimumab. Patients with at least one positive sample were regarded as ADA-positive.
The patients had a median OS of 605 days (range: 62–1365 days) and a median PFS of 133 days (range: 32–1224 days) (Fig. 1).
Figure 1.
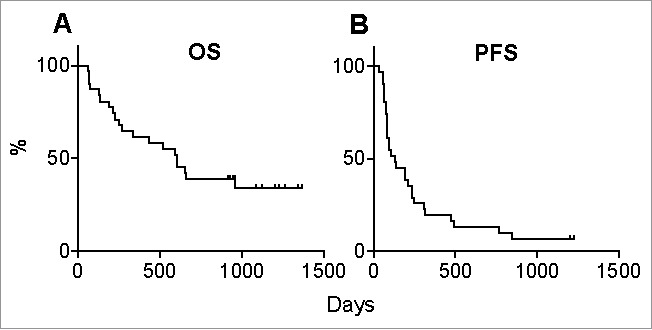
Overall survival and progression-free survival. Thirty-one patients with metastatic melanoma were treated with 1–4 infusions of ipilimumab (3 mg/kg) and followed up for up to 1365 days. Shown are (A) overall survival (OS) and (B) progression-free survival (PFS).
Circulating serum levels of ipilimumab
Blood samples drawn before the 2nd infusion from 24 patients were eligible for analysis of circulating ipilimumab, as were samples drawn before the 4th infusion from 20 patients (Table 2). As expected, ipilimumab levels increased progressively during the treatment period (Fig. 2).
Table 2.
Correlation of serum ipilimumab levels with overall and progression-free survival.
| OS |
PFS |
||||||
|---|---|---|---|---|---|---|---|
| n | s-ipilimumab in µg/ml median (range) | HR (95% CI) per µg/ml | p | HR (95% CI) per µg/ml | p | ||
| Before 2nd infusion | 24 | 11.0 (2.1-18.7) | 1.3 (1.1-1.5) | 0.01 | 1.2 (1.0-1.4) | 0.02 | |
| Before 4th infusion | 20 | 19.0 (4.6-28.3) | 1.0 (0.9-1.1) | 0.69 | 1.0 (1.0-1.1) | 0.30 | |
Serum drug levels of ipilimumab in patients with metastatic melanoma were measured before the 2nd and 4th infusion of ipilimumab. Hazard ratios per decreasing concentration (1 µg/ml) of ipilimumab are shown. Hazard ratios and p-values were calculated using Cox proportional hazards regression model. PFS: Progression-free survival; OS: Overall survival; HR: Hazard ratio.
Figure 2.

Serum levels of ipilimumab. Serum ipilimumab (s-ipilimumab) levels in 31 patients with metastatic melanoma were measured at baseline, and before the 2nd and 4th infusions of ipilimumab. Seven samples drawn before the 2nd infusion were excluded from analysis due to high background noise (n = 3) or a too long or short interval since the previous infusion (n = 4). Eleven samples drawn before the 4th infusion were unavailable for analysis because they were missing (n = 6), or excluded due to high background signal (n = 2) or a too long or short interval since the previous infusion (n = 3).
The level of ipilimumab in blood samples drawn a few days before the 2nd infusion (n = 24) correlated positively with OS (p = 0.01) and PFS (p = 0.02), with hazard ratios per decreasing µg/mL of serum ipilimumab of 1.3 (95% CI: 1.1-1.5) and 1.2 (95% CI: 1.0-1.4) respectively (Table 2). No correlation with OS or PFS was observed for ipilimumab levels in samples drawn before the 4th infusion (n = 20).
Seven patients (23%) developed early progressive disease, identified as discontinuation of ipilimumab before the 4th infusion due to disease progression. Blood samples from five of these patients drawn before the 2nd infusion were eligible for analysis, and three of these contained remarkably low ipilimumab levels (2.1 µg/ml, 3.3 µg/ml and 4.6 µg/ml). The two remaining patients had normal serum ipilimumab levels before the 2nd infusion but low levels before the 4th infusion (8.6 µg/ml and 12.8 µg/ml).
Development of ADAs to ipilimumab
Serum levels of ADAs to ipilimumab were also measured at baseline, and before the 2nd and 4th infusions (Fig. 3). Seven patients were ADA-positive before the 2nd infusion of ipilimumab, and one patient turned positive between the 2nd and 4th infusions. Thus, eight patients (26%) were ADA-positive at any timepoint during the study period (Table 1). One patient had a positive sample at the 2nd infusion but a negative sample at the 4th infusion.
Figure 3.
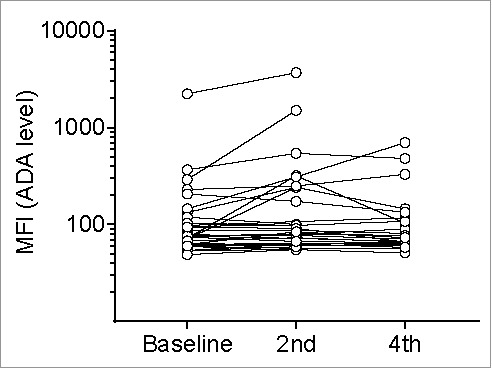
Anti-drug antibodies (ADAs) to ipilimumab. The levels of antibodies to ipilimumab in serum from 31 patients with metastatic melanoma were measured using a Luminex bead-based assay. The median fluorescence intensity (MFI) values, representing the ADA levels, are plotted on a logarithmic axis. Samples were drawn at baseline, and before the 2nd and 4th infusions of ipilimumab. Six samples drawn before 4th infusion were missing. ‘
ADA-positive patients had a significantly shortened OS (p = 0.03) with a median of 235 days (range: 67–927 days) compared to the 658 days (range: 62–1365 days) in ADA-negative patients (Fig. 4A). The corresponding medians for PFS were 80 days (range: 34–476 days) and 191 days (range: 55–1224 days), but this difference was not statistically significant (p = 0.08) (Fig. 4B). Thus, ADA positivity was associated with a hazard ratio of 3.0 (95% CI: 1.2-7.8) for the shorter OS (Table 3).
Figure 4.
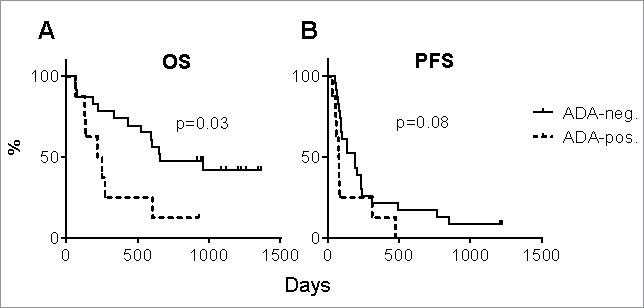
Overall and progression-free survival by ADA status. (A) The overall survival (OS) and (B) progression-free survival (PFS) of eight ADA-positive and twenty-three ADA-negative patients with metastatic melanoma are shown. P-values were calculated using Cox proportional hazards regression analysis.
Table 3.
Clinical outcomes stratified by occurrence of anti-drug antibodies.
| OS |
PFS |
|||||
|---|---|---|---|---|---|---|
| Median (range) | HR (95% CI) | p | Median (range) | HR (95% CI) | p | |
| ADA-positive | 235 (67-927) | 3.0 (1.2-7.8) | 0.03 | 80 (34-476) | 2.1 (0.9-5.0) | 0.08 |
| ADA-negative | 658 (62-1365) | 191 (55-1224) | ||||
Hazard ratios and p-values were calculated using Cox proportional hazards regression model. PFS: Progression-free survival (days); OS: Overall survival (days); HR: Hazard ratio; CI: Confidence interval.
To allow for prejudice in our definition of ADA positivity, we also correlated the log-transformed ADA-derived MFI values directly to the survival data and found a similar statistically significant correlation with OS (p = 0.03), but not with PFS (data not shown).
Of the eight patients who were ADA-positive, four (50%) discontinued therapy before the 4th infusion of ipilimumab due to progressive disease compared to only 3 out of 23 patients (13%) in the ADA-negative group (Table 1).
Influence of ADA development on serum levels of ipilimumab
We observed no association between ADA positivity and low serum levels of ipilimumab neither before the 2nd or the 4th infusion (Fig. 5), nor did ADA positivity before the 2nd infusion correlate with the serum level of ipilimumab before the 4th infusion (data not shown).
Figure 5.
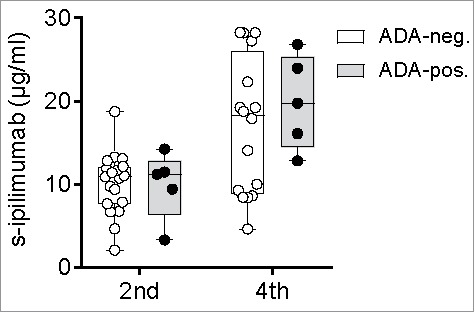
Serum levels of ipilimumab by ADA status. Serum levels of ipilimumab (s-ipilimumab) were measured before the 2nd and 4th infusion of ipilimumab in 24 and 20 patients with metastatic melanoma. The s-ipilimumab values of ADA-positive and ADA-negative patients were compared using the Mann-Whitney U test.
It is noteworthy that two ADA-positive patients had considerably lower serum levels of ipilimumab than the group median. One of these patients had a level of 3.3 µg/mL before the 2nd infusion, as compared to the group median of 10 µg/mL. This patient stopped treatment before the 4th infusion due to progressive disease and died shortly after. The other patient had levels of 11.5 before the 2nd and 12.8 µg/mL before 4th infusion; whereby the latter was markedly lower than the group median of >19 µg/mL. This patient also discontinued therapy before the 4th infusion due to progressive disease and died one month later.
Discussion
The ipilimumab dose and corresponding serum level have previously been shown to affect treatment outcome in MM,12–14 but an influence of ADAs has not been reported. Circulating ADAs may neutralize drugs and enhance drug clearance,26 and a clinical influence of ADAs has been demonstrated after treatment with a number of therapeutic monoclonal antibodies, including tumor necrosis factor-alpha blockers,18–20 natalizumab,27 cetuximab21 and rituximab.28
In keeping with the findings of others,12,13 we found that high serum levels of ipilimumab before the 2nd of four infusions correlate statistically significantly with longer OS and PFS. The absence of statistical significance before the 4th infusion may be a consequence of discontinued treatment and missing samples in some patients with low drug levels or greater heteroscedasticity of drug levels after three infusions than after one.
We detected ADAs in 26% of our ipilimumab treated patients. The FDA and EMA have previously reported frequencies of 5% and <2% respectively.23,24 The higher proportions in our study are presumably a result of the different testing methods and definitions of ADA positivity. Overall, a positive ADA status was associated with statistically significantly shortened OS and a markedly increased HR. These results were confirmed by analysis of the MFI values independent of our definition of ADA positivity. The association was statistically significant for OS but not for PFS. Considering the short PFS intervals, however, statistically significant results cannot be expected in such a small study population as examined here. The rate of therapy discontinuation before the 4th infusion due to progressive disease was also notably higher in ADA-positive patients (50%) than in ADA-negative patients (13%).
To our knowledge, no other studies have demonstrated an influence of ADAs on survival after treatment with ipilimumab. In a recent study on another checkpoint inhibitor, the anti-PD-1 antibody nivolumab, Agrawal et al. showed development of ADAs in 13% of patients with MM and lung cancer25 but, in contrast to our findings, they did not observe a correlation between ADA development and survival. However, the anti-PD-1 antibodies may show a different dose-response relationships from ipilimumab, as recently demonstrated for pembrolizumab.29
We were not able to demonstrate that ADA positivity was associated with lower serum levels of ipilimumab, although two ADA-positive patients with poor clinical responses had remarkably low serum ipilimumab levels. It is noteworthy that ADAs of clinical significance (as indicated by survival data) were measured as early as 16–24 days after the 1st infusion of ipilimumab. At this timepoint, ADAs presumably have low affinity and are unlikely to elicit considerable drug clearance or to neutralize the binding of ipilimumab to CTLA-4 in our assay for drug measurement. Therefore, relatively high serum levels of ipilimumab may be measured in the presence of early ADAs, and the very presence of early ADAs may anticipate affinity maturation and development of high-affinity antibodies that neutralize ipilimumab or promote its clearance from the circulation.
During the follow-up period of this study, treatment with the PD-1 blocking antibody pembrolizumab was introduced as a therapy option for patients with MM. Thus, some patients who developed disease progression during or after ipilimumab therapy were eligible to receive this treatment, which may have prolonged their survival, leading to a potential underestimation of the effects of ADA formation.
Our study has some limitations. Due to the low number of participants, the study can only be regarded as exploratory. The retrospective design limited the possibility of investigating relevant clinical outcomes, such as hypersensitivity. The definition of ADA positivity, based on both absolute levels and an increase from baseline, was not ideal. A validated cut-off level based, for example, on a human anti-ipilimumab positive control antibody would have been desirable. We used an anti-lambda light chain antibody as detecting antibody, which has proved as effective as other methods in the detection of ADAs and prediction of clinical outcomes.30,31 However, it does not detect ADAs with kappa light chains, nor does it distinguish between different antibody isotypes.
Despite its limitations, our study confirms that low drug levels correlate with a poorer clinical outcome of ipilimumab treatment in MM, and introduce the novel finding that early ADAs to ipilimumab have prognostic value. If this can be confirmed in larger prospective studies, early detection of ADAs and recognition of low serum levels of ipilimumab may help clinicians in making timely decisions regarding dosing or switching to other treatments.
Conclusion
This study suggests that the presence of ADAs to ipilimumab is associated with shortened OS. We also confirmed a correlation between low serum drug levels and shortened OS and PFS. These results warrant prospective studies on the clinical consequences of ADAs on checkpoint inhibitors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Morse M. Technology evaluation: Ipilimumab, Medarex/Bristol-Myers Squibb. Curr Opin Mol Ther. 2005;7(6):588–97. PMID:16370382. [PubMed] [Google Scholar]
- 2.Cameron F, Whiteside G, Perry C. Ipilimumab: First global approval. Drugs. 2011;71(8):2079–89. doi: 10.2165/11594010-000000000-00000. PMID:21985171. [DOI] [PubMed] [Google Scholar]
- 3.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways. Am J Clin Oncol. 2016;39(1):98–106. doi: 10.1097/COC.0000000000000239. PMID:26558876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, Day SJO, Mcdermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al.. Improved Survival with Ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. PMID:20525992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolchok JD, Weber JS, Maio M, Neyns B, Harmankaya K, Chin K, Cykowski L, de Pril V, Humphrey R, Lebbé C. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol. 2013;24(8):2174–80. doi: 10.1093/annonc/mdt161. PMID:23666915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Giacomo AM, Calabrò L, Danielli R, Fonsatti E, Bertocci E, Pesce I, Fazio C, Cutaia O, Giannarelli D, Miracco C, et al.. Long-term survival and immunological parameters in metastatic melanoma patients who responded to ipilimumab 10 mg/kg within an expanded access programme. Cancer Immunol Immunother. 2013;62(6):1021–8. doi: 10.1007/s00262-013-1418-6. PMID:23591982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDermott D, Haanen J, Chen TT, Lorigan P, O'Day S. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann Oncol. 2013;24(10):2694–8. doi: 10.1093/annonc/mdt291. PMID:23942774. [DOI] [PubMed] [Google Scholar]
- 8.Bjoern J, Juul Nitschke N, Zeeberg Iversen T, Schmidt H, Fode K, Svane IM. Immunological correlates of treatment and response in stage IV malignant melanoma patients treated with Ipilimumab. Oncoimmunology. 2015;5(4):e1100788. doi: 10.1080/2162402X.2015.1100788. PMID:27141381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Giacomo AM, Danielli R, Calabrò L, Bertocci E, Nannicini C, Giannarelli D, Balestrazzi A, Vigni F, Riversi V, Miracco C, et al.. Ipilimumab experience in heavily pretreated patients with melanoma in an expanded access program at the University Hospital of Siena (Italy). Cancer Immunol Immunother. 2011;60(4):467–77. doi: 10.1007/s00262-010-0958-2. PMID:21170646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al.. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med. 2014;371(23):2189–99. doi: 10.1056/NEJMoa1406498. PMID:25409260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daud AI, Loo K, Pauli ML, Sanchez-rodriguez R, Sandoval PM, Taravati K, Tsai K, Nosrati A, Nardo L, Alvarado MD, et al.. Tumor immune profiling predicts response to anti – PD-1 therapy in human melanoma. J Clin Invest. 2016;126(9):1–6. doi: 10.1172/JCI87324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Y, Roy A, Masson E, Chen TT, Humphrey R, Weber JS. Exposure-response relationships of the efficacy and safety of ipilimumab in patients with advanced melanoma. Clin Cancer Res. 2013;19(14):3977–86. doi: 10.1158/1078-0432.CCR-12-3243. PMID:23741070. [DOI] [PubMed] [Google Scholar]
- 13.Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, Waterfield W, Schadendorf D, Smylie M, Guthrie T Jr, et al.. Ipilimumab monotherapy in patients with pretreated advanced melanoma: A randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11(2):155–64. doi: 10.1016/S1470-2045(09)70334-1. PMID:20004617. [DOI] [PubMed] [Google Scholar]
- 14.Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Chiarion-Sileni V, Arance A, Lebbé C, Bastholt L, Hamid O, Rutkowski P, et al.. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611–22. doi: 10.1016/S1470-2045(17)30231-0. PMID:28359784. [DOI] [PubMed] [Google Scholar]
- 15.Hwang WYK, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36:3–10. doi: 10.1016/j.ymeth.2005.01.001. PMID:15848070. [DOI] [PubMed] [Google Scholar]
- 16.Vultaggio A, Nencini F, Pratesi S, Petroni G, Maggi E, Matucci A. Manifestations of antidrug antibodies response: Hypersensitivity and infusion reactions. J Interf Cytokine Res. 2014;34(12):946–52. doi: 10.1089/jir.2012.0139. [DOI] [PubMed] [Google Scholar]
- 17.Radstake T, Svenson M, Eijsbouts AM, van den Hoogen FHJ, Enevold C, van Riel PLCM, Bendtzen K. Formation of antibodies against infliximab and adalimumab strongly correlates with functional drug levels and clinical responses in rheumatoid arthritis. Ann Rheum Dis. 2009;68(11):1739–45. doi: 10.1136/ard.2008.092833. PMID:19019895. [DOI] [PubMed] [Google Scholar]
- 18.West RL, Zelinkova Z, Wolbink GJ, Kuipers EJ, Stokkers PCF, Van Der Woude CJ. Immunogenicity negatively influences the outcome of adalimumab treatment in Crohn's disease. Aliment Pharmacol Ther. 2008;28(9):1122–6. doi: 10.1111/j.1365-2036.2008.03828.x. PMID:18691349. [DOI] [PubMed] [Google Scholar]
- 19.Baert F, Noman M, Vermeire S, Van Assche G, D'Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348(7):601–8. doi: 10.1056/NEJMoa020888. PMID:12584368. [DOI] [PubMed] [Google Scholar]
- 20.Bendtzen K, Geborek P, Svenson M, Larsson L, Kapetanovic MC, Saxne T. Individualized monitoring of drug bioavailability and immunogenicity in rheumatoid arthritis patients treated with the tumor necrosis factor alpha inhibitor infliximab. Arthritis Rheum. 2006;54(12):3782–9. doi: 10.1002/art.22214. PMID:17133559. [DOI] [PubMed] [Google Scholar]
- 21.Chung CH, Mirakhur B, Chan E, Le Q-T Berlin J, Morse M, Murphy BA, Satinover SM, Hosen J, Mauro D, et al.. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–17. doi: 10.1056/NEJMoa074943. PMID:18337601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolbink GJ, Aarden LA, Dijkmans B. Dealing with immunogenicity of biologicals: Assessment and clinical relevance. Curr Opin Rheumatol. 2009;21(3):211–5. doi: 10.1097/BOR.0b013e328329ed8b. PMID:19399992. [DOI] [PubMed] [Google Scholar]
- 23.FDA Yervoy (ipilimumab) label [Internet]; 2013. Available from: http://www.accessdata.fda.gov/
- 24.European Medicines Agency EPAR Yervoy [Internet]; 2011. Available from: http://www.ema.europa.eu
- 25.Agrawal S, Statkevich P, Bajaj G, Feng Y, Saeger S, Desai DD, Park JS, Waxman IM, Roy A, Gupta M. Evaluation of immunogenicity of nivolumab monotherapy and its clinical relevance in patients with metastatic solid tumors. J Clin Pharmacol. 2016;0(0):1–7. [DOI] [PubMed] [Google Scholar]
- 26.Shankar G, Pendley C, Stein KE. A risk-based bioanalytical strategy for the assessment of antibody immune responses against biological drugs. Nat Biotechnol. 2007;25(5):555–61. doi: 10.1038/nbt1303. PMID:17483842. [DOI] [PubMed] [Google Scholar]
- 27.Vennegoor A, Rispens T, Strijbis EM, Seewann A, Uitdehaag BM, Balk LJ, Barkhof F, Polman CH, Wolbink G, Killestein J. Clinical relevance of serum natalizumab concentration and anti-natalizumab antibodies in multiple sclerosis. Mult Scler J. 2013 Apr 19;19(5):593–600. doi: 10.1177/1352458512460604. [DOI] [PubMed] [Google Scholar]
- 28.Dunn N, Juto A, Ryner M, Manouchehrinia A, Piccoli L, Fink K, Piehl F, Fogdell-Hahn A. Rituximab in multiple sclerosis: Frequency and clinical relevance of anti-drug antibodies. Mult Scler J. 2017;23(2):1–10. [DOI] [PubMed] [Google Scholar]
- 29.Schachter J, Ribas A, Long GV, Arance A, Grob J-J, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al.. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;6736(17):1–10. [DOI] [PubMed] [Google Scholar]
- 30.Kopylov U, Mazor Y, Yavzori M, Fudim E, Katz L, Coscas D, Picard O, Chowers Y, Eliakim R, Ben-Horin S. Clinical utility of antihuman lambda chain-based enzyme-linked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies. Inflamm Bowel Dis. 2012;18(9):1628–33. doi: 10.1002/ibd.21919. PMID:22038899. [DOI] [PubMed] [Google Scholar]
- 31.Steenholdt C, Bendtzen K, Brynskov J, Thomsen OE, Ainsworth MA. Clinical implications of measuring drug and anti-drug antibodies by different assays when optimizing infliximab treatment failure in Crohn's disease: Post hoc analysis of a randomized controlled trial. Am J Gastroenterol. 2014;109(7):1055–64. doi: 10.1038/ajg.2014.106. PMID:24796769. [DOI] [PubMed] [Google Scholar]


