Abstract
BACKGROUND:
Accurate assessment of HER-2 is imperative in selecting patients for targeted therapy. Most commonly used test methods for HER-2 are immunohistochemistry (IHC) and fluorescence in situ hybridisation (FISH). We evaluated the concordance between FISH and IHC for HER-2 in breast cancer samples using Food and Drug Administration approved tests.
MATERIAL AND METHODS:
Archived paraffin tissue blocks from 73 breast cancer patients were used. HER-2 immunostaining was performed using Ventana anti–HER-2 monoclonal antibody. The FISH assay was performed using PathVysion™ HER-2 DNA Probe Kit.
RESULTS:
Of the 73 cases 68.5% were IHC 0/1+, 15.07% were IHC 2+ and 16.44% were IHC 3+. Successful hybridisation was achieved in 72 cases. HER-2 FISH amplification was determined in 16.67% cases. Ten IHC 3+ and two IHC 2+ cases were FISH positive. Two of the IHC 3+ cases were FISH negative. Concordance rate was 100%, 18.18% and 83.33% for IHC 0/1+, 2+ and 3+ group, respectively. Total concordance was 84.72%, kappa 0.598 (p < 0.0001). The sensitivity of IHC in detecting IHC 2+ and IHC 3+ cases was 16.7% and 83.3%, and the specificity was 85% and 96.67%, respectively.
CONCLUSION:
The consistency between the methods was highest for IHC negative and lowest for IHC equivocal cases. The immunohistochemistry showed high sensitivity for IHC 2+/3+ cases and high specificity for IHC 3+ cases. Our results support the view that false-positive rather than false-negative IHC results are a problem with HER-2/IHC testing, and that IHC should be used as an initial screening test, but IHC 2+/ 3+ results should be confirmed by FISH.
Keywords: Breast cancer, HER –2, Fluorescence in situ hybridisation, Immunohistochemistry
Introduction
The human epidermal growth factor receptor gene HER-2 (also known as HER-2/neu, c – erbB-2) is located on chromosome 17q12 and encodes a member of the epidermal growth factor receptor (EGFR) family with tyrosine kinase activity that is responsible for cell-cell or cell-stroma communication through the process of signal transduction [1]. Activation of the protein receptor is associated with increased cell proliferation, tumour invasiveness, progressive regional and distant metastases, increased angiogenesis and reduced apoptosis [1]. HER-2 gene amplification is the primary mechanism of protein overexpression and is found in nearly 15 to 20% of breast cancer patients [1] [2]. HER-2 gene amplification or protein overexpression are molecular targets for specific targeted therapies associated with good results in early and metastatic HER-2 positive breast carcinomas [3] [4] [5]. Therefore, accurate assessment of HER-2, using reliable, highly sensitive and specific test is imperative in the selection of patients for the therapy [3] [4] [5].
To date, there is still no single, universally accepted test for HER-2 assessment. Two most commonly used techniques are immunohistochemistry (IHC) and in situ hybridisation (fluorescence in situ hybridization-FISH and bright field in situ hybridization-BRISH), performed on formalin fixed paraffin embedded (FFPE) tissue samples [6] [7] [8]. Immunohistochemistry uses antibodies to detect HER-2 protein expression on the surface of tumour cells, while FISH is a molecular method that uses fluorescently labelled DNA probes, to determine HER -2 gene copy number. Although both methods are widely used in the routine analysis, both have advantages and disadvantages. Immunohistochemistry is relatively cheap and fast method that uses the light microscope for analysis. Conversely, a FISH method is technically more demanding, time-consuming and expensive assay [9], but is more consistent and more objective [9]. Numerous studies that evaluated the consistency between the IHC and FISH, as well as their effect on the response to trastuzumab therapy, showed contradictory results [10].
In this study we evaluated the concordance between FISH and IHC for HER-2 assessment in breast cancer tissue samples, using Food and Drug Administration (FDA) approved tests.
Material and Methods
In this retrospective study, we used FFPE tissue blocks from 73 patients diagnosed with invasive breast carcinoma, non-special type (NST), during 2014-2015. Patients who underwent radical mastectomy and did not receive neoadjuvant therapy were included.
All the cases were stained and analysed in the standard procedure to determine the histologic type and grade of a tumour, lymph node status and the stage of the disease. Tumour grade was determined based on the recommendations of the Nottingham grading system [11], while the stage of the disease was determined according to the criteria of the American Joint Committee on Cancer (AJCC) [12]. The patients’ age and tumour dimension were obtained from medical records. HER-2/IHC was performed in parallel with ER, PR, and Ki-67 as part of the daily routine work at our Institute. Regardless of the IHC results, additional FISH testing was done in all cases, using parallel sections from the same tissue block as for IHC.
Using 4 micron thick sections mounted on silanized microscopic slides, HER-2 immunostaining was performed on BenchMark GX automated staining instrument (Ventana Medical Systems, Inc., USA) using Ventana anti–HER-2 rabbit monoclonal primary antibody, clone 4B5 and UltraVIEW universal DAB Detection Kit (Ventana), according to the manufacturer’s recommendations. Briefly, after deparaffinization with EZ Prep, slides were pretreated with Cell Conditioning 1 for 36 minutes at 100°C and then incubated with anti–HER-2 primary antibody for 20 minutes at 37°C. The antibody was detected using DAB and then counterstained with hematoxylin and bluing reagent, for 4 minutes in both steps.
The ER, PR and Ki67 immunostainings were performed using DAKO monoclonal antibodies (clone EP1, dilution 1:50; clone PgR 636, dilution 1:100 and clone Mib1, dilution 1:150, respectively), by semiautomated PT Link immunoperoxidase technique. Shortly, after deparaffinization and rehydration, samples were pretreated using Target Retrieval Solution for 20 minutes at 97°C and then incubated with primary antibody for 20 minutes at 25°C. Antibodies were detected using visualisation system (EnVision FLEX, DAKO) for 20 minutes at 25°C and chromogen (di–amino-benzene-DAB) for 5 minutes, also at 25°C. After that slides were counterstained with hematoxylin.
Semiquantitative evaluation of HER-2 protein expression included evaluation of membrane positivity according to the criteria of American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) [7]. The expression of ER, PR and Ki67 was evaluated as a percentage of positive cells of the total number of cells. One percent was the cut-off point for hormone receptors [13], while 20% was taken as a cut-off point that distinguishes cases with low and high proliferative index (Ki67-low/Ki67-high) [14]. The slides were analyzed with a light microscope, Nikon 80i Eclipse (Nikon Instruments, Austria).
The FISH assay was performed by using PathVysion™ HER-2 DNA Probe Kit (Abbott/Vysis, IL, USA) containing two fluorophore-labeled DNA probes allowing simultaneous detection of HER-2 and chromosome 17 (CEP 17) gene copy numbers: Spectrum Orange-labeled DNA probe for HER-2 gene locus and Spectrum Green labelled DNA probe for CEP 17. Samples were tested using two different paraffin pretreatment kits in two different FISH protocols, Vysis/Abbott Paraffin Pretreatment Reagent Kit (40 samples) and DAKO Histology FISH Accessory Kit (33 samples), described in details in a previous paper [15]. Briefly, 4 µm thick tumour sections were mounted on a positively charged microscopic slide, air dried and baked in the oven at 56°C, overnight. After deparaffinization and pretreatment, slides were incubated with protease/pepsin. Then slides were washed, dehydrated and DNA probe was applied. After denaturation (5 minutes at 72°C) and hybridisation (16 hours at 37°C), the slides were washed in preheated post-hybridisation buffer, counterstained with DAPI, and cover slips were applied.
For accurate localisation of the invasive tumour component, the FISH assays were viewed in conjunction with H&E sections, and DAPI counterstain was used to identify tumour nuclei. Signals were analyzed at x1000 magnification, using an appropriate filter. The results were interpreted according to recommendations of ASCO/CAP, where HER-2 status is defined as positive when the HER-2/CEP17 ratio is greater than 2, and negative when the ratio is less than 2 [7]. The tests were analysed using Olympus BX43 fluorescence microscope (Olympus Corporation, Japan) equipped with appropriate filters. Each case was photographed and documented with Olympus XM10 monochrome camera and analysed using the Olympus cell Sens Standard software, Version 1.15.
Analyses were performed by using SPSS for Windows 17.0. The results of HER-2 status by FISH and IHC were compared, and concordance, sensitivity, specificity, negative and positive predictive values were evaluated considering FISH as the gold standard. Kappa test was used to determine the concordance between the methods. Also, Fisher’s exact two-tailed test and Chi-square tests were used to determine the correlation of HER-2 status with ER and PR status along with various clinical and histology parameters. The p-value < 0.05 was considered statistically significant.
Results
The HER-2/ IHC results showed that most of the samples 32 (43.84%), were classified as IHC 0, followed by 18 (24.66%) classified as IHC 1+, 12 (16.44%) classified as IHC 3+ and 11 (15.07%) classified as IHC 2+ (Figure 1A). From 73 cases included in this study, 72 showed successful hybridisation. HER-2 FISH gene amplification was determined in 12 (16.44%) of the cases, while 60 (82.19%) of the cases were FISH negative (Figure 1B and Figure 2). One case with failed hybridisation was excluded from the study. The FISH failure rate was 1.37%.
Figure 1.
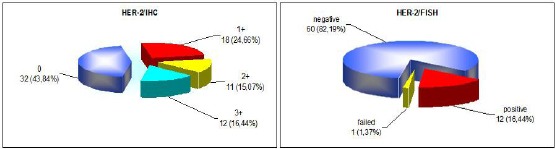
Distribution of HER-2 according to A, IHC (left) and B, FISH (right)
Figure 2.
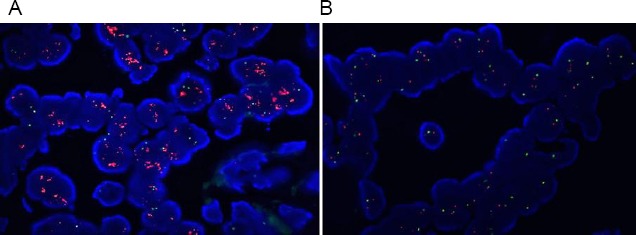
Typical examples of FISH-positive and FISH negative case. A, FISH amplified case, HER-2/Chr 17>2 (DAPI counterstain x 1000); B, FISH non amplified case, HER-2/Chr 17 < 2 (DAPI counterstain x 1000)
Of 12 HER-2 FISH amplified cases, 10 (83.3%) were scored IHC 3+, 2 (16.7%) were scored IHC 2+ and none was scored IHC 1+ or 0. Among the 60 FISH-negative cases, only 2 (3.3%) had IHC score 3+ (Figure 3), and the other samples were either indeterminate 9 (15%) or negative 49 (81.7%). The two IHC 3+ cases that were negative for FISH showed polysomy for chromosome 17.
Figure 3.
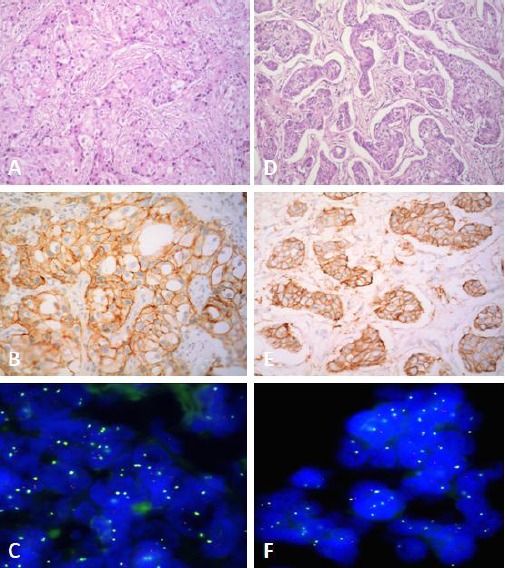
Discordance between IHC and FISH in two cases. Case 1: A, Invasive breast carcinoma (H&E x 200); B, HER-2/IHC 3+ (HER-2 x 200), C, FISH, HER-2/Chr 17 < 2, non amplified (DAPI counterstain x 1000). Case 2: D, Invasive breast carcinoma (H&E x 200); E, HER-2/IHC 3+ (HER-2 x 200); F, FISH, HER-2/Chr 17 < 2, non-amplified (DAPI counterstain x 1000)
In Table 1 we present the rate of concordance for HER-2 results obtained by IHC and FISH. The concordance rate was high (100%) for negative IHC 0/1+ group and low (18.18%) for undetermined IHC 2+ group. The concordance rate for IHC 3+ group was 83.33%.
Table 1.
Comparison of HER-2 results determined by IHC and FISH
| IHC scoring | HER-2/FISH positive | HER-2/FISH negative | Concordance by IHC | Discordance by IHC |
|---|---|---|---|---|
| 0/1+ (n=49) | 0 | 49 | (49/49) 100% | (0/49) 0% |
| 2+ (n=11) | 2 | 9 | (2/11) 18.18% | (9/11) 81.82% |
| 3+ (n=12) | 10 | 2 | (10/12) 83.33% | (2/12) 16.67% |
When IHC 2+ and 3+ positive tumours were grouped, the total concordance between IHC and FISH was 84.72% (61/72), and the Kappa coefficient was 0.598, with a statistical significance of p < 0.0001. After excluding the IHC 2+ cases, the concordance rate improved to 96.72% (59/61).
Table 2 presents the diagnostic performances of the IHC method in determining the HER-2 status using the FISH method as a gold standard. As indicated, the positive predictive value for positive IHC 3+ cases was 83.3%, and for 2+/3+ cases was 52.2%. The immunohistochemical method showed the sensitivity of 100% and 83.3% in detecting IHC 2+/3+ and 3+ cases, and specificity of 81.67% and 96.7% in detecting IHC 2+/3+ and IHC 3+ cases, accordingly.
Table 2.
Sensitivity, specificity, positive and negative predictive values of IHC according to FISH as gold standard
| IHC scoring | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) |
|---|---|---|---|---|
| 2+/3+ positive | 100(75.8-100) | 81.67(70.1-89.4) | 52.2(33.01-70.8) | 100(92.7-100) |
| 3+ positive | 83.3(55.2-95.3) | 96.7(88.6-99.1) | 83.3(55.2-95.3) | 96.7(88.6-99.1) |
| 2+ positive | 16.7(4.7-44.8) | 85(73.9-91.9) | 18.2(5.1-47.7) | 83.6(72.4-90.8) |
In table 3 we present a correlation between HER-2 amplification and clinico-pathological characteristics. The mean age of the patients included in the study was 57.98 ± 10.3 years (range, 41-86 years), and the mean tumour size was 27.01 ± 14.8 mm (range, 8-75 mm). There was no significant correlation between HER-2 amplification and patients’ age, tumour size, tumour grade (G), nuclear grade (NG), tumour status (pT), lymph node status (pN) or stage of the disease. A significant correlation (p < 0.05) was detected between HER-2 and biological markers (ER, PR, Ki 67). ER and PR were more commonly detected in the HER-2/FISH negative than in HER-2/FISH positive tumours (90% vs 58.33%; 78.33% vs 41.67%, respectively). Conversely, the high proliferative index was more frequently found in HER -2 positive tumours (91.67% vs 56.67%).
Table 3.
Correlation of clinical and pathological features with HER-2 amplification status
| Variable | Total n (%) | HER-2/FISH | p-value | ||
|---|---|---|---|---|---|
| Negative (n = 60) | Positive (n = 12) | ||||
| Age | ≤50 >50 | 23 (31.94) 49 (68.06) | 17 (28.33) 43 (71.67) | 6 (50) 6 (50) | 0.18 |
| Tumor size (mm) | ≤20 >20 | 24 (33.33) 48 (85.42) | 19 (79.17) 41 (85.42) | 5 (20.83) 7 (14.58) | 0.5 |
| G | 1 | 3 (4.17) | 3 (5) | 0 | 0.26 |
| 2 | 19 (26.39) | 18 (30) | 1 (8.33) | ||
| 3 | 50 (69.44) | 39 (65) | 11 (91.67) | ||
| NG | 1 | 1 (1.39) | 1 (1.67) | 0 | 0.12 |
| 2 | 15 (20.83) | 15 (25) | 0 | ||
| 3 | 56 (77.78) | 44 (73.33) | 12 (100) | ||
| pT | 1 | 24 (33.33) | 19 (31.67) | 5 (41.67) | 0.34 |
| 2 | 41 (56.94) | 35 (58.33) | 6 (50) | ||
| 3 | 2 (2.78) | 1 (1.67) | 1 (8.33) | ||
| 4 | 5 (6.94) | 5 (8.33) | 0 | ||
| pN | 0 | 27 (37.56) | 24 (40) | 3 (25) | 0.13 |
| 1 | 21 (29.17) | 18 (30) | 3 (25) | ||
| 2 | 12 (16.67) | 11 (18.33) | 1 (8.33) | ||
| 3 | 12 (16.67) | 7 (11.67) | 5 (41.67) | ||
| Stage | I | 16 (22.22) | 14 (23.33) | 2 (16.67) | 0.66 |
| II | 30 (41.67) | 26 (43.33) | 4 (33.33) | ||
| III | 26 (36.11) | 20 (33.33) | 6 (50) | ||
| ER | N | 11 (15.28) | 6 (10) | 5 (41.67) | 0.015 |
| P | 61 (84.72) | 54 (90) | 7 (58.33) | ||
| PR | N | 20 (27.78) | 13 (21.67) | 7 (58.33) | 0.016 |
| P | 52 (72.22) | 47 (78.33) | 5 (41.67) | ||
| Ki 67 | high | 45 (62.5) | 34 (56.67) | 11 (91.67) | 0.025 |
| low | 27 (37.5) | 26 (43.33) | 1 (8.33) | ||
G- histological grade, NG- nuclear grade, pT- tumour status, pN- lymph node status, ER- estrogen receptor, PR- progesterone receptor, Ki67- a marker of proliferation.
Discussion
Breast cancer is the most common malignant tumour and second leading cause of cancer death in women [16]. Prognosis and treatment of breast cancer patients depend on several factors, such as histological type, grade, stage, hormone receptor status and HER-2 status. Determination of HER-2 status is a strong indicator of response to treatment with trastuzumab [17] [18]. Considering the benefits and side effects that patients would have from targeted therapy, the use of the appropriate test for HER -2 assessment is essential in selecting patients for treatment [3] [5]. Immunohistochemistry and FISH are most widely used routine test methods in pathology laboratories. Both methods have their advantages and disadvantages. To date, it is still under debate which single method is the best for HER-2 determination. According to some authors, the use of IHC and FISH methods in combination is the most effective strategy even though it is not cost effective [19] [20]. Immunohistochemistry is widely used, relatively inexpensive and easy to perform test method for HER-2. It is affected by variations in tissue fixation and processing and variations in testing methodologies that can influence the final results [21]. Other disadvantages of IHC are subjectivity in interpretation of the results and absence of internal control, which calls into question the reliability of the analysis, especially when the HER-2/IHC results are negative [3] [21] [22].
Fluorescence in situ hybridisation is expensive, technically demanding molecular assay that requires special equipment for evaluating the results, and its performance is limited to a smaller number of pathology laboratories [9]. However, the FISH method has several advantages over immunohistochemistry: it is less affected by artefacts associated with tissue processing, is more objective because the results are quantitative, and there are internal positive controls [9]. Fluorescence in situ hybridisation is a method of choice when selecting patients for HER-2 targeted therapy regarding accuracy, reproducibility, and predictivity [3]. It provides 96.5% sensitivity and 100% specificity for detection of HER-2 gene amplification in breast cancer patients [23].
In this study, we evaluated the concordance between FISH and IHC for HER-2 detection in breast cancer patients using FDA approved tests. Most of our cases (68.5%) were classified IHC 0/1+, 16.44% were classified IHC 3+ and 15.07% were classified IHC 2+. None of IHC 0/1+ cases was FISH positive. 16.67% of cases in our study showed amplification for HER-2: two cases of IHC 2+ group were FISH positive, and two cases from IHC 3+ group were FISH negative. Concordance rate in our study was 100%, 18.18% and 83.33% for IHC 0/1+, 2+ and 3+ group, respectively. When 2+ and 3+ positive tumours were grouped together, the concordance rate between IHC and FISH was 84.72%, kappa 0.598 (p < 0.0001), but after excluding the IHC 2+ cases form the group, the total concordance rate improved to 96.72%. According to literature, concordance rate between IHC and FISH ranges from 79% to 100% for 3+ cases [24] [25] and from 12% to 36% for 2+ cases [26] [27].
Gokhale et al., [10] showed high concordance between FISH and IHC 3+ groups and poor concordance in the 0, 1+ and 2+ groups. Contrary to these results, other authors [28] [29] have shown that the concordance rate between IHC and FISH is highest for the IHC negative cases and lowest for the IHC 2+ and 3+ cases. Our results also confirmed high concordance rate in IHC 0/1+ group, followed by IHC 3+ group with the lowest concordance rate in the IHC 2+ group. Other authors reported low concordance rates of only 51% between IHC and FISH [21] owing to subjectivity in interpretation, chromosome 17 aneuploidy and technical aspects of tissue processing and IHC. Sarode et al. [30] showed significant improvement in concordance rate in 10 year period due to an overall improvement in standardisation of pre-analytic and analytic variables and experience in HER-2 scoring. The finding of IHC 3+ staining without gene amplification is attributed to false -positive immunostaining when using an unstandardized or unvalidated immunohistochemical method, or chromosome 17 polysomy [31] [32]. Several studies have shown that chromosome 17 polysomy is responsible for discrepancies between protein expression and gene amplification [23] and that these patients have similar clinical outcomes to patients without the HER-2 gene alteration. The rate of discordance in our study may be the result of variability in tissue fixation (time to fixation and time in fixative) because almost half of the cases included were from other city hospitals where tissue fixation started. However, we cannot exclude the influence of aneusomy 17 because two IHC 3+/FISH- cases in our study, showed chromosome 17 polysomy.
Taking the FISH method as a gold standard, sensitivity rate in our study was 16.7% and 83.3% for IHC 2+ and 3+ cases. The specificity rate was 96.67% and 85% for 3+ and 2+ cases, respectively. When 2+/3+ cases were analyzed as a group, the sensitivity was 100%, but the specificity was 81.67%. The positive predictive value of positive IHC 3+ and IHC 2+/3+ cases was 83.3% and 52.2% respectively, and negative predictive value for negative IHC 0/1+ cases was 100%. The immunohistochemical method showed the highest sensitivity of 100% in detecting IHC 2+/3+ positive tumours as one group. Other authors reported high specificity (94%), but low sensitivity (43%), of immunohistochemistry [10].
According to some authors, the lowest cost -effective HER-2 testing is to screen all breast cancer patients with immunohistochemistry (because of its high negative predictive value) and to confirm only IHC 2+ and 3+ scores with the FISH assay [29]. Although FISH testing is much more expensive than IHC, it never exceeds the cost of treating patients who are not likely to benefit because of a false-positive IHC [29]. Our findings support the view that false -positive rather than false-negative IHC results are a major issue with HER-2 IHC testing. HER-2 positive status is a bad prognostic marker, and these tumours are associated with high histological grade, negative hormone receptor status and positive regional lymph nodes at the time of diagnosis [30] [33] [34]. Our results did not show a significant correlation between HER-2 amplification and other clinico-pathological parameters like patient’s age, tumour size, tumour grade, nuclear grade, lymph node status and stage of the disease. Although statistically non-significant HER-2 amplified, tumours were more frequently poorly differentiated with high nuclear grade, positive lymph node status and high postoperative stage indicating biologically more aggressive tumours. Other authors found no association between HER-2 and patients age [35] [36] [37] [38] tumor size [35] [36] [37] or lymph node status [35], too. Contrary to our results, other authors noted significant correlation of HER-2 with tumour size [39] tumour grade [35] [39] or lymph node metastasis [40] [41].
Statistically significant association in our study was detected between HER-2 positive tumors and negative estrogen (p = 0.015), progesterone receptor (p = 0.016) status, and high proliferative index Ki67 (p = 0.025). Some authors also reported significant correlation with negative hormone receptor status [35] [39] and high proliferative index [30] [37] [38], while other authors showed correlation with positive hormone receptor status [41] [42] or low Ki67 [41].
In conclusion, the overall concordance between IHC and FISH was 84.72%. The consistency between the two methods was highest for IHC negative and lowest for IHC equivocal cases. With FISH as the gold standard, the positive predictive value of positive (IHC 3+) cases was 83.3%, and negative predictive value for negative (IHC 0/1+) cases was 100%. The immunohistochemical method showed high sensitivity in IHC 2+/3+ cases and high specificity in IHC 3+ group. Our results support the view that false -positive rather than false-negative IHC results are a bigger problem with HER-2/IHC testing, and that IHC should be used as an initial screening test, but that FISH should confirm IHC 2+ and 3+ results. Standardization of tissue processing is necessary to improve the specificity of the IHC assay.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalised medicine. Oncologist. 2009;14(4):320–368. doi: 10.1634/theoncologist.2008-0230. https://doi.org/10.1634/theoncologist.2008-0230. PMid:19346299. [DOI] [PubMed] [Google Scholar]
- 2.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Richard J, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131(1):18–43. doi: 10.5858/2007-131-18-ASOCCO. PMid:19548375. [DOI] [PubMed] [Google Scholar]
- 3.Sauter G, Lee J, Bartlett JM, Slamon DJ, Press MF. Guidelines for human epidermal growth factor receptor 2 testing: Biologic and methodologic considerations. J Clin Oncol. 2009;27(8):1323–1333. doi: 10.1200/JCO.2007.14.8197. https://doi.org/10.1200/JCO.2007.14.8197. PMid:19204209. [DOI] [PubMed] [Google Scholar]
- 4.Sapino A, Goia M, Recupero D, Marchiò C. Current challenges for HER2 testing in diagnostic pathology: State of the art and controversial issues. Front Oncol. 2013;3:129. doi: 10.3389/fonc.2013.00129. https://doi.org/10.3389/fonc.2013.00129. PMid:23734345. PMCid: PMC3659312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lottner C, Schwarz S, Diermeier S, Hartmann A, Knuechel R, Hofstaedter F, et al. Simultaneous detection of HER2/neu gene amplification and protein overexpression in paraffin-embedded breast cancer. J Pathol. 2005;205(5):577–584. doi: 10.1002/path.1742. https://doi.org/10.1002/path.1742. PMid:15732132. [DOI] [PubMed] [Google Scholar]
- 6.Dowsett M, Hanna WM, Kockx M, Penault-Llorca F, Ruschoff J, Gutjahr T, et al. Standardization of HER2 testing: Results of an international proficiency-testing ring study. Mod Pathol. 2007;20(5):584–591. doi: 10.1038/modpathol.3800774. https://doi.org/10.1038/modpathol.3800774. PMid:17396141. [DOI] [PubMed] [Google Scholar]
- 7.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/College of American pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. https://doi.org/10.1200/JCO.2013.50.9984. PMid:24101045. [DOI] [PubMed] [Google Scholar]
- 8.Perez EA, Cortes J, Gonzalez-Angulo AM, Bartlett JM. HER2 testing: Current status and future directions. Cancer Treat Rev. 2014;40(2):276–284. doi: 10.1016/j.ctrv.2013.09.001. https://doi.org/10.1016/j.ctrv.2013.09.001. PMid:24080154. [DOI] [PubMed] [Google Scholar]
- 9.Schnitt SJ, Jacobs TW. Current status of HER2 testing: caught between a rock and a hard place. Am J Clin Pathol. 2001;116(6):806–810. doi: 10.1309/WMN8-VTR5-DUGF-X12L. https://doi.org/10.1309/WMN8-VTR5-DUGF-X12L. PMid:11764067. [DOI] [PubMed] [Google Scholar]
- 10.Gokhale S, Gatalica Z, Mohammad A, Rampy AI, Velagaleti Gopalrao VN. FISH for HER-2/neu in breast cancer: Standardization makes the difference! Indian J Cancer. 2004;41(4):152–158. PMid:15659867. [PubMed] [Google Scholar]
- 11.Elston CW, Ellis IO, Pathological prognostic factors in breast cancer I The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. https://doi.org/10.1111/j.1365-2559.1991.tb00229.x. PMid:1757079. [DOI] [PubMed] [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Handbook. 7th ed. New York: Springer-Verlag; 2010. [Google Scholar]
- 13.Anderson E. The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Res. 2002;4(5):197–201. doi: 10.1186/bcr452. https://doi.org/10.1186/bcr452. PMid:12223124. PMCid: PMC138744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. https://doi.org/10.1093/annonc/mdt303. PMid:23917950. PMCid: PMC3755334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogdanovska-Todorovska M, Petrushevska G, Janevska V, Spasevska L, Kostadinova-Kunovska S. Standardization and optimization of fluorescence in situ hybridization (FISH) for HER-2 assessment in breast cancer: A single center experience. Bosnian journal of basic medical sciences. 2018 Jan 30; doi: 10.17305/bjbms.2018.2519. https://doi.org/10.17305/bjbms.2018.2519. PMid:29389309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–156. doi: 10.1002/ijc.1440. https://doi.org/10.1002/ijc.1440. PMid:11668491. [DOI] [PubMed] [Google Scholar]
- 17.Gong Y, Sweet W, Duh YJ, Greenfield l, Fang Y, Zhao J, et al. Chromogenic in situ hybridization is a reliable method for detecting HER2 gene status in breast cancer. Am. J. Clin. Pathol. 2009;131(4):490–497. doi: 10.1309/AJCPI00TVGIGYXAA. https://doi.org/10.1309/AJCPI00TVGIGYXAA. PMid:19289584. [DOI] [PubMed] [Google Scholar]
- 18.Slamon D, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against Her-2 for metastatic breast cancer that overexpresses Her-2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. https://doi.org/10.1056/NEJM200103153441101. PMid:11248153. [DOI] [PubMed] [Google Scholar]
- 19.Ellis CM, Dyson MJ, Stephenson TJ, Maltby EL. HER2 amplification status in breast cancer: a comparison between immunohistochemical staining and fluorescence in situ hybridisation using manual and automated quantitative image analysis scoring techniques. J Clin Pathol. 2005;58(7):710–714. doi: 10.1136/jcp.2004.023424. https://doi.org/10.1136/jcp.2004.023424. PMid:15976337. PMCid: PMC1770709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilous M, Dowsett M, Hanna W, et al. Current Perspectives on HER2 Testing: A Review of National Testing Guidelines. Mod Pathol. 2003;16(2):173–182. doi: 10.1097/01.MP.0000052102.90815.82. https://doi.org/10.1097/01.MP.0000052102.90815.82. PMid:12591971. [DOI] [PubMed] [Google Scholar]
- 21.Hammock L, Lewis M, Phillips C, Cohen C. Strong HER-2/neu Protein Overexpression by Immunohistochemistry Often Does Not Predict Oncogene Amplification by Fluorescence In Situ Hybridization. Hum Pathol. 2003;34(10):1043–1047. doi: 10.1053/s0046-8177(03)00409-x. https://doi.org/10.1053/S0046-8177(03)00409-X. [DOI] [PubMed] [Google Scholar]
- 22.Nitta H, Kelly BD, Allred C, Jewell S, Banks P, Dennis E, Grogan TM. The assessment of HER2 status in breast cancer: the past, the present, and the future. Pathol Int. 2016;66(6):313–324. doi: 10.1111/pin.12407. https://doi.org/10.1111/pin.12407. PMid:27061008. [DOI] [PubMed] [Google Scholar]
- 23.Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, Slamon DJ. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: A direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000;18(21):3651–64. doi: 10.1200/JCO.2000.18.21.3651. https://doi.org/10.1200/JCO.2000.18.21.3651. PMid:11054438. [DOI] [PubMed] [Google Scholar]
- 24.Lebeau A, Deimling D, Kaltz C, Sendelhofert A, Iff A, Luthardt B, et al. HER-2/neu analysis in archival tissue samples of human breast cancer: Comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol. 2001;19(2):354–363. doi: 10.1200/JCO.2001.19.2.354. https://doi.org/10.1200/JCO.2001.19.2.354. PMid:11208826. [DOI] [PubMed] [Google Scholar]
- 25.Tubbs RR, Pettay JD, Roche PC, Stoler MH, Jenkins RB, Grogan TM. Discrepancies in clinical laboratory testing of eligibility for trastuzumab therapy: Apparent immunohistochemical false-positives do not get the message. J Clin Oncol. 2001;19(10):2714–2721. doi: 10.1200/JCO.2001.19.10.2714. https://doi.org/10.1200/JCO.2001.19.10.2714. PMid:11352964. [DOI] [PubMed] [Google Scholar]
- 26.Perez EA, Roche PC, Jenkins RB, Reynolds CA, Halling KC, Ingle JN, Wold LE. HER-2/neu testing in patients with breast cancer: Poor correlation between weak positivity by immunohistochemistry and gene amplification by fluorescence in situ hybridization. Mayo Clin Proc. 2002;77(2):148–154. doi: 10.4065/77.2.148. https://doi.org/10.1016/S0025-6196(11)62329-X. [DOI] [PubMed] [Google Scholar]
- 27.Ridolfi RL, Jamehdor MR, Arber JM. HER-2/neu testing in breast carcinoma: A combined immunohistochemical and fluorescence in situ hybridization approach. Mod Pathol. 2000;13(8):866–873. doi: 10.1038/modpathol.3880154. https://doi.org/10.1038/modpathol.3880154. PMid:10955453. [DOI] [PubMed] [Google Scholar]
- 28.Dybdal N, Leiberman G, Anderson S, McCune B, Bajamonde A, Cohen RL, et al. Determination of HER2 gene amplification by fluorescence in situ hybridization and concordance with the clinical trials immunohistochemical assay in women with metastatic breast cancer evaluated for treatment with trastuzumab. Breast Cancer Res Treat. 2005;93(1):3–11. doi: 10.1007/s10549-004-6275-8. https://doi.org/10.1007/s10549-004-6275-8. PMid:16184453. [DOI] [PubMed] [Google Scholar]
- 29.Dendukuri N, Khetani K, McIsaac M, Brophy J. Testing for HER2-positive breast cancer: a systematic review and cost-effectiveness analysis. CMAJ. 2007;176(10):1429–1434. doi: 10.1503/cmaj.061011. https://doi.org/10.1503/cmaj.061011. PMid:17485695. PMCid: PMC1863543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarode VR, Xiang QD, Christie A, Collins R, Rao R, Leitch AM, et al. Evaluation of HER2/neu status by immunohistochemistry using computer-based image analysis and correlation with gene amplification by fluorescence in situ hybridization assay: A 10-year experience and impact of test standardization on concordance rate. Arch Pathol Lab Med. 2015;139(7):922–928. doi: 10.5858/arpa.2014-0127-OA. https://doi.org/10.5858/arpa.2014-0127-OA. PMid:26125432. [DOI] [PubMed] [Google Scholar]
- 31.Brunelli M, Manfrin E, Martignoni G, Bersani S, Remo A, Reghellin D, et al. HER-2/neu assessment in breast cancer using the original FDA and new ASCO/CAP guidelines recommendation: impact in selecting patients for Herceptin therapy. Am J Clin Pathol. 2008;129(6):907–911. doi: 10.1309/MD79CDXN1D01E862. https://doi.org/10.1309/MD79CDXN1D01E862. PMid:18480007. [DOI] [PubMed] [Google Scholar]
- 32.Roche PC, Suman VJ, Jenkins RB, Davidson NE, Martino S, Kaufman PA, et al. Concordance between local and central laboratory HER2 testing in the Breast Intergroup Trial N9831. J Natl Cancer Inst. 2002;94(11):855–857. doi: 10.1093/jnci/94.11.855. https://doi.org/10.1093/jnci/94.11.855. PMid:12048274. [DOI] [PubMed] [Google Scholar]
- 33.Ross JS, Fletcher JA, Bloom KJ, Linette GP, Stec J, Clark E, et al. Her- 2/ neu testing in breast cancer. Am J Clin Pathol. 2003;120(Suppl 1):S53–S71. doi: 10.1309/949FPQ1AQ3P0RLC0. https://doi.org/10.1309/949FPQ1AQ3P0RLC0. [DOI] [PubMed] [Google Scholar]
- 34.Makar AP, Desmedt EJ, De Potter CR, Vanderheyden JS, Schatteman EA. Neu (C-erbB-2) oncogene in breast cancer and its possible association with the risk of distant metastases. A retrospective study and review of literature. Acta Oncol. 1990;29(7):931–934. doi: 10.3109/02841869009096392. https://doi.org/10.3109/02841869009096392. PMid:1979748. [DOI] [PubMed] [Google Scholar]
- 35.Panjwani P, Epari S, Karpate A, Shirsat H, Rajsekharan P, Basak R, et al. Assessment of HER-2/neu status in breast cancer using fluorescence in situ hybridization & immunohistochemistry: Experience of a tertiary cancer referral centre in India. Indian J Med Res. 2010;132:287–294. PMid:20847375. [PubMed] [Google Scholar]
- 36.Onody P, Bertrand F, Muzeau F, Bieche I, Lideteau R. Fluorescence In Situ Hybridization and Immunohistochemical Assays for HER-2/ neu Status Determination. Arch Pathol Lab Med. 2001;125(6):746–750. doi: 10.5858/2001-125-0746-FISHAI. PMid:11371225. [DOI] [PubMed] [Google Scholar]
- 37.Qiao E-Q,, Ji M, Wu J, Li J, Xu X, Ma R, et al. Joint detection of multiple immunohistochemical indices and clinical significance in breast cancer. Mol Clin Oncol. 2013;1(4):703–710. doi: 10.3892/mco.2013.111. https://doi.org/10.3892/mco.2013.111. PMid:24649232. PMCid: PMC3915321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shokouh TZ, Ezatollah A, Barand P. Interrelationships Between Ki67, HER2/neu, p53, ER, and PR Status and Their Associations With Tumor Grade and Lymph Node Involvement in Breast Carcinoma Subtypes. Retrospective-Observational Analytical Study Feng Y Medicine. 2015;94(32):e1359. doi: 10.1097/MD.0000000000001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartlett JM, Ellis IO, Dowsett M, Mallon EA, Cameron DA, Johnson S, et al. Human epidermal growth factor receptor 2 status correlates with lymph node involvement in patients with estrogen receptor (ER) negative, but with grade in those with ER-positive early-stage breast cancer suitable for cytotoxic chemotherapy. J Clin Oncol. 2007;25(28):4423–4430. doi: 10.1200/JCO.2007.11.0973. https://doi.org/10.1200/JCO.2007.11.0973. PMid:17906205. [DOI] [PubMed] [Google Scholar]
- 40.Ali EM, Ahmed ARH, Ali AMA. Correlation of Breast Cancer Subtypes Based on ER PR and HER2 Expression with Axillary Lymph Node Status. Cancer and Oncology Research. 2014;2(4):51–57. [Google Scholar]
- 41.Payandeh M, Shahriari-Ahmadi A, Sadeghi1 M, Sadeghi E. Correlations between HER2 Expression and Other Prognostic Factors in Breast Cancer: Inverse Relations with the Ki-67 Index and P53 Status. Asian Pac J Cancer Prev. 2016;17(3):1015–1018. doi: 10.7314/apjcp.2016.17.3.1015. https://doi.org/10.7314/APJCP.2016.17.3.1015. PMid:27039719. [DOI] [PubMed] [Google Scholar]
- 42.Zhou P, Jiang YZ, Hu X, Sun W, Liu RY, Liu F, et al. Clinicopathological characteristics of patients with HER2-positive breast cancer and the efficacy of trastuzumab in the People's Republic of China. Onco Targets Ther. 2016;18(9):2287–2295. doi: 10.2147/OTT.S97583. https://doi.org/10.2147/OTT.S97583. PMid:27143924. PMCid: PMC4846044. [DOI] [PMC free article] [PubMed] [Google Scholar]


