Abstract
All of the most important guaiacol-type peroxidase (POX) isoforms accumulated in the culture medium of BY-2 tobacco (Nicotiana tabacum L. cv Bright Yellow 2) cells have been isolated. Five basic and two acidic isoforms were found. The four major isoforms (B2, B3, P1, and P2), all strongly basic, have been purified to homogeneity and partially sequenced. B2 and B3 are new isoforms showing high homology to only one POX isolated so far. Amino acid sequencing and specific activities indicated that basic isoPOXs constitute two pairs of strictly related isoforms (P1/P2 and B2/B3). Their specific activities measured in the presence of different substrates, as monolignols and NAD(P)H, indicated possible specialized functions in cell wall metabolism. Only P-type POXs were able to oxidize indoleacetic acid. Variations in pH could play a regulatory role by changing the relative contribution of different isoforms to total POX activity. Apart from cell culture medium, polyclonal antibodies obtained against P1 and P2 detected P1 in roots and in lower parts of stems. Immunocytochemical labeling indicated that P-type POXs were expressed in stem phloem and in phloem and epidermal cells of roots.
Peroxidases belong to a large family of enzymes able to oxidize several different substrates in the presence of H2O2. In higher plants the number of isoforms may be extremely high, up to 40 genes corresponding to isoperoxidases for each plant, and several other isoforms can be generated by posttranscriptional modifications (Van Engelen et al., 1991; Welinder et al., 1996). Classic plant POXs can be distinguished by their nonspecific use of phenolic derivatives and their involvement in polymerizing reactions (Eberhardt et al., 1993), whereas peroxidases of different phylogenetic origin have a higher affinity for ascorbate (Chen and Asada, 1989) or glutathione (Eshdat et al., 1997). The latter are involved as scavengers in the control of active oxygen species or in biomembrane repair (Asada, 1992). POXs also play a regulatory role in the phytohormone metabolism, because they take part in the catabolism of IAA (Gazaryan et al., 1996) and in ethylene biosynthesis (Boyer and De Jaegher, 1986).
A peculiar feature of POXs is their apparent redundancy, i.e. several isoforms can ensure the same reactions. At present, the available information does not allow the prediction of the putative in vivo function of any POX, given its amino acid sequence, or an understanding of the reason for the high number of isoforms (Esnault and Chibbar, 1997), which apparently is not substrate specific, but often is tissue and development specific (Criqui et al., 1992; Østergaard et al., 1996; Kjærsgård et al., 1997). Yet, the speculation that it could be possible to infer cell localization and function of isoPOXs from their pI values has been questioned; for instance, the presence of both basic and acidic extensin POXs in the cell culture medium has been reported (Brownleader et al., 1995; Schnabelrauch et al., 1996), and both acidic and basic POXs accumulate in protoplast culture medium (de Marco and Roubelakis-Angelakis, 1997). Interesting insights concerning the physiological roles of plant POX have been obtained by correlating the induction of specific isoforms to particular external stimuli. By using this approach, POXs that are involved in wound healing, NaCl-stress control, pathogen-defense reaction, and the hypersensitivity response have been characterized (Lagrimini, 1991; Bradley et al., 1992; Thordal-Christensen et al., 1992; Botella et al., 1994; Scott-Craig et al., 1995). POXs are also specifically expressed during protoplast regeneration and cell development (Criqui et al., 1992; de Marco and Roubelakis-Angelakis, 1996a), and they probably play a key physiological role in cell wall assembly and in the control of cell wall plasticity during cell elongation (Hoson et al., 1995). Both proteins and monolignols are polymerized by POXs (Everdeen et al., 1988; Eberhardt et al., 1993), which are also able to provide the H2O2 necessary for the reaction (Gross et al., 1977; de Marco and Roubelakis-Angelakis, 1996a). The inhibition of POX activity prevented normal cell wall reconstitution and protoplast division, even though the short-term viability of the protoplasts was not altered (de Marco and Roubelakis-Angelakis, 1996b).
The difficulty of recognizing a relationship between POXs with conserved sequences and specific physiological functions is mostly due to the reactivities that isoPOXs show toward several potential substrates, at least in in vitro conditions (Lagrimini et al., 1997). On the other hand, to our knowledge, no systematic survey has been attempted to determine the substrate specificity and in vivo reaction conditions for each isoform. Cell-suspension culture is a suitable source of POXs involved in cell wall metabolism (Buffard et al., 1990; Schnabelrauch et al., 1996; Melo et al., 1997). BY-2 tobacco (Nicotiana tabacum L. cv Bright Yellow 2) cells have been chosen because they are commonly used in cell-cycle studies (Nagata et al., 1992) and therefore several aspects of their metabolism are well known. However, their POXs have been only partially investigated (Narita et al., 1995), in spite of the possible regulatory effect of cell wall reconstitution on the rate of cell division (Cooper et al., 1994). In this paper we describe the isolation of the most representative isoPOXs accumulated in the culture medium of tobacco BY-2 cells and their biochemical characterization to determine whether they make a temporal or substrate-specific contribution to cell wall structural development.
MATERIALS AND METHODS
Plant, Cell, and Protoplast Material
Tobacco (Nicotiana tabacum cv Samsun NN) plants were grown in a greenhouse at 25°C. Protoplasts were isolated from mesophyll after 4 h of maceration in hydrolytic solution (1% cellulase R-10, 0.5% macerozyme R-10; Yakult Pharmaceutical, Tokyo, Japan) and cultured as described by Koop and Schweiger (1985). BY-2 tobacco (cv Bright Yellow 2) cells were cultured for 7 d in Murashige and Skoog basal medium (Duchefa, Haarlem, The Netherlands), pH 5.8, supplemented with 3% Suc, 1 mg/L thiamine, 100 mg/L myoinositol, 200 mg/L KH2PO4, and 0.2 mg/L 2,4-D at 25°C under constant agitation. Cell viability was tested by fluorescein diacetate.
Protein Extraction and Enzyme Assays
Leaf, stem, root, cell, and callus crude extracts were obtained by grinding tissues with a mortar and pestle in the presence of 4 volumes of extraction buffer (50 mm K-phosphate, pH 7.5, 1 mm ascorbate, 1 mm EDTA, 1 mm DTT, 0.3% Triton X-100, and 0.8 m NaCl). Homogenate was filtered through cheesecloth and centrifuged for 30 min at 12,000g, and supernatants were recovered. Protoplast and derived-cell lysates were obtained by adding 3 volumes of extraction buffer to pelleted protoplasts or cells in Eppendorf tubes and by grinding the suspension with a minipestle. Spent media from protoplast and cell cultures were concentrated by freeze drying and dialyzed against 25 mm Tris-HCl, pH 7.5, buffer. Crude extracts and lysates were washed and concentrated using Centricon-10 tubes (Amicon) and the same buffer. Resulting samples were exploited for native- and SDS-PAGE, western blotting, and determination of control POX enzymic activity using o-dianisidine as a substrate, according to the method of Church and Galston (1988). Purified isoPOXs were used to calculate specific activities in the presence of IAA, NAD(P)H, and monolignols. One unit of activity is defined as the amount of enzyme that oxidizes 1 μmol of substrate per minute in standard conditions. NADH and NADPH POX-dependent oxidation were followed in the spectrophotometer by decrease in A340 at pH 7.5 and 25°C, using 0.2 mm H2O2 and 0.15 mm NAD(P)H, and an extinction coefficient of 6.22 mm−1 cm−1 was used to calculate activity units. Sinapyl and coniferyl alcohol oxidation were followed at A272 and A264, respectively, in the presence of 20 mm K-phosphate buffer, pH 6.7, 0.3 mm monolignols, and 2 mm H2O2, at 25°C; extinction coefficient values of 8.09 and 13.09, respectively, were exploited (Sterjiades et al., 1993). IAA oxidase activity was detected at A261, according to Smith et al. (1982), by using 50 mm Na-acetate buffer, pH 5.0, 0.2 mm IAA. pH-dependent isoPOX specific activities were measured in the range of 4.2 to 7.7, using 50 mm citrate Na2HPO4 (pH 4.2–5.2), 50 mm Na-acetate (pH 5.7–6.7), or 50 mm Tris-HCl (pH 7.2–7.7) buffers.
Purification of Isoperoxidases
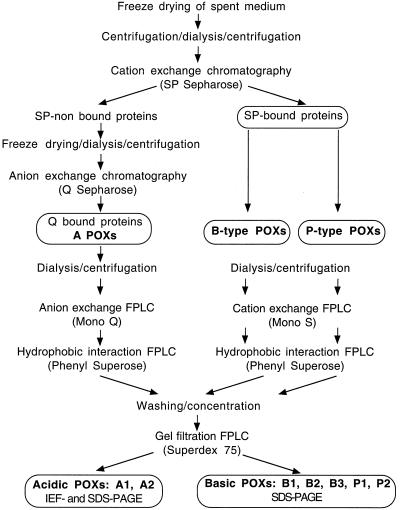
Columns and substrates used for protein purification (Q and SP Sepharose, Mono-Q, Mono-S, Phenyl-Superose, and Superdex 75) were from Pharmacia Biotech. Seven-day tobacco BY-2 cell-culture medium was recovered by filtration through nylon membrane and its volume reduced by freeze drying. Most of the pectic polysaccharides were removed by ultracentrifugation, and spent medium was dialyzed against ion-exchange chromatography buffer (25 mm Na-acetate, pH 5.2, or 25 mm Tris-HCl, pH 8.0, for SP and Q Sepharose columns, respectively) at 4°C, ultracentrifuged again to remove additional particulates, and loaded onto ion-exchange chromatography columns in the cold room. Alternatively, filtered cells were washed with water and resuspended in 0.5 m CaCl2. Ion-extracted proteins were recovered by filtration and processed as described above; no other isoPOXs were recovered, even though a different ratio among isoforms was observed. Moreover, no additional POX was found in NaCl-washed cells. Proteins were eluted with a NaCl linear gradient of 0 to 250 mm; fractions corresponding to successive peaks of POX activity were pooled separately, dialyzed overnight against the corresponding chromatography buffers, passed through a 0.22 μm protein filter (Millipore), and further purified using fast-protein liquid chromatography at room temperature. Samples separated by SP and Q Sepharose columns were loaded onto Mono-S and Mono-Q columns, respectively, and eluted with linear gradients of different NaCl concentrations (from 0 to 100 or 200 mm) to maximize peak separation.
In an alternative protocol acidic isoPOXs were recovered from the SP flow-through fraction; the sample was freeze-dried, resuspended in water, dialyzed against Q medium, and loaded onto the Q column. This method did not alter the qualitative recovery of acidic isoforms and was exploited routinely to economize cell material. Fractions corresponding to single peaks of POX activity were combined and concentrated, and ammonium sulfate was added to reach a final concentration of 1.2 m (POX B1, B2, B3, and A1) or 1.8 m (POX P1 and P2). Samples were loaded onto the Phenyl-Superose column and eluted with a descending gradient of 1.2 to 0 m or 1.8 to 0 m ammonium sulfate, respectively. POX fractions were washed, buffer exchanged, concentrated using Centricon-10 tubes (Amicon) and 25 mm Tris-HCl, pH 6.8, and 150 mm NaCl buffer. Sample solution (200 μL) was loaded onto a Superdex 75 column for a final step of purification and Mr determination. Fractions corresponding to a POX peak were pooled and stored at −20°C.
IEF-, Native-, and SDS-PAGE
Ampholine carrier ampholytes, an IEF calibration kit, and SDS-PAGE molecular-mass standards were purchased from Pharmacia Biotech Europe. IEF-PAGE was run as described by Robertson et al. (1987) over a pH range of 3.5 to 10.0. Native- and SDS-PAGE (10% when not indicated otherwise) were run according to the method of Laemmli (1970). SDS gels were silver stained (Eschenbruck and Bürch, 1982), and POXs were visualized in native and IEF gels using chloro-1-naphthol (Lagrimini and Rothstein, 1987). Glycoproteins were stained by immersion of gels in Schiff's reagent (Merck, Darmstadt, Germany) after periodic oxidation, according to the manufacturer's instructions.
Western Blotting
SDS-PAGE was run in standard conditions, and gels were removed, equilibrated in transfer buffer (25 mm Tris-HCl, pH 8.0, 192 mm Gly, and 15% methanol) for 10 min, and mounted in a transblotting sandwich using an Immobilon-P membrane (Millipore). After electrotransfer (45 min at 400 mA), membranes were stained with Ponceau S solution (Serva, Heidelberg, Germany) for protein detection. Immunodetection was performed according to the method of Geoffroy et al. (1990).
Protein Microsequencing
Purified proteins were run in standard conditions on denaturing gels, equilibrated in 10 mm 3-cyclohexylamino-1-propanesulfonic acid, pH 11.0, 10% methanol buffer, and electroblotted onto a Problott membrane (Perkin-Elmer Applied Biosystems, Courtaboeuf, France) for 30 min at 50 V, at room temperature. The membrane was washed and stained with Coomassie Blue R-250 (Merck), and protein bands were recovered for N-terminal sequencing, according to the method of Heitz et al. (1994). Partial proteolysis was used to sequence N-terminal blocked proteins. Purified proteins (30–50 μg) were run on denaturing gels and stained in Coomassie Blue R-250, and bands corresponding to the proteins were cut, soaked in 100 mm Tris-HCl, pH 6.8, 1 mm EDTA, and 0.1% SDS, and loaded into a well of a polyacrylamide denaturing gel. Into the same well was deposited 2 to 5 μg of endoproteinase Glu-C (Boehringer Mannheim) dissolved in the equilibration buffer plus 10% glycerol. The migration front was allowed to reach the interface between stacking and running (15%) gels by using standard PAGE conditions before the power was switched off. After 30 min, the products of the proteolysis reaction were separated in the running gel and transferred onto a Problott membrane, as described above. Microsequencing of peptides was performed as described by Geoffroy et al. (1990).
Antibody Preparation
A P1/P2 isoPOX mixture was dialyzed against 0.9% NaCl. Two-hundred micrograms of protein was dissolved in a final volume of 300 μL and combined with an equal volume of Freund's complete adjuvant, and the emulsion was injected subcutaneously into a rabbit. Eighty micrograms of protein was combined with Freund's incomplete adjuvant and injected intramuscularly three times (every 2 weeks for 6 weeks) to booster the antibody reaction. IgG was purified from sera by chromatography, exploiting a Fractogel EMD TA column (Merck), and stored with 20% glycerol at −20°C.
Immunocytolocalization
Tissues were fixed and subsequently embedded in paraffin and 10-μm-thick cuts were performed. After deparaffination and dehydration, samples were washed in PBS in the presence of 0.05% Triton X-100 and 1% BSA. Surfaces were saturated using the same buffer plus 5% goat serum (Boehringer Mannheim) and incubated at 4°C overnight in PBS, 0.01% Triton X-100, 1% BSA, and primary antibodies diluted 1:1000. Samples were washed in the same buffer, incubated for 1 h at room temperature in the presence of anti-rabbit alkaline phosphatase-conjugated secondary antibodies (1:1000 in PBS; Boehringer Mannheim), washed in PBS, and equilibrated with 100 mm Tris-HCl, pH 8.2. Color reaction was developed using Fast Red (Sigma) and blocked by 10 mm Tris-HCl, pH 8.0, 1 mm EDTA. Finally, samples were washed in water and mounted in glycerol. Controls had no antibodies or preimmune serum added instead of primary antibodies. Callose in cell walls of phloem cells was visualized by fluorescence with decolorized aniline blue according to the method of Currier and Strugger (1956).
Other Methods
Protein concentration was determined by the Bio-Rad protein assay kit using BSA as a protein standard. Sequence data were analyzed using the FASTA search service (Pearson and Lipman, 1988) and the Genetics Computer Group (Madison, WI) package programs (Devereux et al., 1984).
RESULTS
Purification of Tobacco IsoPOXs
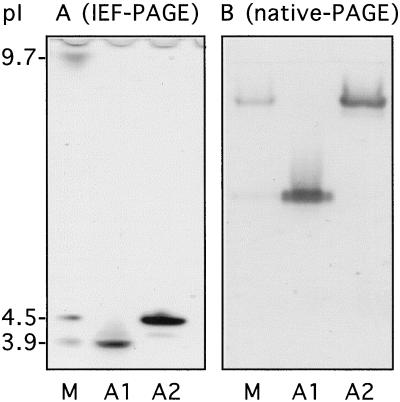
We chose to work with isoforms accumulated in BY-2 cell-suspension culture medium and to study their role in cell wall metabolism. Preliminary tests established that 7-d-old cultures gave the highest yield of POXs and that POX specific activity increased 4-fold between d 2 and 7 of cell culture, even though the pattern of expression of the isoforms did not change (data not shown). The standard protocol of purification for the different isoPOXs is described in Figure 1. Two steps of centrifugation, before and after dialysis against ion-exchange chromatography buffer, were necessary to eliminate most of the abundant polysaccharides present in the filtrate that resulted from the freeze-drying concentration of the medium. About 95% of POX activity preferentially bound to a cation exchanger (SP Sepharose column), whereas the recovery of Q Sepharose-bound fractions (anion exchanger) was very low, regardless of the protocol (standard or alternative) used for their purification (see Methods). This indicates that very basic isoforms were predominant in the cell-suspension culture medium (Table I). The two most important acidic isoforms, A1 and A2, were purified according to the protocol described in Figure 1. They had pI values of 3.9 (A1) and 4.5 (A2), respectively (Fig. 2A), and very different mobility in native gel (Fig. 2B). Even though A2 showed stronger activity than A1 using chloro-1-naphthol in native gels loaded with filtered medium (Fig. 2A), the scarce amount obtained after the Mono-Q elution step prevented any further purification. In contrast, enough A1 was available to purify an isoform of 45 kD to homogeneity (Table II; Fig. 3A).
Figure 1.
Protocol of purification for peroxidase isoforms recovered from 7-d spent medium of tobacco BY-2 cell-suspension culture. FPLC, Fast-protein liquid chromatography.
Table I.
Preliminary steps of purification of tobacco POXs from BY-2 cell-suspension culture medium
| Fractionation Step | Volume | Concentration | Total Activity | Protein | Specific Activity | Yield | Purification Factor |
|---|---|---|---|---|---|---|---|
| mL | units mL−1 | units | μg mL−1 | units μg−1 protein | % | ||
| BY-2 spent medium filtrate (Fmd) | 1000 | 125.0 | 125,000 | 167 | 0.7 | 100 | 1 |
| Dialyzed/centrifuged medium filtrate | 150 | 162.0 | 24,300 | 111 | 1.5 | 19.4 | 2.1 |
| SP-nonbound proteins | 60 | 26.5 | 1,590 | 63 | 0.4 | 1.3 | 0.6 |
| Q-bound proteins | 45 | 14.5 | 650 | 5 | 2.9 | 0.5 | 4.1 |
| SP-bound proteins | 110 | 186.0 | 20,460 | 42 | 4.4 | 16.4 | 6.3 |
Here the steps of concentration and separation between acidic (Q-bound proteins) and basic (SP-bound proteins) isoPOXs are described. POX activity was measured using o-dianisidine as a substrate, as described in Methods.
Figure 2.
POX isoforms identified in IEF- and native-PAGE. IEF-PAGE (A) and native-PAGE (B) gels were loaded with filtrate medium of 7-d tobacco BY-2 cell-suspension culture (lanes M) and purified fractions corresponding to POX acidic isoforms A1 and A2. The amounts of proteins loaded onto the IEF-PAGE gels were 1 μg (lane M) and 250 ng (lanes A1 and A2), and the amounts loaded onto the native-PAGE gels were 1 μg (lane M), 250 ng (lane A1), and 150 ng (lane A2). Peroxidase activity was revealed using chloro-1-naphthol as a substrate.
Table II.
Purification of tobacco POXs from BY-2 cell-suspension culture medium: fast-protein liquid chromatography steps
| Protein | Anionic Exchanger Mono-Q
|
Cationic Exchanger Mono-S
|
Hydrophobic-Interaction Phenyl Superose
|
Gel-Filtration Superdex 75
|
Purification Factor | ||||
|---|---|---|---|---|---|---|---|---|---|
| Elution peak | Specific activity | Elution peak | Specific activity | Elution peak | Specific activity | Molecular mass | Specific activity | ||
| mM NaCl | units μg−1 protein | mM NaCl | units μg−1 protein | m (NH4)2SO4 | units μg−1 protein | kD | units μg−1 protein | ||
| A1 | 60 | 59.1 | 0.57 | 165.3 | 45 | 180.6 | 258 | ||
| A2 | 38 | 45.2 | |||||||
| B1 | 16 | 1.7 | 0.80 | 5.6 | 33.5 | 7.1 | 10 | ||
| B2 | 46 | 23.4 | 0.44 | 93.5 | 33 | 107.8 | 154 | ||
| B3 | 68 | 74.1 | 0.59 | 122.0 | 33 | 137.2 | 196 | ||
| P1 | 130 | 112.1 | 1.36 | 133.2 | 42 | 146.4 | 209 | ||
| P2 | 143 | 168.0 | 1.09 | 198.8 | 40 | 213.7 | 305 | ||
NaCl concentrations corresponding to peaks of elution of each isoPOX are indicated, together with specific activities using o-dianisidine as a substrate, as described in Methods.
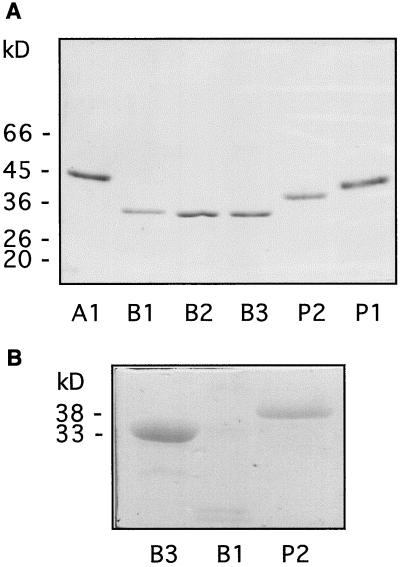
Figure 3.
Molecular masses and glycosylation state of purified POX isoforms. A, Two-hundred nanograms of purified isoPOXs A1, B1, B2, B3, P2, and P1 was visualized by silver staining after SDS-PAGE. Molecular masses of protein standards are indicated in kilodaltons. B, One microgram of partially purified B3, B1, and P2 isoPOXs was stained for their carbohydrate content after SDS-PAGE. Note that no band is visible in lane B1 at the expected position of 33.5 kD.
For basic isoforms, two peaks of activity could be resolved after cation-exchange chromatography on a SP Sepharose column. They corresponded to peak I and peaks II/III reported previously by Narita et al. (1995). Their contributions to the total POX activity were about 50% and 45%, respectively. The first peak of activity corresponded to a single band of about 33 kD in a silver-stained gel. It was not characterized further by Narita et al. (1995), probably because it contained a mixture of several proteins, as indicated by microsequencing data. Hence, fractions eluted from the SP Sepharose column that corresponded to this POX peak were recovered (B-type POXs), dialyzed, and loaded separately onto the Mono-S column. Such a protocol allowed resolution of five different peaks inside the original main peak, three of which showed POX activity: isoforms B1, B2, and B3 (Table II). Hydrophobic affinity and gel filtration allowed us to purify these isoPOXs to homogeneity (Fig. 3A). B1 had a molecular mass of 33.5 kD, whereas B2 and B3 had a molecular mass of 33 kD (Table II). B2 was the only isoform able to enter an IEF gel, because it had a pI of 9.7 (Fig. 2A). In contrast to B2 and B3, B1 was a minor isoform and its amount was not sufficient for microsequencing. It was the only basic isoPOX that showed no staining after treatment with Schiff's reagent (Fig. 3B), indicating that its degree of glycosylation was lower than that of B- and P-type isoPOXs or that it was not glycosylated. As shown previously (Narita et al., 1995), two other basic isoPOXs could be isolated from the second peak resulting from SP Sepharose elution (P-type POXs) and purified (Fig. 1). The molecular masses of P1 and P2 were 42 and 40 kD, respectively, as calculated from gel filtration, and 40 and 38 kD, respectively, after comparison with Mr standards on SDS gels (Table II; Fig. 3A).
Microsequencing of IsoPOXs B2, B3, P1, and P2
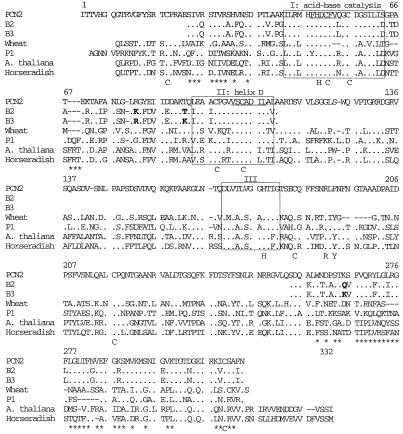
B2, B3, and A1 were N blocked; this feature is quite frequent in POXs, due to a cyclization of a Gln in position 1 to pyroglutamate (Kjærsgård et al., 1997). B2 and B3 were partially digested to obtain peptides that could be sequenced. Sequences of 83 and 60 amino acids located at their N and C termini, respectively, were determined for each isoPOX, which represent almost one-half of the length of a common POX (Fig. 4). The two isoforms were very similar; they differed in only 3 amino acids over the available sequence. B2 and B3 have not been described and were very close to a POX isolated from peanut cell-culture medium (Buffard et al., 1990): 68% identity and 84% similarity in the N-terminal region; 77% identity and 92% similarity in the C-terminal region (Fig. 4). B2 and B3 showed less identity to other basic POXs, such as wheat POX, P1/P2, Arabidopsis, and horseradish POXs (about 50% identity and 70% similarity in the N-terminal region; about 40% identity and between 51% and 59% similarity in the C-terminal region). P1 and P2 were N-terminal microsequenced for 48 and 42 amino acids, respectively. They showed perfect homology to the predicted amino acid sequences corresponding to the previously reported clones D42064 and D42065 (Narita et al., 1995), except that they contained an additional Val in position 23 of the mature protein that was not reported by Narita et al. (1995).
Figure 4.
Amino acid sequence comparison between newly identified B2 and B3 tobacco POXs and other basic plant POXs. Alignment of the two tobacco POXs B2 and B3 is with peanut PCN2 (Buffard et al., 1990), wheat (Hertig et al., 1991), tobacco P1 (Narita et al., 1995; this work), Arabidopsis ATPEa (Intapruk et al., 1991), and horseradish (Fujiyama et al., 1988) POXs. Identical amino acids are indicated by dots, and gaps introduced to improve alignment and are indicated by dashes. Boxed regions correspond to conserved regions around His residues (I and III) and around helix D, according to the method of Buffard et al. (1990). Conserved His (H) and Cys (C) residues are indicated below POX sequences, as is the putative substrate-binding site (R and Y). Regions conserved in PCN2, B2, and B3 are underlined with asterisks. The accession numbers for the amino acid sequences of tobacco POXs B2 and B3 are P81512 and P81513, respectively.
The two newly characterized POXs, B2 and B3, showed conservation of amino acids essential for either catalytic activity or tertiary structure (Fig. 4): Cys and His residues, which belong to the active site; the acid-base catalysis region, including the functional His; and helix D according to Buffard et al. (1990), which seems to be important for tertiary structure (Welinder, 1985). Sequence comparison between B2, B3, and the PCN2 POX isolated from peanut (Buffard et al., 1990) showed remarkable homology in sequences usually not conserved among POXs, especially in the C-terminal region. A peculiar feature of B2/B3 is the presence of five Gly residues between positions 273 and 287, which may confer an extremely high flexibility on their spatial structures (Fig. 4).
pH Dependence of IsoPOX Activity
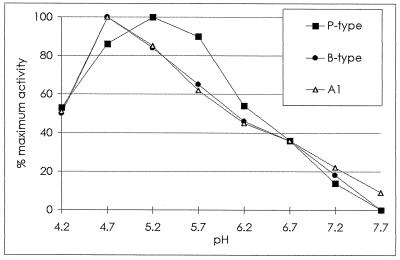
The activities of the four major basic isoPOXs (B2, B3, P1, and P2) and that of A1 were measured while varying the pH of the buffer in the reaction mixture from 4.2 to 7.7. B2/B3 isoPOXs and A1 had a maximum of activity at pH 4.7, and A1 conserved a low activity at slightly alkaline pH values (Fig. 5). P1/P2 showed a wider range of suitable pH values than B2/B3 and A1 POXs, with a maximum of activity at pH 5.2.
Figure 5.
pH-dependent activity of A1 (▵), B-type (•), and P-type (▪) POXs. Specific activities were measured as described in Methods. Results are expressed as a percentage of maximum activity arbitrarily fixed to 100 for each type of peroxidase. Values reported are the average of three independent experiments, and relative sd values are not higher than 7%.
Substrate Specificity of IsoPOXs in Vitro
Three types of substrates were tested: (a) NADP and NADPH, because POXs can trigger the nonenzymic production of H2O2 in the presence of NAD(P)H, which is later used as a substrate for the oxidation of phenolic groups (Halliwell, 1978); (b) monolignols, such as coniferyl and sinapyl alcohol, which are polymerized in secondary cell walls (Eberhardt et al., 1993); and (c) IAA, which POXs may catabolize in vivo (Gazaryan et al., 1996). The activity of B1 was barely detectable and then only in the presence of monolignols (Table III). The other purified POXs could use both NAD(P)H and monolignols as the substrates. A1 had the highest specific activity with both NAD(P)H and monolignols compared with B- and P-type isoPOXs (Table III). A fraction enriched in A2 and containing negligible contamination due to other POXs (Fig. 2) showed relatively low affinity for NAD(P)H, but a higher specific activity with monolignols than other isoPOXs (data not shown). P-type isoPOXs showed higher affinity for NADPH than B-type isoPOXs, which, in contrast, could use monolignols with more elevated efficiency than P-type isoPOXs (Table III). Furthermore, P-type isoPOXs were the only ones that possessed IAA oxidase activity (Table III).
Table III.
Specific activities of tobacco POXs purified from BY-2 cell-suspension culture medium in the presence of different substrates
| Protein | NADH | NADPH | Coniferyl Alcohol | Sinapyl Alcohol | IAA |
|---|---|---|---|---|---|
| units μg−1 protein | change in optical density min−1 μg−1 protein | ||||
| A1 | 72.3 | 218.7 | 62.3 | 40.8 | 0 |
| B1 | 0 | 0 | 2.0 | 0.4 | 0 |
| B2 | 32.9 | 63.6 | 31.0 | 15.5 | 0 |
| B3 | 38.6 | 77.2 | 33.6 | 19.8 | 0 |
| P1 | 36.6 | 104.2 | 11.9 | 3.6 | 2.4 |
| P2 | 41.8 | 102.6 | 15.6 | 4.7 | 5.6 |
The specific activities of purified POXs A1, B1, B2, B3, P1, and P2 were measured in vitro as described in Methods.
Immunodetection Using Polyclonal Antibodies against P1 and P2
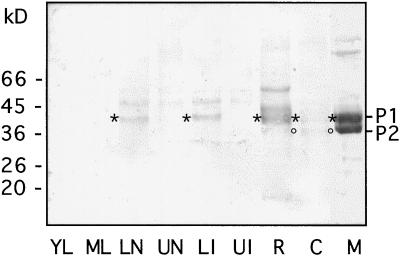
A mixture of the strictly related isoPOXs P1 and P2 was used as antigen for the production of polyclonal antibodies. Protein extracts from different plant organs and from BY2 cells were analyzed by western blotting. Specific signals corresponding to P1 could be detected in the lower parts of stems, both nodes and internodes, as well as in roots (Fig. 6, lanes LN, LI, and R). No signal could be detected in leaves and in the upper parts of stems (Fig. 6, lanes YL, ML, UN, and UI). Finally, weak signals corresponding to P1 and P2 appeared in the cell extract, whereas very strong signals were detected in the spent medium (Fig. 6, lanes C and M). It should be noted that the amount of protein loaded in lane M was only one-tenth of that loaded in the other lanes. P2 was also detected in extracts from BY2 calli grown on solid medium, whereas only a faint signal corresponding to P1 appeared (data not shown). The other weak signals detected in the different samples may correspond to other POXs sharing common epitopes with P-type isoPOXs. Regenerating protoplasts isolated from tobacco leaves were used to determine if P-type isoPOXs accumulated at specific times during culture. Separate aliquots of both protoplasts and their corresponding media were recovered daily during the 1st week of culture and used for western blotting, but no protein was recognized by anti-P-type antibodies (data not shown). In conclusion, apart from BY2 cells and culture medium, the presence of the P1 protein was detected in roots and in stems, whereas the P2 protein could not be found.
Figure 6.
Detection of P-type isoPOXs in extracts of different plant organs and BY-2 cells and in cell-suspension culture medium. Soluble proteins were extracted from young leaves (lane YL), mature leaves (lane ML), lower stem nodes (lane LN), upper stem nodes (lane UN), lower stem internodes (lane LI), upper stem internodes (lane UI), roots (lane R), and 7-d BY-2 cells (lane C). Ten micrograms of these extracts was analyzed by western blotting together with an aliquot of cell-suspension culture medium (lane M) containing 1.2 μg of protein. Polyclonal antibodies raised against P-type isoPOXs were used; the asterisks and circles indicate the positions of signals corresponding to P1 and P2, respectively. The control with preimmune serum did not show any signal.
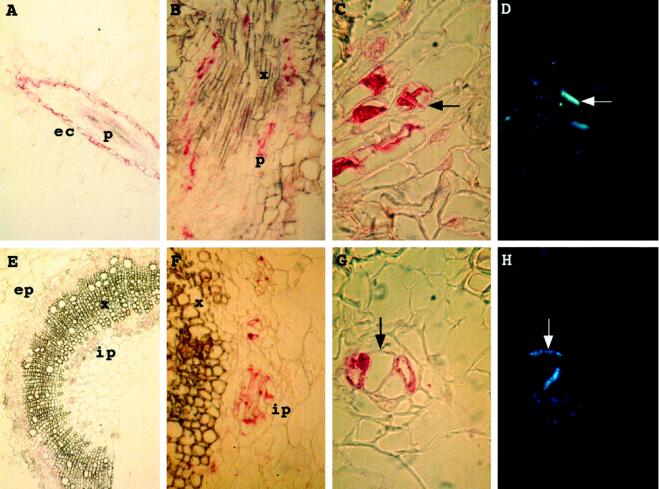
Immunocytolocalization experiments were then performed on root and stem sections (Fig. 7). In roots a signal was detected in epidermal cells and in phloem cells surrounding xylem sieves (Fig. 7, A and B). In stems a signal was detected in both internal and external phloem (Fig. 7, E and F). Staining of root and stem sections with aniline blue showed the presence of callose in the walls of the groups of cells labeled by the antibodies (Fig. 7, compare C and D, and G and H). This confirmed that these cells were phloem cells, either companion cells or phloem sieves. Results from the western blotting suggest that these signals probably reveal the presence of P1 and/or of related peroxidases.
Figure 7.
Immunocytolocalization assays using anti-P-type POX antibodies. A, Root longitudinal section; B, close-up of root longitudinal section; C, close-up of transversal root section; D, same as C but stained with aniline blue; E, stem transversal section; F, close-up of stem section shown in E; G, close-up of stem transversal section; and H, same as G but stained with aniline blue. ec, Epidermal cells; ep, external phloem; ip, internal phloem; p, phloem; and x, xylem. Arrows indicate the localization of walls visualized by fluorescence with aniline blue.
DISCUSSION
Cell wall metabolism is very important for protoplast and cell development, and POXs are considered to play a primary role in polymerizing structural elements and regulating cell wall elasticity during cell elongation (Hoson et al., 1995). POX specific activity increases several times in culture media of both regenerating protoplasts (de Marco and Roubelakis-Angelakis, 1997) and cell-suspension cultures (this work). In this paper we have described the purification to homogeneity and biochemical characterization of six isoPOXs and the partial purification of another one from the spent medium of tobacco BY-2-cultured cells. These represent all of the most important POXs detectable in the medium. The data concerning amino acid sequences and specific activities indicate that two pairs of strictly related basic isoforms (P1/P2 and B2/B3) are present, representing more than 95% of the total POX activity. In particular, B2 and B3 have not been described previously and have a quite peculiar sequence, for which a high homology has been found only to a POX recovered from peanut culture medium (Buffard et al., 1990; Breda et al., 1993). P1 and P2 were isolated previously (Narita et al., 1995), but scant biochemical information was available from that study. The existence of two very similar isoforms for P- and B-type isoPOXs might be explained by the phylogenesis of N. tabacum, which is an amphidiploid originating from an interspecific hybridization between Nicotiana sylvestris and Nicotiana tomentosiformis (Goodspeed, 1954). We also propose that the basic isoform B1, which has a much lower degree of glycosylation than B- and P-type isoPOXs, is a transient degradation product of another POX that is rapidly digested after loss of the carbohydrate protection (Marañon and van Huystee, 1994). In fact, it does not accumulate in the medium and its activity is very low and detectable only with substrates of simple structure. This may be due to partial unfolding of the protein, which may cause loss of specialized activity, as suggested for the pI-4.6 extensin peroxidase upon storage (Schnabelrauch et al., 1996). It is interesting that its Mr corresponds to the Mr of P1 apoprotein. Electrophoretic mobility and pI values show an evident heterogeneity between the two acidic isoPOXs, but their scarcity and the blocking of the N terminus did not allow sequencing. A1 has been characterized biochemically, whereas for A2 it was only possible to measure a higher affinity for monolignols than for the other substrates with respect to P-type isoPOXs.
To date, only limited evidence has accumulated to suggest that POXs can discriminate among classes of potential substrates (Brisson et al., 1994; Schnabelrauch et al., 1996), and the simple observation of an isoPOX pattern of expression under the action of different factors is not sufficient to suggest specific physiological roles for any POX. In fact, different stimuli believed to influence cell wall metabolism induce different sets of isoPOXs. To our knowledge, this is the first systematic survey in which substrate and pH specificities were recognizable for isoPOXs expressed under a specific condition. Except for B1, the isolated POXs were able to use both NAD(P)H and monolignols as the substrates in vitro, but a certain specificity and a different affinity were recognizable. A1 showed the highest specific activities. P-type isoPOXs had higher affinity for NADPH than for monolignols, compared with B-type isoPOXs, and were the only isoforms able to oxidize IAA. It was already shown that POXs are able to use NAD(P)H to produce H2O2 (Halliwell, 1978), the limiting cosubstrate in the polymerizing activity, and can control cell elongation by modulating the IAA concentration. In fact, auxin stimulates cell wall glycosidases responsible for cell wall carbohydrate breakdown (Hoson et al., 1995) and induces cell swelling by stimulating monovalent cation uptake (Keller and Van Volkenburgh, 1996). Even though pH optima were similar among isoPOXs, P-type isoPOXs had almost a 50% higher specific activity than B-type isoPOXs and A1 at pH 5.7 (the pH value of culture medium), and only A1 retained low activity at neutral pH values. Therefore, small variations in pH values could represent efficient regulatory means in vivo to shift optimal conditions from one POX to another and thereby favor the different processes connected to cell wall metabolism and preferentially catalyzed by specialized isoPOXs.
Our results also suggest that different isoPOXs could ensure specialized activities related to specific steps in cell wall metabolism. In a previous work (Narita et al., 1995), an attempt was made to correlate isoPOX expression and successive steps of cell wall metabolism during cell culture, but the cultured cells were not synchronized and, therefore, the results explained the relative accumulation of isoPOXs rather than a strict relationship with specific phases of cell wall reconstitution. Protoplasts are a more homogeneous material than cells, and we tried to determine if P-type POXs were expressed during specific steps of the regeneration of the cell wall around protoplasts. Unfortunately, no protein was recognized by antibodies in protoplast extracts and media recovered daily during the 1st week of culture. Actually, protoplasts, cells, and their corresponding culture media shared only a few of the POXs visualized in IEF gels (data not shown). Cultured cells probably need different POXs to ensure cell division and elongation compared with protoplasts, which have to completely reconstitute cell wall structure.
POXs may be regulated during development or in response to wounding or pathogen attack (Mohan et al., 1993; Intapruk et al., 1994; Klotz et al., 1998). Immunotests performed with extracts from different plant tissues confirmed that P-type isoPOXs are preferentially expressed in culture media, but also showed that P1 and/or POXs related to the P type are expressed in roots and in the lower parts of stems. The immunocytolocalizations showed that POXs related to the P type accumulate in the phloem and epidermal root cells, as well as in the internal and external phloem of stems. This localization, together with results from the analysis of substrate specificity in vitro detailed above, suggest that these enzymes are probably not involved in lignification. The P-type isoPOXs, rather, may contribute to strengthening of the walls of phloem and epidermal cells. This type of tissue-specific expression differs from that described previously for other POX genes. The tomato anionic peroxidase gene (tap1) was shown to be expressed in the epidermis and trichomes in the aerial parts of plants, as well as in stem nodes at the level of leaf traces (Mohan et al., 1993). The tobacco anionic peroxidase was expressed in epidermis and trichomes at nearly all developmental stages, as well as in ground tissues and parenchyma cells associated with vascular tissues (Klotz et al., 1998). The Arabidopsis prxCa gene was found to be expressed in all organs, and especially in root xylem, whereas the prxEa gene encoding a cationic peroxidase was expressed in phloem and in cortical root cells (Intapruk et al., 1994). Together, these results suggest that each type of POX is expressed in a specific way and plays a particular role during development. However, POX antisense transformed plants did not show apparent morphological differences (Sherf et al., 1993; Lagrimini et al., 1997), and these results have been explained by suggesting that a higher activity/amount of nonspecialized POX was able to recover the lost specialized enzymic contribution.
The accession numbers for the sequences reported in this article are P81512 (B2) and P81513 (B3).
ACKNOWLEDGMENTS
The authors thank Dr. Roberte Bronner for her assistance in microscopy, Pierrette Geoffroy for her collaboration with fast-protein liquid chromatography, and Monique Le Ret for microsequencing of proteins.
Abbreviation:
- POX
guaiacol-type peroxidase
Footnotes
This work was supported by the Centre National de la Recherche Scientifique, by a fellowship from the Consiglio Nazionale delle Ricerche in Rome to A.d.M., and by a grant from the Ministère de l'Éducation Nationale de la Recherche et de la Technologie to P.G.
LITERATURE CITED
- Asada K. Ascorbate peroxidase: a hydrogen peroxide-scavenging enzyme in plants. Physiol Plant. 1992;85:235–241. [Google Scholar]
- Botella MA, Quesada MA, Kononowicz AK, Bressan RA, Pliego F, Hasagawa PM, Valpuesta V. Characterization and in situ localization of a salt-induced tomato peroxidase mRNA. Plant Mol Biol. 1994;25:105–114. doi: 10.1007/BF00024202. [DOI] [PubMed] [Google Scholar]
- Boyer N, De Jaegher G (1986) Direct or indirect role of peroxidases in ethylene biosynthesis? In H Greppin, C Penel, T Gaspar, eds, Molecular and Physiological Aspects of Plant Peroxidases. University of Geneva Press, Geneva, pp 47–60
- Bradley DJ, Kjellbom P, Lamb CJ. Elicitor- and wound-induced oxidative cross-linking of a plant cell wall proline-rich protein: a novel, rapid defense response. Cell. 1992;70:21–30. doi: 10.1016/0092-8674(92)90530-p. [DOI] [PubMed] [Google Scholar]
- Breda C, Buffard D, van Huystee RB, Esnault R. Differential expression of two peanut peroxidase cDNA clones in peanut plants and cells in suspension culture in response to stress. Plant Cell Rep. 1993;12:268–272. doi: 10.1007/BF00237133. [DOI] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb C. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell. 1994;6:1703–1712. doi: 10.1105/tpc.6.12.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownleader MD, Ahmed N, Trevan M, Chaplin MF, Dey PM. Purification and partial characterization of tomato extensin peroxidase. Plant Physiol. 1995;109:1115–1123. doi: 10.1104/pp.109.3.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffard D, Breda C, van Huystee RB, Asemota O, Pierre M, Dang Ha DB, Esnault R. Molecular cloning of complementary DNAs encoding two cationic peroxidases from cultivated peanut cells. Proc Natl Acad Sci USA. 1990;87:8874–8878. doi: 10.1073/pnas.87.22.8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G-H, Asada K. Ascorbate peroxidase in tea leaves: occurrence of two isozymes and their differences in enzymatic and molecular properties. Plant Cell Physiol. 1989;30:987–998. [Google Scholar]
- Church DL, Galston AW. 4-Coumarate coenzyme A ligase and isoperoxidase expression in Zinnia mesophyll cells induced to differentiate into tracheary elements. Plant Physiol. 1988;88:679–684. doi: 10.1104/pp.88.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JB, Heuser JE, Varner JE. 3,4-Dehydroproline inhibits cell wall assembly and cell division in tobacco protoplasts. Plant Physiol. 1994;104:747–754. doi: 10.1104/pp.104.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criqui M-C, Plesse B, Durr A, Marbach J, Parmentier Y, Jamet E, Fleck J. Characterization of genes expressed in mesophyll protoplasts of Nicotiana sylvestris before the re-initiation of the DNA replicational activity. Mech Dev. 1992;38:121–132. doi: 10.1016/0925-4773(92)90004-4. [DOI] [PubMed] [Google Scholar]
- Currier H, Strugger S. Aniline blue and fluorescence microscopy of callose in bulbscales of Allium cepa L. Protoplasma. 1956;45:552–559. [Google Scholar]
- de Marco A, Roubelakis-Angelakis KA. The complexity of enzymic control of hydrogen peroxide may affect the regeneration potential of plant protoplasts. Plant Physiol. 1996a;110:137–145. doi: 10.1104/pp.110.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marco A, Roubelakis-Angelakis KA. Hydrogen peroxide plays a bivalent role in the regeneration of protoplasts. J Plant Physiol. 1996b;149:109–114. doi: 10.1104/pp.110.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marco A, Roubelakis-Angelakis KA. Laccase activity could contribute to cell-wall reconstitution in regenerating protoplasts. Phytochemistry. 1997;46:421–425. doi: 10.1016/s0031-9422(97)00301-4. [DOI] [PubMed] [Google Scholar]
- Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt TL, Bernards MA, He L, Davin LB, Wooten JB, Lewis NG. Lignification in cell suspension cultures of Pinus taeda. J Biol Chem. 1993;28:21088–21096. [PubMed] [Google Scholar]
- Eschenbruck M, Bürch RM. Experimentally improved relationship of ultrasensitive silver staining of protein in polyacrylamide gels. Anal Biochem. 1982;125:96–99. doi: 10.1016/0003-2697(82)90387-6. [DOI] [PubMed] [Google Scholar]
- Eshdat Y, Holland D, Faltin Z, Ben-Hayyim G. Plant glutathione peroxidases. Physiol Plant. 1997;100:234–240. [Google Scholar]
- Esnault R, Chibbar RN. Peroxidases: at the gene expression level. Plant Peroxidase Newsletter. 1997;10:7–14. [Google Scholar]
- Everdeen DS, Kiefer S, Willard JJ, Muldoon EP, Dey PM, Li X-B, Lamport DTA. Enzymic crosslinkage of monomeric extensin precursors in vitro. Plant Physiol. 1988;87:616–621. doi: 10.1104/pp.87.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama K, Takemura H, Shibayama S, Kobayashi K, Choi JK, Shinmio A, Takano M, Yamada Y, Okada M. Structure of the horseradish peroxidase isozyme C genes. Eur J Biochem. 1988;73:681–687. doi: 10.1111/j.1432-1033.1988.tb14052.x. [DOI] [PubMed] [Google Scholar]
- Gazaryan IG, Lagrimini LM, Ashby GA, Thorneley R. The mechanism of indole-3-acetic acid oxidation by plant peroxidases: anaerobic stopped-flow spectrophotometric studies on horseradish and tobacco peroxidases. Biochem J. 1996;313:841–847. doi: 10.1042/bj3130841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy P, Legrand M, Fritig B. Isolation and characterization of a proteinaceous inhibitor of microbial proteinases induced during the hypersensitive reaction of tobacco to tobacco mosaic virus. Mol Plant Microbe Interact. 1990;3:327–333. doi: 10.1094/mpmi-3-327. [DOI] [PubMed] [Google Scholar]
- Goodspeed TH. Origins, relationships and evolution of its species in the light of their distribution, morphology and cytogenetics. In: Goodspeed TH, editor. The Genus Nicotiana. Chronica Botanica. Waltham, MA: Acta; 1954. pp. 357–391. [Google Scholar]
- Gross GG, Janse C, Elstner EF. Involvement of malate, monophenols and the superoxide radical in hydrogen peroxide formation by isolated cell wall from horseradish (Armoracia lapathifolia Gilib) Planta. 1977;136:271–276. doi: 10.1007/BF00385995. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Lignin synthesis: the generation of hydrogen peroxide and superoxide by peroxidase and its stimulation by manganese (II) and phenols. Planta. 1978;140:81–88. doi: 10.1007/BF00389384. [DOI] [PubMed] [Google Scholar]
- Heitz T, Segond S, Kauffmann S, Geoffroy P, Prasad V, Brunner F, Fritig B, Legrand M. Molecular characterization of a novel tobacco pathogenesis-related (PR) protein: a new plant chitinase/lysozyme. Mol Gen Genet. 1994;245:246–254. doi: 10.1007/BF00283273. [DOI] [PubMed] [Google Scholar]
- Hertig C, Rebmann G, Bull J, Mauch F, Dudler R. Sequence and tissue-specific expression of a putative peroxidase gene from wheat (Triticum aestivum L.) Plant Mol Biol. 1991;16:171–174. doi: 10.1007/BF00017928. [DOI] [PubMed] [Google Scholar]
- Hoson T, Wakabayashi K, Masuda Y. Inhibition of the breakdown of xyloglucans in azuki bean epicotyls by concanavalin A. Plant Cell Physiol. 1995;36:897–902. [Google Scholar]
- Intapruk C, Higashimura N, Yamamoto K, Okada N, Shinmyo A, Takano M. Nucleotide sequences of two genomic DNAs encoding peroxidases of Arabidopsis thaliana. Gene. 1991;98:237–241. doi: 10.1016/0378-1119(91)90179-f. [DOI] [PubMed] [Google Scholar]
- Intapruk C, Yamamoto K, Sekine M, Takano M, Shinmyo A. Regulatory sequences involved in the peroxidase gene expression in Arabidopsis thaliana. Plant Cell Rep. 1994;13:123–129. doi: 10.1007/BF00239877. [DOI] [PubMed] [Google Scholar]
- Keller CP, Van Volkenburgh E. Osmoregulation by oat coleoptile protoplasts. Plant Physiol. 1996;110:1007–1016. doi: 10.1104/pp.110.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjærsgård IVH, Jespersen HM, Rasmussen SK, Welinder KG. Sequence and RT-PCR expression analysis of two peroxidases from Arabidopsis thaliana belonging to a novel evolutionary branch of plant peroxidases. Plant Mol Biol. 1997;33:699–708. doi: 10.1023/a:1005707813801. [DOI] [PubMed] [Google Scholar]
- Klotz KL, Liu T-TY, Liu L, Lagrimini LM. Expression of the tobacco anionic peroxidase gene is tissue-specific and developmentally regulated. Plant Mol Biol. 1998;36:509–520. doi: 10.1023/a:1005939600344. [DOI] [PubMed] [Google Scholar]
- Koop HV, Schweiger HG. Regeneration of plants from individually cultivated protoplasts using an improved microculture system. J Plant Physiol. 1985;121:245–257. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagrimini LM. Wound-induced deposition of polyphenols in transgenic plants overexpressing peroxidase. Plant Physiol. 1991;96:577–583. doi: 10.1104/pp.96.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini LM, Gingas V, Finger F, Rothstein S, Liu T-TY. Characterization of antisense transformed plants deficient in the tobacco anionic peroxidase. Plant Physiol. 1997;114:1187–1196. doi: 10.1104/pp.114.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrimini LM, Rothstein S. Tissue specificity of tobacco peroxidase isozymes and their induction by wounding and tobacco mosaic virus infection. Plant Physiol. 1987;84:438–442. doi: 10.1104/pp.84.2.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marañon MJR, van Huystee RB. Plant peroxidases: interaction between their prosthetic groups. Phytochemistry. 1994;37:1217–1225. [Google Scholar]
- Melo NS, Larsen E, Welinder KG, Fevereiro PS. Characterization of two major cationic peroxidases from cell suspension cultures of Vaccinum myrtillus. Plant Sci. 1997;122:1–10. [Google Scholar]
- Mohan R, Vijayan P, Kolattukudy PE. Developmental and tissue-specific expression of a tomato anionic peroxidase (tap1) gene by a minimal promoter, with wound and pathogen induction by an additional 5′-flanking region. Plant Mol Biol. 1993;22:475–490. doi: 10.1007/BF00015977. [DOI] [PubMed] [Google Scholar]
- Nagata T, Nemoto Y, Kasezawa S. Tobacco BY2 cell line as the “Hela” cell in the cell biology of higher plants. Int Rev Cytol. 1992;132:1–30. [Google Scholar]
- Narita H, Asaka Y, Ikura K, Matsumoto S, Sasaki R. Isolation, characterization and expression of cationic peroxidase isozymes released into the medium of cultured tobacco cells. Eur J Biochem. 1995;228:855–862. [PubMed] [Google Scholar]
- Østergaard L, Abelskov AK, Mattsson O, Welinder KG. Structure and organ specificity of an anionic peroxidase from Arabidopsis thaliana cell suspension culture. FEBS Lett. 1996;398:243–247. doi: 10.1016/s0014-5793(96)01244-6. [DOI] [PubMed] [Google Scholar]
- Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EF, Dannelly HK, Malloy PJ, Reeves HC. Rapid isoelectric focusing in a vertical polyacrylamide minigel system. Anal Biochem. 1987;167:290–294. doi: 10.1016/0003-2697(87)90166-7. [DOI] [PubMed] [Google Scholar]
- Schnabelrauch LS, Kieliszewski M, Upham BL, Alizedeh H, Lamport DTA. Isolation of pI 4.6 extensin peroxidase from tomato cell suspension cultures and identification of Val-Tyr-Lys as putative intermolecular cross-link site. Plant J. 1996;9:477–489. doi: 10.1046/j.1365-313x.1996.09040477.x. [DOI] [PubMed] [Google Scholar]
- Scott-Craig JS, Kerby K, Stein BD, Sommerville SC. Expression of an extracellular peroxidase that is induced in barley (Hordeum vulgare) by the powdery mildew pathogen (Erysiphe graminis) Physiol Mol Plant Pathol. 1995;47:407–418. [Google Scholar]
- Sherf BA, Bajar AM, Kolattukudy PE. Abolition of an inducible highly anionic peroxidase activity in transgenic tomato. Plant Physiol. 1993;101:201–208. doi: 10.1104/pp.101.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Morrison WL, Milham PJ. Oxidation of indole-3-acetic by peroxidase: involvement of reduced peroxidase and compound III with superoxide as a product. Biochemistry. 1982;21:4414–4419. doi: 10.1021/bi00261a034. [DOI] [PubMed] [Google Scholar]
- Sterjiades R, Dean JFD, Gamble G, Himmelsbach DS, Erikson K-EL. Extracellular laccase and peroxidases from sycamore maple (Acer pseudoplatanus) cell suspension cultures: reactions with monolignols and lignin model compounds. Planta. 1993;190:75–87. [Google Scholar]
- Thordal-Christensen H, Brandt J, Cho BH, Rasmussen SK, Gregersen PL, Smedegaard-Petersen V, Collinge DB. cDNA cloning and characterization of two barley peroxidase transcripts induced differentially by the powdery mildew fungus Erysiphe graminis. Physiol Mol Plant Pathol. 1992;40:395–409. [Google Scholar]
- Van Engelen FA, Sterk P, Booij H, Cordewener JHG, Rook W, van Kammen A, deVries SC. Heterogeneity and cell type-specific localization of a cell wall glycoprotein from carrot suspension cells. Plant Physiol. 1991;96:705–712. doi: 10.1104/pp.96.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welinder KG. Plant peroxidases: their primary, secondary and tertiary structures, and relation to cytochrome c peroxidase. Eur J Biochem. 1985;151:497–504. doi: 10.1111/j.1432-1033.1985.tb09129.x. [DOI] [PubMed] [Google Scholar]
- Welinder KG, Jespersen HN, Kjærsgård IVH, Østergaard L, Abelskov AK, Hansen LN, Rasmussen SK (1996) What can we learn from Arabidopsis peroxidases? In C Obinger, U Burner, R Ebermann, C Penel, H Greppin, eds, Plant Peroxidases: Biochemistry and Physiology. University of Geneva Press, Geneva, pp 173–178