Abstract
BACKGROUND:
Human immunodeficiency virus (HIV) infection is an epidemic worldwide, despite the marked benefits of antiretroviral therapy (ARV) in reducing severe HIV-associated dementia. A milder form of neurocognitive disorders are still prevalent and remain a challenge.
AIM:
This study aimed to determine the correlation between plasma cluster of differentiation 4 (CD4) lymphocyte, duration of ARV treatment, opportunistic infections, and cognitive function in HIV-AIDS patients.
METHODS:
A cross-sectional study involving 85 HIV-AIDS patients was conducted at Adam Malik General Hospital Medan, Indonesia. All subjects were subjected to physical, neurologic examination and Montreal Cognitive Assessment-Indonesian Version (MoCA-INA) to assess cognitive function and measurement of lymphocyte CD4 counts.
RESULTS:
Out of the 85 subjects evaluated, the proportion concerning sexes include 52 males (61.2 %) and 33 females (38.8%). The mean age was 38.53 ± 9.77 years old. There was a significant correlation between CD4 lymphocyte counts and MoCA-INA score (r = 0.271, p = 0.012), but there was no significant correlation between duration of ARV treatment and MoCA-INA score. There was also no difference in MoCA-INA score based on the presence of opportunistic infection.
CONCLUSION:
Lymphocyte CD4 count was independently correlated with cognitive function in HIV-AIDS patients.
Keywords: CD4, HIV – AIDS, Cognitive function
Introduction
Human immunodeficiency virus (HIV) infection is a global epidemic. According to estimates from the 2017 UNAIDS report on the global Acquired Immunodeficiency Syndrome (AIDS) epidemic, there were approximately 36.7 million people living with HIV worldwide [1]. There were 15,534 people with HIV infection and 1700 AIDS cases at the end of 2014 in Indonesia, with a cumulative number from April 1987 to 2014 stands at 142,950 HIV cases and 55,623 AIDS cases. The cumulative number of HIV-AIDS cases in Sumatera Utara province ranked tenth in Indonesia, with 8,794 HIV cases and 1,573 AIDS cases at the end of 2014, with a prevalence of 12.12 per 100.000 population [2].
Neurologic disorders in HIV infection can be divided into primary and secondary disorders. The secondary disorders are caused by opportunistic infections resulting from immunosuppression and have become less frequently found since the establishment of antiretroviral (ARV) treatment. The primary disorders, including HIV-associated neurocognitive disorders (HAND) representing the deleterious effects of HIV on brain [3] [4] [5] There are three severity categories for HAND: HIV-associated asymptomatic neurocognitive impairment (ANI), HIV -associated mild neurocognitive disorder (MND), and HIV-associated dementia (HAD). Although the use of ARV has made severe forms of cognitive impairment less common, mild to moderate degrees of cognitive disturbances have become more prevalent and remain a challenge [6] [7] [8]. ARV has improved survival substantially and may also reverse neurocognitive deficits in many cases. The impact of recognising ANI in clinical practice and research would be to encourage more frequent neuropsychologic assessment for individuals with ANI, to detect early cognitive changes, functional impairments and potential transition to MND or HAD. It might also promote the initiation of ARV independent of CD4 count or plasma HIV RNA levels, especially in the developing countries where measurement of plasma viral load are not readily available, or in a limited resource setting [8].
This study aimed to determine the correlation between plasma CD4 lymphocyte count, ARV duration, opportunistic infection and cognitive function in HIV-AIDS patients.
Methods
This was a cross-sectional study conducted in Adam Malik General Hospital Medan North Sumatera Indonesia, between June and November 2017. HIV -AIDS patients 18 yrs and above were included in this study, with the following profiles: compos mentis, speak Bahasa Indonesia fluently, ability to read and write, and gave written consent. We excluded patients who had psychiatric disorders, aphasia, dementia, traumatic brain injury and central nervous system opportunistic infection. The evaluation encompassed standard physical and neurologic examination, Montreal Cognitive Assessment-Indonesian Version (MoCA-INA) and CD4 lymphocyte measurement.
All statistical procedures were performed with SPSS version 20. We used Pearson’s or Spearman correlation test to evaluate the correlation between CD4 count, age and cognitive function (MoCA-INA score), ANOVA or Kruskal Wallis to evaluate the difference on MoCA INA score based on level of education and t-test or Mann Whitney to evaluate the differences of CD4 count and ARV duration based on MoCA-INA score.
The study was performed with approval obtained from the Health Research Ethical Committee Medical Faculty of Universitas Sumatera Utara/H. Adam Malik General Hospital with number 325/TGL/KEPK FK USU-RSUP HAM/2017.
Results
Out of the 85 subjects evaluated, the proportion concerning sexes includes 52 males (61.2 %) and 33 females (38.8%). The mean age was 38.53 ± 9.77 years old, ranging from 19 to 65 years old. The mean MoCA INA score was 22.94 ± 3.38, ranging from 15 to 28. The mean CD4 counts were 432.58 ± 332.24 cells/μL. Table 1 shows characteristics of subjects.
Table 1.
Characteristics of Subjects
| Characteristic | Number (n = 85) | Percentage |
|---|---|---|
| Age (years), mean ± SD | 38.53 ± 9.77 | |
| Sex | ||
| Male | 52 | 61.2 |
| Female | 33 | 38.8 |
| Level of education | ||
| Elementary | 7 | 8.2 |
| Junior High School | 43 | 50.6 |
| High School | 25 | 29.4 |
| College/University | 10 | 11.8 |
| Occupation | ||
| Entrepreneur | 37 | 43.5 |
| Employee | 5 | 5.9 |
| Housewife | 23 | 27.1 |
| Farmer | 9 | 10.6 |
| Student | 4 | 4.7 |
| Unemployed | 7 | 8.2 |
| Ethnic | ||
| Batak | 38 | 44.7 |
| Melayu | 6 | 7.1 |
| Javanese | 18 | 21.2 |
| Tionghoa | 6 | 7.1 |
| Karo | 17 | 20.0 |
| MoCA-INA Score | ||
| ≥ 26 | 21 | 24.7 |
| < 26 | 64 | 75.3 |
| Opportunistic Infection | ||
| Yes | 60 | 70.6 |
| No | 25 | 29.4 |
| ARV duration (months), mean±SD, | 37.5 ± 25.91 | |
| MoCA-INA Score, mean± SD | 22.94 ± 3.38 | |
| Absolute CD4 count (cell/μL), mean ± SD | 432.58 ± 332.24 | |
| CD4 percentage (%), mean±SD | 18.80 ± 9.93 |
There was no significant difference on MoCA-INA score based on the level of education (Table 2). There was also no significant correlation between age and MoCA-INA score (r = 0.034, p = 0.76).
Table 2.
Comparison of MoCA-INA Scores based on the level of education
| Level of education | Mean ± SD | p |
|---|---|---|
| Elementary school | 23.14 ± 3.80 | 0.849* |
| Junior high school | 22.74 ± 3.37 | |
| High school | 23.40 ± 3.58 | |
| College | 22.50 ± 2.95 |
SD = standar deviation;
Kruskal-Wallis Test.
Table 3.
Differences in the CD4 absolute count, CD 4 percentage and ARV duration based on MoCA-INA Score
| MoCA-INA Score | Lymphocyte CD4 absolute count (cell/μL) | Lymphocyte CD4 percentage (%) | ARV Duration (months) | |||
|---|---|---|---|---|---|---|
| Mean ± SD | p | Mean ± SD | p | Mean ± SD | p | |
| Normal (n = 21) | 591.35 ± 369.33 | 0.003 | 22.94 ± 9.35 | 0.010 | 45.12 ± 38.54 | 0.914 |
| Abnormal (n = 64) | 283.00 ± 258.18 | 14.83 ± 9.29 | 38.39 ± 24.3 | |||
Mann Whitney.
The absolute lymphocyte CD4 count and lymphocyte CD4 percentage were significantly different based on MoCA INA score, but there was no significant difference in ARV duration based on MoCA-INA score.
There was a significant correlation between absolute CD4 lymphocyte count and MoCA-INA score (r = 0.271, p = 0.015) and between percentage CD4 count and MoCA-INA Score (r = 0.227, p = 0.037). Figure 1 shows the MoCA-INA Score plotted against absolute CD4 lymphocyte counts. The normal MoCA INA score should be above or equal to 26. In this study, most of the subjects had scored lower than 26 (n = 64), and from that 64 subjects, most of them had scored between 21-25, but the overall mean score was 22.94 ± 3.38.
Figure 1.
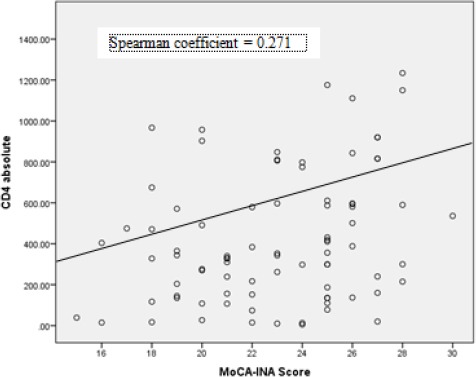
MoCA-INA Score plotted against CD4 lymphocyte counts
The duration of ARV treatment was 37.5 ± 25.91 months. There was no significant correlation between duration of ARV treatment with MoCA-INA score (r = 0.091, p = 0.407). Most of the subjects (60 patients, 70.6%) had an opportunistic infection which was lung tuberculosis. There was no significant difference on MoCA-INA score based on the presence of opportunistic infection (Table 4).
Table 4.
Comparison of MoCA-INA Scores based on the presence of opportunistic infection
| Opportunistic Infection | Mean ± SD | p |
|---|---|---|
| Yes | 23.02 ± 3.29 | 0.625 |
| No | 22.76 ± 3.64 |
Mann Whitney Test.
Discussion
Our data show that there was a significant correlation between CD4 lymphocyte count with cognitive function in HIV-AIDS patients as measured by MoCA-INA score. This correlation was independent of age and level of education. This is in line with several previous studies [9] [10] [11] [12]. Valcour et al. (2006) [9] found that after adjusting for age and duration of HIV, lowest historical CD4 was related to a current diagnosis of HAD (odds ratio 1.395 (1.106-1.761) Patients with CD4 lymphocyte below 200 cells/mm3 were considered highly vulnerable to neurological complications associated with infection, including cognitive impairment and the risk increased with further reductions in CD4 lymphocyte count [9]. Ellis et al. (2011) [10] found that lowest historical CD4 was a predictor of HIV neurocognitive impairment in the era of a combination of antiretroviral therapy [10]. A study by Childs et al. (1999) [11] found that levels of plasma CD4 counts and HIV RNA were predictive of HAD and it develops in parallel with immunodeficiency and with more advanced stages of HIV [11]. This finding could partially reflect the effect of marked immunodeficiency in HIV patients to cognitive function. The correlation between CD4 count and cognitive function was also independent of the duration of ARV treatment and the presence of opportunistic infection. A study by Reger et al. (2005) [12]. Suggested that plasma HIV RNA levels may not predict the degree of neurobehavioral disturbances in HIV infection among patients receiving ARV treatment [12]. HIV causes damage in the central nervous system through two separate but linked processes: the first is a host-derived cascade of inflammatory molecules released by activated or infected microglia, macrophages and astrocytes and the second process involves the direct neurotoxic effects of HIV-encoded proteins such as gp120, Vpr, and Tat, which also modulate transcription of HIV-1 long terminal repeat (LTR) promoter activity and affects several cellular functions [5]. The neuronal damage that occurs is likely caused by shedding viral proteins such as gp120 and Tat or indirectly through the elevated production of neurotoxic molecules released by activated astrocytes macrophages and microglia [6]. Although neurons are not directly infected by HIV, secondary neuronal damage caused by other infected cells is probably required to cause cognitive impairment [4]. HIV infection causes damage to the elaborate network of connections between neurons that take place at dendrites and synapses. This synaptodendritic injury disrupts the highly integrated functioning of neural systems that are required to process information, leading to HIV -associated neurocognitive disorders (HAND), which consist of asymptomatic neurocognitive impairment (ANI), HIV-associated mild neurocognitive disorder (MND), and HIV-associated dementia (HAD) [8]. ANI describes individuals with usually mild impairment in two or more cognitive areas, demonstrated by neuropsychological testing, without a clear effect on everyday functioning. MND refers to the presence of mild to moderate deficits in two or more cognitive areas which create at least mild interference in everyday functioning. Finally, HAD describes individuals with documented moderate to severe deficits in two or more cognitive areas, with substantial impairment in everyday functioning making the person incapable of employment and often unable to live independently [6] [8].
Our study did not classify the cognitive impairment further based on the cognitive domains. MoCA-INA was only used to assess cognitive function. It can assess several domains, including executive function, visuospatial function, attention and concentration, memory, language, calculation and orientation [13]. The Indonesian version of MoCA, namely MoCA-INA has been developed and validated in Indonesia, and so it can be used as a cognitive tool [14]. HIV infection characteristically generates a “sub-cortical” pattern of neurocognitive dysfunction with deficits predominantly affecting executive functions, speed of information processing, attention/working memory, motor speed, new learning and retrieval of new information, while long-term (semantic) memory, many language skills, and visuo -spatial abilities may remain intact [6]. The pattern of neurocognitive dysfunction, however, is not consistent across individuals and may be even less consistent across individuals from markedly different backgrounds [5]. MND is often an antecedent syndrome that can precede the onset of frank dementia but presents with the clinical hallmarks of HAD albeit with less-severe signs and symptoms and at higher CD4þ T-cell levels. Symptoms of HAD typically begin once an individual’s lymphocyte CD4 count drops below 200 cells/mL. Clinical risk factors for HAD include low CD4 T-cell levels, high viral load in cerebrospinal fluid (CSF) or plasma, anaemia, extremes of age, injection drug use, and several host genetic polymorphisms [6]. This study did not analyse the factors that could associate with CD4 counts. A study by Li et al (20110 [15] found several important modifiable risk factors were associated with lower CD4 counts, including older age (> 50 years), lower CD4 counts at the time of HAART initiation, the number of switches in HAART regimen in the first 5 years and hepatitis B and C virus infection [15].
An interesting finding from this study was the fact that the number of patients with abnormal cognitive function (MoCA-INA score < 26) was higher than the number of patients with normal cognitive function (MoCA-INA score ≥ 26), although most of the patients did not have any subjective cognitive complaint. Although this finding could be attributed to the fact that most of the patients had a level of education lower than 12 years, there were no significant differences in MoCA-INA score based on the level of education, also there was no significant correlation between age and cognitive function. This could emphasise the importance of cognitive assessment in HIV-AIDS patients to evaluate HIV effects on the CNS, to detect early cognitive changes, functional impairments and potential transition to MND or HAD.
This study concludes that CD4 lymphocyte count correlates with cognitive function in HIV-AIDS patients. This finding could reflect that immune status as marked partly by CD4 count might contribute to the development of CNS complications, including cognitive dysfunction.
Acknowledgements
We thank all the staff of Division of Tropical Infectious Diseases, Department of Internal Medicine of Faculty of Medicine Universitas Sumatera Utara/Adam Malik General Hospital and staff of Care, Support and Treatment (CST) of Adam Malik General Hospital.
This research is funded by Lembaga Penelitian Universitas Sumatera Utara according to Kontrak Penelitian TALENTA Universitas Sumatera Utara, Tahun Anggaran 2017. Nomor: 5338/UN5.1.R/PPM/2017 tanggal, 22 Mei 2017.
Footnotes
Funding: This research is funded by Lembaga Penelitian Universitas Sumatera Utara according to Kontrak Penelitian TALENTA Universitas Sumatera Utara Tahun Anggaran 2017. Nomor: 5338/UN5.1.R/PPM/2017 tanggal, 22 Mei 2017
Competing Interests: The authors have declared that no competing interests exist
References
- 1.UNAIDS. Joint United Nations Programme on HIV/AIDS 2017. Report on the global AIDS epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2017. Available from www.unaids.org/sites/default/files/media_asset/20170720_Data_book_y2017_en.pdf . [Google Scholar]
- 2.Departemen Kesehatan RI. 2014 Statistik Kasus HIV/AIDS di Indonesia sampai dengan 30 Juni 2014. Direktorat Jendral Pengendalian Penyakit dan Penyehatan Lingkungan Kemenkes RI. Jakarta. 2014 [Google Scholar]
- 3.Ellis RJ, Calero P, Stockin MD. HIV infection and the central nervous system: a primer. Neuropsychol Rev. 2009;19:144–51. doi: 10.1007/s11065-009-9094-1. https://doi.org/10.1007/s11065-009-9094-1. PMid:19415500. PMCid: PMC2690832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzales-Duarte A, Cikurel K, Simpson DM. Selected neurologic complication of HIV and antiretroviral therapy. The PRN Notebook. 2006;11(2):24–29. [Google Scholar]
- 5.Boiss L, Gill J, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin. 2008;7:799–819. doi: 10.1016/j.ncl.2008.04.002. https://doi.org/10.1016/j.ncl.2008.04.002. PMid:18657727. [DOI] [PubMed] [Google Scholar]
- 6.Robertson K, Liner J, Heaton R. Neuropsycological Assessment of HIV-Infected Populations in International Settings. Neuropsychol Rev. 2009;19:232–49. doi: 10.1007/s11065-009-9096-z. https://doi.org/10.1007/s11065-009-9096-z. PMid:19455425. PMCid: PMC2690834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cysique LA, Bain MPA, Lane T, Brew BJ. Management issues in HIV-associated neurocognitive disorders. Neurobehavioral HIV Medicine. 2012;4:63–73. https://doi.org/10.2147/NBHIV.S30466. [Google Scholar]
- 8.Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M. 2007 Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. https://doi.org/10.1212/01.WNL.0000287431.88658.8b. PMid:17914061. PMCid: PMC4472366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valcour V, Yee P, Williams AE, Shiramizu B, Watters M, Selnes O, Paul R, Shikuma C, Sacktor N. Lowest ever CD4 lymphocyte count (CD4 nadir) as a predictor of current cognitive and neurological status in HIV- 1 infection—The Hawaii Aging with HIV Cohort. J Neurovirol. 2006;12(5):387–91. doi: 10.1080/13550280600915339. https://doi.org/10.1080/13550280600915339. PMid:17065131. [DOI] [PubMed] [Google Scholar]
- 10.Ellis RJ, Badiee J, Vaida F, Letendr S, Heatona RK, Clifford D, Collier AC, Gelmand B, McArthure J, Morgellof S, McCutchana JA, Granta I. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–51. doi: 10.1097/QAD.0b013e32834a40cd. https://doi.org/10.1097/QAD.0b013e32834a40cd. PMid:21750419. PMCid: PMC3867631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Childs EA, Lyles RH, Selnes OA, Chen B, Miller EN, Cohen BA, Becker JT, Mellors J, McArthur JC. Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy. Neurology. 1999;52:607–13. doi: 10.1212/wnl.52.3.607. https://doi.org/10.1212/WNL.52.3.607. PMid:10025796. [DOI] [PubMed] [Google Scholar]
- 12.Reger MA, Martin DJ, Cole SL, Strauss G. The relationship between plasma viral load and neuropsychological functioning in HIV-1 infection. Archives of clinical neuropsychology. 2005;20(2):137–43. doi: 10.1016/j.acn.2004.03.009. https://doi.org/10.1016/j.acn.2004.03.009. PMid:15708723. [DOI] [PubMed] [Google Scholar]
- 13.Nasreddine Z.S, Phillips N.A, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. https://doi.org/10.1111/j.1532-5415.2005.53221.x. PMid:15817019. [DOI] [PubMed] [Google Scholar]
- 14.Husein N, Lumempouw S, Ramli Y. Herqutanto Uji validitas dan reliabilitas montreal cognitive assessment versi Indonesia (MoCA-Ina) untuk skrining gangguan fungsi kognitif. Neurona. 2010;27(4):15–22. [Google Scholar]
- 15.Li X, Margolick J, Jamieson B, Rinaldo C, Phair J, Jacobson L. CD4 T-cell counts and plasma HIV-1 RNA levels beyond 5 years of highly antiretroviral therapy (HAART) J Acquir Immune Defic Syndr. 2011;57(5):421–8. doi: 10.1097/QAI.0b013e31821e9f21. https://doi.org/10.1097/QAI.0b013e31821e9f21. PMid:21602699. PMCid: PMC3293185. [DOI] [PMC free article] [PubMed] [Google Scholar]


