Abstract
INTRODUCTION:
Type 1 diabetes mellitus (T1DM) is one of the most common chronic diseases in children that may be complicated by micro or macrovascular complications. Measurement of the carotid intima-media thickness (CIMT) allows the early detection of atherosclerotic alterations of blood vessels that may complicate T1DM.
SUBJECTS AND METHODS:
This study is a case-control study. Participants were classified into two groups. The first group included 40 children with T1DM and the second group included 30 matched healthy controls. The studied cases were recruited from Endocrinology and Diabetology Unit, Pediatric Hospital, Ain Shams University. Serum apelin, cholesterol, TG, LDL were measured for every case. Also, albumin level was analyzed in urine. Measurement of the carotid intima-media thickness (CIMT) was done for all cases.
RESULTS:
Comparison between T1DM patients and controls revealed that serum apelin, cholesterol, TG, LDL and albuminuria were significantly increased in cases compared to controls. Significant positive correlations were detected between HbA1C, albuminuria and lipid profile with apelin in the diabetic group (p < 0.05). CIMT has significant positive correlation with serum apelin levels (r = 0.36, p = 0.05). Also, this study found positive correlations between CIMT and some variables as LDL, SBP z-score and duration of the illness.
CONCLUSION:
Increased levels of serum apelin in T1DM patients may be considered as predicting factor for the ongoing development of vascular sequels. This study highlighted the possible validity of apelin assay as an early predictor of atherosclerosis in T1DM children. Evaluating CIMT in these patients is of at most important for early detection of subclinical atherosclerosis.
Keywords: Type 1 diabetes mellitus, carotid intima-media thickness, Serum apelin, cholesterol, TG, LDL, case-control study
Introduction
Type 1 diabetes mellitus (T1DM) is a multifactorial health problem worldwide. It is one of the most common chronic diseases in children that may be complicated by micro or macrovascular complications [1]. Endothelial dysfunction and subclinical organ damage indices, such as greater Carotid Intima-Media Thickness (CIMT) and arterial atherosclerotic changes, reflecting higher cardiovascular diseases (CVD) risk in adulthood, are increased with hyperglycemia, hypertension, high LDL and TG concentrations, insulin resistance, increased BMI, proinflammatory status and disturbances in adipocytokines [2]. The direct impedance of adipokines on the endothelium needs further investigations. Endothelial dysfunction is considered the early stage of atherosclerotic changes that may affect blood vessels in T1DM [3]. It is characterised by abnormalities in the lumen and endothelium of the blood vessels, resulting in vasodilatation response. These changes in vascular endothelium result in increased secretion of inflammatory cytokines that augment adhesion of cellular molecules and other biologically active substances and finally lead to a proinflammatory and prothrombotic state [4]. These mechanisms represent an important step in the development of the initial atherosclerotic changes and subsequent complications in diabetic children [5]. Measurement of the carotid intima-media thickness (CIMT) allows the early detection of atherosclerotic alterations of blood vessels. It is considered a strong predictor of vascular abnormalities in high-risk individuals, such as those with T1DM [6]. Measurement of the intima-media thickness of the large arteries, especially the carotids, has considered as the method of choice for detecting the anatomical extent of arterial wall deterioration and for assessing cardiovascular risk [7]. Several investigators have recommended the clinical use of this technique for detecting subclinical (asymptomatic) atherosclerosis and for identifying subjects at high-risk.
Apelin is one of the adipokines that is secreted from white adipose tissue and has various functions as insulin sensitivity. It has an important role in diabetes mellitus. Furthermore, its concentration is changed according to insulin resistance. It has been shown that apelin has a crucial role in energy metabolism and pathogenesis of diabetes mellitus because white adipose tissue that secretes apelin acts as an endocrine organ [8].
Apelin had been discovered by Tatemoto et al., in 1998 [9]. They suggested that it is the endogenous ligand for angiotensin II protein J (APJ) receptor [10]. Apelin and its receptor APJ are expressed in several tissues like heart, lung, stomach and skeletal muscles. In the past few years, it has been found the possible roles played by apelin in human physiology, It is considered as regulating peptide of cardiovascular, hypothalamus-hypophysis, gastrointestinal and immune systems [11]. Scientists stated that apelin might be a risk indicator in young children prone to atherosclerosis and type 2 diabetes [12]. Moreover, some previous studies found apelin as a novel biomarker for predicting diabetes especially T1DM [13]. In healthy individuals, the level of apelin depends on the nutritional state. It was reduced in fasting and increased by feeding, while in diabetic patients and impaired glucose tolerance it may increase due to insulin resistance [14]. Insulin increased the expression of apelin in adipocytes [15].
This study aims to analyse the role of the adipokine apelin in the pathogenesis and complications of type I diabetes mellitus in children. Also, we aimed to clarify the relation between serum apelin and lipid concentrations and to verify the presence of CIMT and initial structural atherosclerotic changes in children with Type 1 diabetes mellitus (T1DM).
Subjects and Methods
This study is a case-control study. Participants were classified into two groups. The first group included 40 children with T1DM, their mean age was 9.15 ± 3.64 years, and the mean duration of disease was 4.5 years. The second group included 30 matched healthy controls, of same age group. Cases with T1DM were classified into two subgroups according to HbA1c, group I with HbA1C of 8% or less (well-controlled T1DM) and group II with HbA1c greater than 8% (poorly controlled T1DM).
The studied cases were recruited from Endocrinology and Diabetology Unit, Pediatric Hospital, Ain Shams University. Healthy children were peers of diabetic patients and from local schools. Cases with the chronic or inflammatory disease, cases which were on medications or hormones (other than insulin), cases with known renal disease and systemic disease and acute infection at the time of testing were excluded from the study. The study was approved by the local ethics committee of National Research centre, and written informed consent was obtained from both parents of every participant.
All participants were subjected to:
- Full history with special emphasis on the onset of diabetes.
- Clinical examination and anthropometric measurements including Height (in centimetres) using Harpenden stadiometer and Weight (in kilograms) using an electronic weight scale.
- calculation of BMI: weight in kg/(height in meters)2.
- Weight for age, height for age and BMI Z -score were determined using the new WHO reference [16].
- Measuring blood pressure (BP) by a sphygmomanometer.
Venous blood samples (3 ml) were taken from each child participating in the study and divided into two parts: the first part was added to a tube containing EDTA for glycosylated haemoglobin determination by cation-exchange resin and the second part was put in a serum separator tube. The separated serum was stored at – 20°C for determination of Apelin, fasting blood sugar, total cholesterol, and triglyceride (TG), HDL and LDL. Fasting blood glucose level was performed on automated clinical chemistry analyser (Olympus AU400). Total cholesterol, triglyceride and HDL were determined using colourimetric techniques on Synchron Cx7 (Beckman Instruments Inc., California, USA). LDL cholesterol was measured by Friedwald formula [17]. For random urinary albumin measurement, an early morning mid-stream specimen was used. The cloudy samples were centrifuged before use, and the clear supernatant was stored at -20°C until analysis. Albumin concentrations were measured in urine using a Minineph microalbumin kit based on nephelometry method on Mininephnephlometer (AD200) (The Binding Site, Birmingham, UK) [18]. We compared albumin in the sample against its creatinine concentration (measured by Jaffe reaction) on a Synchron Cx7 autoanalyser, and the albumin/creatinine ratio was calculated [19]. Serum Apelin was measured by quantitative commercial enzyme-linked immunosorbent assay ELISA kit supplied from Elabscience Biotechnology Co., Ltd, Wu Han, China-Catalog No: E – EL-H0456 (www.elabscience.com), detection range was between 62.5-400 pg/ml.
Measurement of carotid intima-media thickness (CIMT) was performed using high-resolution B mode ultrasound to detect the thickness of Common Carotid Arteries. All of the carotid scans were done using carotid Doppler ultrasound scanner (Toshiba Ultrasonography machine [Xario], Tokyo, Japan) with a 10.0-MHz linear array transducer following a predetermined standardised scanning protocol [20].
The data were coded, entered and processed by computer using Statistical Program for Social Science version 22 (SPSS Inc., Chicago, IL, USA). Quantitative variables were described in the form of mean and SD concerning age, BMI Z-score, systolic and diastolic BP Z-score, level of HbA1C, cholesterol, TG, and level of Apelin and qualitative variables were described as number and percent concerning sex distribution and presence of albuminuria. To compare quantitative parametric variables between two groups, Student t-test was used. Pearson correlation coefficient was employed to measure the strength and direction of the linear relationship between two variables. A p-value < 0.05 was considered the cut-off value for significance in all analyses.
Results
Subjects in this study were classified into two groups. The first group is cases which involve 40 children with type I diabetes mellitus, of them 17 were males and 23 were females. The second group controls which involve 30 healthy children, of them 11 were males, and 19 were females. No significant difference was found between cases and controls in relation to age.
Table 1 shows a comparison of anthropometric and clinical parameters between cases and controls; there was highly significant difference between the two groups regarding SBP and DBP z-score (p < 0.01). No significant difference was found between the two groups as regards weight, height and BMI z-score.
Table 1.
Comparison of anthropometric and clinical parameters between cases and controls
| Variable | Cases (n = 40) Mean ± sd | Control (n = 30) Mean ± sd | P |
|---|---|---|---|
| Age (years) | 9.15 ± 3.64 | 9.70 ± 4.38 | 0.63 |
| Weight z-score | 0.61 ± 0.47 | -0.42 ± 0.67 | 0.12 |
| Height z-score | -0.48 ± 1.21 | -0.27 ± 1.14 | 0.77 |
| Bmi z- score | 0.67 ± 1.28 | 0.41 ± 0.83 | 0.52 |
| Sbp z-score | 0.76 ± 0.44 | 0.16 ± 0.36 | 0.001* |
| Dbp z-score | 1.1 ± 0.49 | 0.41 ± 0.38 | 0.000* |
Table 2 shows a comparison of laboratory markers between cases and controls. Serum apelin, cholesterol, TG, LDL and albuminuria were significantly increased while HDL was significantly decreased in cases compared to controls.
Table 2.
Comparison of laboratory markers between cases and controls
| Variable | Cases (n = 40) Mean ± sd | Control (n = 30) Mean ± sd | P |
|---|---|---|---|
| Apelin (pg/ml) | 1040 ± 576 | 741.8 ± 231.6 | 0.015* |
| Albuminuria (mg/gm creatinine) | 46.46 ± 32.08 | 9.83 ± 5.07 | 0.02* |
| Cholesterol (mg/dl) | 217.6 ± 44.30 | 139.27 ± 19.3 | 0.000* |
| TG (mg/dl) | 156.8 ± 18.94 | 76.53 ± 7.12 | 0.000* |
| LDL (mg/dl) | 183.32 ± 46.71 | 58.16 ± 13.45 | 0.000* |
| HDL (mg/dl) | 23.27 ± 5.43 | 46.14 ± 10.2 | 0.000* |
Table 3 shows correlations between Apelin and anthropometric, clinical and laboratory data in the Diabetic group. DBP z-score has significant positive correlation with apelin in diabetic group (p < 0.05). HbA1C, albuminuria and lipid profile of diabetic children had a highly significant positive correlation with apelin (p < 0.001). Mean CIMT in diabetic children was 0.5 ± 0.1 mm.
Table 3.
Correlation between Apelin and anthropometric, clinical and laboratory data in the Diabetic group
| variable | Diabetic (N = 40) | |
|---|---|---|
| r | p | |
| DBP z-score | 0.292 | 0.02* |
| HbA1C | 0.441 | 0.001* |
| Cholesterol | 0.404 | 0.001* |
| TG | 0.401 | 0.001* |
| LDL | 0.402 | 0.001* |
| CIMT | 0.362 | 0.05* |
| Albuminuria | 0.887 | 0.000* |
CIMT had significant positive correlation with serum apelin levels as shown in figure 1 (r = 0.36, p = 0.05). Also, this study found a positive correlation between CIMT and some variables as LDL, SBP z -score and duration of the illness (p < 0.05). Also, Duration of diabetes was positively correlated with LDL (p = 0.03). HbA1C was positively correlated with DBP z-score and albuminuria (p < 0.05).
Figure 1.
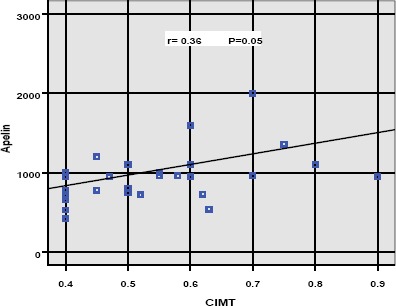
Correlation between serum apelin levels and CIMT
We also investigated the influence of different clinical and laboratory variables on diabetic nephropathy occurrence. It revealed that serum apelin and Hb1C had a significant influence on the appearance of diabetic nephropathy. This is discovered by detecting a positive correlation between albuminuria and serum apelin, HbAc (p < 0.05).
Discussion
Apelin is an adipokine that is secreted from adipocytes in different organs. It has been shown that serum apelin was involved in glucose homeostasis and also it regulates insulin secretion. In this study, we found that serum apelin levels are significantly increased in children with T1DM in comparison to healthy subjects, this is in agreement with previous studies [21] [22]. This is maybe explained by that apelin action on glucose metabolism is additive to insulin as it increases glucose uptake and transport in tissues [15], also it increases intestinal absorption of glucose [23]. All reported previous actions of apelin make it an anti-diabetic agent. Thus, our study hypothesised that increased levels of apelin in T1DM is a result of compensatory mechanism devoted to decreased insulin levels and to overcome insulin resistance in these patients. This study also found a highly significant positive correlation between serum apelin and HbA1c in diabetic subjects; this is in contrary with a previous study [22], who stated that serum apelin levels were negatively correlated to HbA1c in type 2 diabetic patients, suggesting that circulating apelin is associated with the better glycemic control. This difference may be explained by the fact that patients with T1DM in our study were treated with insulin which plays an important role in apelin secretion and expression.
In this study, we found a significant influence of serum apelin on diastolic blood pressure, it increases with increased serum apelin levels, but it does not affect systolic blood pressure. This is in disagreement with a previous study [12], which discovered that there was not a significant influence of serum apelin on SBP, but there was a tendency to lower DBP with higher levels of serum apelin, this discrepancy is due to that they studied elderly subjects with an increased risk to develop T2DM. To check the further influence of serum apelin on vascular integrity, we studied the CIMT as a parameter for early atherosclerotic changes in blood vessels in T1DM patients. CIMT has been described as a mirror of the atherosclerotic burden and a predictor for the subsequent sequel as myocardial infarction and stroke [24]. From the previous advantages of this technique, we decided to use it as a gold standard for early detection of subclinical atherosclerosis in T1DM patients. We discovered positive correlations between CIMT and LDL; this ensures our recommendation that CIMT should be done for every T1DM patient for early detection of atherosclerosis. We found that serum apelin levels have a significant positive correlation with CIMT in T1DM. Thus, we can consider increased levels of serum apelin in T1DM as predicting factor for the further development of vascular complications of DM in these patients. In our study, HbA1C was positively correlated with DBP z-score. This may be explained by that increased glycemic state is associated with increased vasoconstriction and hypertension due to inflammatory state that induces oxidative stress that alters nitric oxide secretion and degradation and finally has a deleterious effect on vascular endothelial cells. Our results concerning hyperglycemia and increased blood pressure are in agreement with a previous study [25]. Furthermore, this study also clarifies the role of apelin in the occurrence of diabetic nephropathy. We found that serum apelin and Hb1C have a significant influence on the appearance of these complications. We discovered that serum apelin was associated with increased diabetic nephropathy which correlated with microalbuminuria. This is ensured by another study [26], which stated that apelin induce glomerular endothelial cells proliferation and then nephropathy so we can state that increased serum apelin may worsen the condition in T1DM through its influence on diabetic nephropathy.
In our study, we reported that serum cholesterol; TG and LDL were significantly increased in T1DM patients than healthy subjects, but serum HDL was significantly decreased in T1DM patients than healthy subjects. From the previous results, we can consider children with T1DM at higher risk of developing premature atherosclerosis because of hyperlipidemia and thus, should be screened well for this serious complication. Also, we found significant positive correlations between serum apelin and serum cholesterol, LDL and TG in diabetic patients. In support of these results, other study found statistical differences between diabetic and non-diabetic groups as regards serum levels of TG and cholesterol [25]. Also, other study reported the same results as regards serum TG [13]. Few studies describe the effects of apelin on lipid metabolism; one of them stated that apelin was shown to inhibit lipolysis [27]. This was ensured by another study [28] that found that apelin increases the stability of lipid vacuoles making them more resistant to lipases. All these findings support our results that apelin is associated with increased serum lipids and thus can be used as a predictor of premature atherosclerosis in T1DM patients.
In conclusion, increased levels of serum apelin in T1DM patients may be considered as predicting factor for the ongoing development of vascular sequels so measuring serum apelin in these patients is of benefit for early detection of disease complications. Premature subclinical atherosclerosis was documented among T1DM patients due to hyperlipidemia detected in these patients, so we recommend evaluating their CIMT for early detection of subclinical atherosclerosis. CIMT was correlated well with dyslipidemia and serum apelin, a finding that highlighted the possible validity of apelin assay as an early predictor of atherosclerosis in T1DM children.
Acknowlegement
Authors are thankful to the Department of Pediatrics and Radiology Department, Faculty of Medicine, Ain Shams University, Egypt.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Giannini C, Mohn A, Chiarelli F, Kelnar CJ. Macrovascular angiopathy in children and adolescents with type 1 diabetes. Diabetes Metab Res Rev. 2011;27(5):436–60. doi: 10.1002/dmrr.1195. https://doi.org/10.1002/dmrr.1195. PMid:21433262. [DOI] [PubMed] [Google Scholar]
- 2.Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. J Am CollCardiol. 2013;62(15):1309–19. doi: 10.1016/j.jacc.2013.07.042. https://doi.org/10.1016/j.jacc.2013.07.042. PMid:23954339. [DOI] [PubMed] [Google Scholar]
- 3.El Wakeel MA, El-Kassas GM, Amer AF, Elbatal WH, Sabry RN, EL-Ghaffar Mohammed NA. E-selectin and vascular complications in children with Type 1 diabetes mellitus. Med Res J. 2014;13(1):27–32. https://doi.org/10.1097/01.MJX.0000446937.40653.3d. [Google Scholar]
- 4.El Wakeel MA, Abou-el-asrar M, El-kassas GM, Elabd MA, Zeid DA, Sabry RN, Awadallah E. Urinary Markers of Oxidative DNA Damage in Type 1 Diabetic Children: Relation to Microvascular Complications. Asian J Pharm Clin Res. 2017;10(10):318–22. https://doi.org/10.22159/ajpcr.2017.v10i10.18930. [Google Scholar]
- 5.Sheetz MJ, King GI. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA. 2002;288(20):2579–88. doi: 10.1001/jama.288.20.2579. https://doi.org/10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 6.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, Jr, Cardiovascular health study collaborative research group Carotid artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. https://doi.org/10.1056/NEJM199901073400103. PMid:9878640. [DOI] [PubMed] [Google Scholar]
- 7.Manios E, Tsivgoulis G, Koroboki E, et al. Impact of prehypertension oncommon carotid artery intima-media thickness and left ventricular mass. Stroke. 2009;40:1515–8. doi: 10.1161/STROKEAHA.108.528174. https://doi.org/10.1161/STROKEAHA.108.528174. PMid:19164793. [DOI] [PubMed] [Google Scholar]
- 8.Fairbridge N.A, Southall T.M, Ayre D.C, Komatsu Y, Raquet P.I, Brown R.J, Randell E, Kovacs C.S, Christian S.L. Loss of CD24 in Mice Leads to Metabolic Dysfunctions and a Reduction in White Adipocyte Tissue. PLoS One. 2015:10. doi: 10.1371/journal.pone.0141966. https://doi.org/10.1371/journal.pone.0141966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatemoto K, Hosoya M, habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res commun. 1998;251:471–6. doi: 10.1006/bbrc.1998.9489. https://doi.org/10.1006/bbrc.1998.9489. PMid:9792798. [DOI] [PubMed] [Google Scholar]
- 10.Habata Y, Fujii R, Hosoya M, Fukusumi S, Kawamata Y, Hinuma S, Kitada C, Nishizawa N, Murosaki S, Kurokawa T, Onda H, Tatemoto K, Fujino M. Apelin, the natural ligand of the orphan receptor APJ is abundantly secreted in the colostrum. Biochim Biophys Acta. 1999;1452:25–35. doi: 10.1016/s0167-4889(99)00114-7. https://doi.org/10.1016/S0167-4889(99)00114-7. [DOI] [PubMed] [Google Scholar]
- 11.Ladeiras-Lopes R, Ferreira-Martins J, Leite-Moreira AF. The apelinergic system: the role played in human physiology and pathology and potential therapeutic applications. Arq Bras Cardiol. 2008;90(5):343–9. doi: 10.1590/s0066-782x2008000500012. PMid:18516406. [DOI] [PubMed] [Google Scholar]
- 12.Rittig K, Hildebrandt U, Thamer C, Staiger H, Peter A, Stefan N, Fritsche A, Häring HU, Balletshofer BM, Siegel-Axel D. Apelin serum levels are not associated with early atherosclerosis or fat distribution in young subjects with increased risk for type 2 diabetes. Exp Clin Endocrinol Diabetes. 2011;119(6):358–61. doi: 10.1055/s-0030-1268466. https://doi.org/10.1055/s-0030-1268466. PMid:21264801. [DOI] [PubMed] [Google Scholar]
- 13.Ma WY, Yu TY, Wei JN, Hung CS, Lin MS, Liao YJ, et al. Plasma apelin: a novel biomarker for predicting diabetes. Clin Chim Acta. 2014;435:18–23. doi: 10.1016/j.cca.2014.03.030. https://doi.org/10.1016/j.cca.2014.03.030. PMid:24721640. [DOI] [PubMed] [Google Scholar]
- 14.Yu S, Zhang Y, Li M.Z, Xu H, Wang Q, Song J, Lin P, Zhang L, Liu Q, Huang Q. X, Wang K, Hou W.K. Chemerin and apelin are positively correlated with inflammation in obese type 2 diabetic patients. Chin Med J (Engl) 2012;125:3440–3444. [PubMed] [Google Scholar]
- 15.Attane C, Daviaud D, Dray C, et al. Apelin stimulates glucose uptake but not lipolysis in human adipose tissue ex vivo. J Mol Endocrinol. 2011;46:21–8. doi: 10.1677/JME-10-0105. https://doi.org/10.1677/JME-10-0105. PMid:21062936. [DOI] [PubMed] [Google Scholar]
- 16.Members of the WHO Multicenter Growth Reference Study Group (WMGRS) WHO child growth standards: length/height-forage, weight-for-age, weight-for-length, weight-for-height and body mass index for age: methods and development. WHO Press. WHO Child Growth Standards. Available in http://www.who.int/childgrowth/standards/Technical_report.pdf .
- 17.Friedwald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Showell PJ, Matters DJ, Long JM, Carr Smith HD, Bradwell AR. Evaluation of latex-enhanced nephelometric reagents for measuring free immunoglobulin light-chains on a modified MININEPHTM. Clin Chem. 2002;48(Suppl):A22, E. [Google Scholar]
- 19.Comper WD, Osicka TM, Jerums G. High prevalence of immuno-unreactive intact albumin in urine of diabetic patients. Am J Kidney Dis. 2003;41:336–342. doi: 10.1053/ajkd.2003.50041. https://doi.org/10.1053/ajkd.2003.50041. PMid:12552494. [DOI] [PubMed] [Google Scholar]
- 20.DallaPozza R, Ehringer-Schetitska D, Fritsch P, Jokinen E, Petropoulos A, Oberhoffer R, Association for European Paediatric Cardiology Working Group Cardiovascular Prevention Intima media thickness measurement in children: A statement from the Association for European Paediatric Cardiology (AEPC) Working Group on Cardiovascular Prevention endorsed by the Association for European Paediatric Cardiology. Atherosclerosis. 2015;238(2):380–7. doi: 10.1016/j.atherosclerosis.2014.12.029. https://doi.org/10.1016/j.atherosclerosis.2014.12.029. PMid:25555270. [DOI] [PubMed] [Google Scholar]
- 21.Alexiadou K, Kokkinos A, Liatis S, Perrea D, Katsilambros N, Tentolouris N. Differences in plasma apelin and visfatin levels between patients with type 1 diabetes mellitus and healthy subjects and response after acute hyperglycemia and insulin administration. Hormones (Athens) 2012;11:444–450. doi: 10.14310/horm.2002.1376. https://doi.org/10.14310/horm.2002.1376. [DOI] [PubMed] [Google Scholar]
- 22.Habchi M, Duvillard L, Cottet V, Brindisi M C, Bouillet B, Beacco M, et al. Circulating apelin is increased in patients with type 1 or type 2 dia-betes and is associated with better glycaemic control. Clin Endocrinol (Oxf) 2014;81:696–701. doi: 10.1111/cen.12404. https://doi.org/10.1111/cen.12404. PMid:24417455. [DOI] [PubMed] [Google Scholar]
- 23.Dray C, Sakar Y, Vinel C, Daviaud D, Masri B, Garrigues L, et al. The intestinal glucose-apelin cycle controls carbohydrate absorption in mice. Gastroenterology. 2013;144:771–780. doi: 10.1053/j.gastro.2013.01.004. https://doi.org/10.1053/j.gastro.2013.01.004. PMid:23313268. [DOI] [PubMed] [Google Scholar]
- 24.Fin AV, Kolodgie VR. Correlation between carotid intima-media thickness and atherosclerosis: a point of view from pathology. Atheriscler Throm Vasc Biol. 2010;30:177–81. doi: 10.1161/ATVBAHA.108.173609. https://doi.org/10.1161/ATVBAHA.108.173609. PMid:19679833. [DOI] [PubMed] [Google Scholar]
- 25.Nascimento A, Sequeira I, Vasconcelos D, Gandolfi L, Pratesi R, Nóbrega Y. Endothelial dysfunction in children with type 1 diabetesmellitus. Arch Endocrinol Metab. 2017;260 doi: 10.1590/2359-3997000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo C, Liu Y, Zhao W, Wei S, Zhang X, Wang W, Zeng X. Apelin promotes diabetic nephropathy by inducing podocyte dysfunction via inhibiting proteasome activities. J Cell Mol Med. 2015;19:2273–2285. doi: 10.1111/jcmm.12619. PMid:26103809. PMCid: PMC4568931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yue P, Jin H, Xu S, Aillaud M, Deng AC, Azuma J, et al. Apelin decreases lipolysis via Gq, Gi, and AMPK-Dependent mechanisms. Endocrinology. 2011;152:59–68. doi: 10.1210/en.2010-0576. https://doi.org/10.1210/en.2010-0576. PMid:21047945. PMCid: PMC3033059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Than A, Cheng Y, Foh LC, Leow MK, Lim SC, Chuah YJ, et al. Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol. Cell. Endocrinol. 2012;362:227–241. doi: 10.1016/j.mce.2012.07.002. https://doi.org/10.1016/j.mce.2012.07.002. PMid:22842084. [DOI] [PubMed] [Google Scholar]


