Abstract
INTRODUCTION:
The correction of the gingival recession is of esthetical and functional significance, but the tissue regeneration can only be confirmed by a histological examination.
AIM:
This study aims to make a comparison between the free gingival graft and the autograft.
MATERIAL AND METHODS:
This study included 24 patients with single and multiple gingival recessions. Twelve patients were treated with a free gingival graft and the other twelve with a micrograft. Six months after the surgical procedure, a micro-punch biopsy of the transplantation area was performed. The tissue was histologically evaluated, graded in 4 categories: immature, mature, fragmented and edematous collagen tissue. The elastic fibres were also examined and graded in three categories: with a normal structure, fragmented rare and fragmented multiplied.
RESULTS:
Regarding the type of collagen tissue that was present, there was a significant difference between the two groups of patients, with a larger number of patients treated with a micrograft showing a presence of mature tissue, compared to the patients treated with a free gingival graft. A larger number of patients in both of the groups displayed elastic fibres with a rare fragmented structure; 33.3% of the patients showed a normal structure; 50% demonstrated a normal structure.
CONCLUSION:
The patients treated with a free gingival graft showed a larger presence of fragmented collagen tissue and fragmented elastic fibres, whereas a mature tissue was predominantly present in the surgical area where a Geistlich Mucograft was placed.
Keywords: Gingival recession, Free gingival graft, Mucograft, Elastic fibres, Collagen fibres, Connective tissue
Introduction
Despite the form, size and colour of the teeth [1], the anatomical characteristics of the gums have a great impact on the aesthetics and visual appearance of every individual. Regarding the previously stated, it is only logical that the recession of the gums is responsible for problems in the physiognomy, root hypersensitivity and the patient’s fear of tooth loss.
However, the treatment of gingival recession is a quite complicated procedure, where the successful outcome depends on many factors, such as the initial condition of the gums, the biological capacity of the tissue, the type of surgical technique, the blood supply of the tissue, the regenerative potential of the periodontal tissue, etc. [2].
The biotype of the gums is also a critical factor that determines the outcome of the dental treatment, i.e. it has an impact on the therapeutic prognosis, which is based on the restorative, regenerative and implant treatment. The biotype of the gums is in a direct correlation with the appearance of gingival recession and the surgical techniques that can be used in the corrective procedures.
In the studies, guided tissue regeneration (GTR) is mostly recommended as a method of choice, because its main benefit is the formation of a new periodontal tissue attachment, which is also histologically confirmed. The newly formed attachment is colonised by ligament cells that eventually produce a new connective tissue [3].
According to Chambrone et al., [4] from a histological point of view, the use of CTG increases the amount of keratinised tissue, providing much better protection from marginal inflammation and trauma.
From a histological aspect, the use of periodontal surgical methods should provide full or partial coverage of the exposed root surfaces with an actual periodontal regeneration [5]. Despite the quite predictable clinical results that derive from the use of various periodontal flaps in combination with a free gingival graft or a Mucograft, the histological results can be compared only by performing a biopsy from the transplantation area.
Many foreign doctors face difficulties when it comes to convincing the patients to agree on a tissue biopsy, despite the fact that this procedure is minimally invasive. Most of the comparisons derive from animal studies and biopsies [6] performed after extracting the teeth that have previously been treated with a free gingival graft or a micrograft. These biopsies display an obvious periodontal regeneration, which is confirmed by a histological examination.
The adhesion between the root surface and the graft indicates that the tissue recovery occurs primarily with the formation of a new periodontal tissue attachment between the root and the graft [7].
The goal of this study was to make a histological evaluation and comparison of the surgical area, six months after the procedure, between the uses of two types of graft: a free gingival graft and a Mucograft.
Material and Methods
The surgical and histological protocols were approved by the Ethics Committee of the Faculty of Dentistry in the University “St. Cyril and Methodius” in Skopje - Republic of Macedonia. Also, every patient received a thorough explanation, concerning the surgical procedure, prognosis and possible complications. If all of this were in order with the patient’s expectations, we would receive their consent to start the procedure.
This study includes a total of 24 patients with single and multiple recessions of the gums, divided into two groups: group A (12 patients treated with a free gingival graft) and group B (12 patients treated with a Mucograft).
Every oral - surgical procedure was performed with the application of a 3% anaesthetic - Scandonest in the form of local infiltration anaesthesia with the use of a carpal syringe for the maxillary and mandibular nerves. The single and multiple gingival recessions were treated with Carl Martin GmbH instruments for periodontal surgery (Solingen - Germany). With number 15 surgical scalpels, an incision was made 2-3 mm from the gingival margin on the medial side of the upper first molar, going in depth right down to the periosteum, while maintaining a parallel course with the tooth position. In length, the incision spread all the way to the distal side of the canine, without including the palatal rugae, as not to compromise the esthetic results.
Because the path of the incision was parallel to the longitudinal axis of the tooth, damage to the palatal artery was avoided, and a separation of the connective tissue from the periosteum and epidermis was made in the desired length, according to the size of the recession, where the graft will be later placed. By size, the obtained graft was compatible to the recipient location and fixated to it with Vicryl absorbable sutures with a 5-0 thickness. The remaining part of the gingiva was sutured with non-absorbable sutures.
On the market, the Geistlich Mucograft can be found packed in two sizes: 15 mm x 20 mm and 20 mm x 30 mm, and which size will be used depending on the dimensions of the surgical region. The advantage of this collagen matrix is that it provides a simple application without any need of previous preparation and hydration.
After the dimensions of the surgical area are measured, the dry autograft is easily cut in the appropriate size and placed in the area of interest. Its hydrophilic capability enables it to be easily hydrated by the patient’s blood. The sutures are absorbable and fixate the graft with the adjacent tissue without any tension.
The micro punch gum technique was used for performing a biopsy of the area of transplantation (2 mm length), after which the tissue sample was sent for histological examination. The biopsy was performed six months after the surgical procedure with a previous application of a 3% local anaesthetic -Scandonest. The tissue sample was processed with a fixative agent in a formalin solution that was neutrally buffered and placed inside Eppendorf tubes for 6 to 18 hours and processed with embedding it in paraffin. 4 - 6 microsections were coloured with hemalaon eosin of LEICA automatic stainer. Afterwards, the tissue sample was submitted to a histological analysis to determine the structural characteristics, or more precisely the collagen and elastic fibres.
Histological verification of the tissue sample of every examinee was made, thus grading it in four categories: a) immature collagen tissue; b) mature (normal) collagen tissue; c) fragmented collagen tissue; d) edematous tissue. Regarding the structure of the elastic fibres, the tissue samples were divided into three groups: a) with a normal structure; b) rare fragmented structure; c) fragmented multiplied structure.
SPSS 17.0 was used for processing the acquired data. Factors of relations, proportions and rates were used for analysing the attribute series, thus presenting them as absolute and relative numbers. The numerical (quantitative) series were processed with the use of measures for central tendency (average, median, minimal value, maximal value) and a measure of dispersion (standard deviation). Pearson, Chi-square test, Fisher – Freeman - Halton exact test and Fischer exact test were used to help determine the difference between certain attribute marks. The Mann Whitney U test was used for analysing the significance of the difference between certain numeric variables. The significance of the p-value was set to 0.05.
Results
From a total of 12 patients, who were treated with a free gingival graft, a fragmented collagen tissue (Figure 1a) was present in 8 (66.7%) of them, whereas a normal (mature) collagen was seen in the remaining four patients (33.3%). None of the patients from this group displayed immature collagen tissue in the histological examination in the following checkup period.
Figure 1.
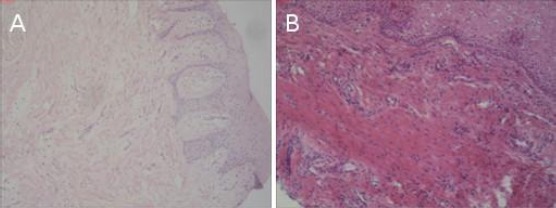
Fragmented collagen fibres in tissue samples, staining is H & E, shot with a 200x magnification Nikon Eclipse 80, six months after the surgical procedure: A) in a free gingival graft; B) in a Mucograft
In the other group of patients, where an autograft was used, in 9 (75%) of them was present a mature (normal) collagen tissue, whereas only 3 (25%) had an immature collagen tissue. There was no patient from this group that was registered with a fragmented collagen tissue.
Evidently, in the two groups of patients, there was no case with an appearance of edematous changes (not regarding its presence in the early stages of tissue formation). For a value of p < 0.05, a significant difference was confirmed between the two groups, regarding the present type of collagen tissue (Fisher – Freeman -Halton exact test: p = 0.0009), displaying a significant increase in the number of patients with a mature (normal) tissue, where a Mucograft was a method of choice (Table 1).
Table 1.
Histological verification according to the type of collagen tissue in both of the groups, six months after the surgical procedure
| Type of collagen tissue | Group | Total | ||
|---|---|---|---|---|
| Examined (FGG) | Controlled (Mucograft) | |||
| Immature | Number | 0 | 3 | 3 |
| % | 0% | 25% | ||
| Mature (normal) | Number | 4 | 9 | 13 |
| % | 33,33% | 75% | ||
| Mature fragmented | Number | 8 | 0 | 8 |
| % | 66,67% | 0% | ||
| Total | Number | 12 | 12 | 24 |
| % | 50% | 50% | 100% | |
Fisher - Freeman - Halton exact test: p = 0.0009*; *significance for a value of p < 0.05.
Table 2.
Prevalence of the elastic fibres in the tissue samples according to their type, six months after the surgical procedure
| Type of collagen tissue | Group | Total | ||
|---|---|---|---|---|
| Examined (FGG) | Controlled (Mucograft) | |||
| Normal structure | Number | 4 | 6 | 10 |
| % | 33,33% | 50% | ||
| Fragmented rare | Number | 8 | 6 | 14 |
| % | 66,67% | 50% | ||
| Total | Number | 12 | 12 | 24 |
| % | 50% | 50% | 100% | |
Fisher exact two tailedtest: p = 0.6802; *significance for a value of p < 0.05.
The analysis of the elastic fibres structure proved that no fragmented increased elastic fibres were present in any of the tissue samples. In the first group of patients, where an autograft was placed, 8 of them showed elastic fibres that were fragmented rare, whereas in 4 of them the elastic fibres were with a normal structure. In the second group of patients, where a Mucograft was used 6 of them had elastic fibres with a normal structure, whereas the other 6 had fragmented rare elastic fibres (Figure 2a).
Figure 2.
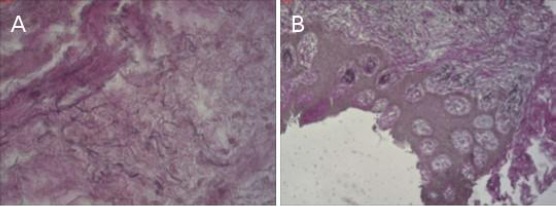
Elastic fibres with a rare fragmented structure in patients tissue samples, staining is H & E, shot with a 400x magnification Nikon Eclipse 80 six months after the surgical procedure: A) in an FGG; B) in a Mucograft
For a value of p > 0.05, no significant difference was present between the two groups, regarding the present type of elastic fibres (Fisher exact two-tailed test: p = 0.6802).
Discussion
The free gingival graft is the oldest surgical technique that is used in the periodontal surgery, where the graft is obtained from the palate or the maxillary tuber. Obtaining the graft from the maxillary tuber region is justified with the fact that the operative site is small; the healing of the donor place is much more simple and faster than when the graft is taken from the palate. The only problem in obtaining a palatal graft is the risk of damaging the palatal artery, so a very precise preoperative evaluation of the maximal dimension of the palatal tissue is required [8].
Ensuring the stability of the postoperative area is a key factor that has a direct impact on the successful outcome of the surgical procedure. The initial adhesion of the blood clot on the root surface is of a great significance because it provides the much-needed pressure and support for the healing process. This is why the surgical method of choice and the first week after the procedure are crucial for accomplishing therapeutic success [9].
In this study, the final results were noted six months after the surgical procedure. Unfortunately, the assessment of the healing process and the histological characteristics of the tissue samples are not well documented in the available dental literature. Because of this, we had no possibility of comparing the results we detected in this study with results from other authors.
For determining the thickness of the palatal tissue, the CT [10] and ultrasound are considered to be the most advanced methods of choice in the everyday practice.
Other types of graft material that are used in the periodontal surgery are the collagen grafts, of which the most preferable is the Geistlich Mucograft, because its composition of collagen matrix is specifically designed to initiate soft tissue regeneration and ensure stability for the sutures with immediate support for the blood clot and early colonization of soft tissue cells (blood and nerve cells) [11]. The compact outer layer, which is comprised of collagen fibres with protective cell capabilities, doesn’t only protect against bacterial invasion but also has a certain elasticity that simplifies the suturing process. The second layer is comprised of a dense, porous, spongy collagen structure, which enables an easy coagulum formation and helps promote angiogenesis and tissue integration [12].
The Geistlich Mucograft ® 3D collagen matrix is the ideal biomaterial for soft tissue regeneration. The collagen from the graft membrane provokes the host’s fibroblasts to start producing new collagen fibres [13] [14]. The porosity of the Mucograft enables an enhanced infiltration of mesenchymal cells inside the area of transplantation. Unlike the typical reaction to foreign bodies (production of gigantic multinuclear cells, lymphocytes and granulation tissue), the host doesn’t reject the Mucograft tissue and accepts it with no severe consequences.
The data that was gathered in this study has shown similarities with the results in Schmitt, et al., a study [15], in which he also examined the differences between a Mucograft and a free gingival graft, from a histological point of view. Schmitt suggests that the membrane of the Mucograft [16] is a promising alternative for regeneration of the keratinised mucosa, i.e. the Mucograft is a sufficient substitute for the free gingival graft when it comes to increasing the keratinised mucosa. Our study has shown concordance with Schmitt, regarding the fact that the Mucograft provokes similar clinical reactions in the early stages of regeneration with the natural tissue and that it has a more natural histological and clinical appearance that the free gingival graft. Schmitt also declared that a precise prediction of the duration and stability of the transplanted graft tissue cannot be proven yet, due to the lack of scientific studies in this area. Similar results are also found in McGuire and Scheyer study [17]. Their study confirms that the Mucograft collagen matrix is characterised by a shorter epithelium and an ability to successfully incorporate itself into the adjacent connective tissue of the host.
In conclusion, six months after the surgical procedure, a fragmented collagen tissue and fragmented elastic fibres were dominantly present in the tissue samples of the patients that were treated with a free gingival graft. However, a mature (normal) collagen tissue was found in the tissue samples of the patients that were treated with a Mucograft.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Chambrone L, Sukekava F, Araujo MG, Pustiglioni FE, Chambrone LA, Lima LA. Root-coverage procedures for thetreatment of localized recession-type defects. Cochrane Database of Systematic Reviews. 2009;15(2):CD007161. doi: 10.1002/14651858.CD007161.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Van Dyke TE. The management of inlamation in periodontal disease. J Periodontol. 2008;79(8 Suppl):1601–1608. doi: 10.1902/jop.2008.080173. https://doi.org/10.1902/jop.2008.080173. PMid:18673016. PMCid: PMC2563957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Souza SL, Macedo GO, Tunes RS, Silveira e Souza AM, Novaes AB, Jr, Grisi MF, et al. Subepitelial connective tissue graft for root coverage in smokers and non smokers: a clinical and histologic controlled study in humans. J Periodontol. 2008;79(6):1014–1021. doi: 10.1902/jop.2008.070479. https://doi.org/10.1902/jop.2008.070479. PMid:18533778. [DOI] [PubMed] [Google Scholar]
- 4.Chambrone L, Chambrone D, Pustiglioni FE, Chambrone LA, Lima LA. Can subepitelial connective tissue grafts be considered the gold standard procedure in the treatment of Miller Class I and II recession-typedefect? J Dent. 2008;36(9):659–671. doi: 10.1016/j.jdent.2008.05.007. https://doi.org/10.1016/j.jdent.2008.05.007. PMid:18584934. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein M, Boyan BD, Cochran DL, Schwartz Z. Human histology of a new attachment after root coverageusingsubepithelial connective tissue graft. J Clin Periodontol. 2001;28(7):657–662. doi: 10.1034/j.1600-051x.2001.028007657.x. https://doi.org/10.1034/j.1600-051x.2001.028007657.x. PMid:11422587. [DOI] [PubMed] [Google Scholar]
- 6.Mcguire MK, Cochran DL. Evaluation of human recessiondefects treated with coronally advanced flaps and eitherenamel matrix derivative or connective tissue. Part 2: Histologicalevaluation. J Periodontol. 2003;74(8):1126–1135. doi: 10.1902/jop.2003.74.8.1126. https://doi.org/10.1902/jop.2003.74.8.1126. PMid:14514225. [DOI] [PubMed] [Google Scholar]
- 7.Raspperini G, Silvestri M, Schenk RK, Nevins ML. Clinicaland histologic evaluation of human gingival recessiontreated with a subepithelial connective tissue graft andenamel matrix derivative (Emdogain): a case report. Int J Periodontics Restorative Dent. 2000;20(3):269–275. [PubMed] [Google Scholar]
- 8.Wara-aswapati N, Pitiphat W, Chandrapho N, Rattanayatikul C, Karimbux N. Thickness of palatal masticatory mucosa associated with age. Journal of periodontology. 2001;72(10):1407–12. doi: 10.1902/jop.2001.72.10.1407. https://doi.org/10.1902/jop.2001.72.10.1407. PMid:11699483. [DOI] [PubMed] [Google Scholar]
- 9.Vitkov L, Krautgartner WD, Hannig M. Surfacemorphology of pocket epithelium. Ultrastruct Pathol. 2005;29(2):121–127. doi: 10.1080/01913120590916832. https://doi.org/10.1080/01913120590916832. PMid:16028668. [DOI] [PubMed] [Google Scholar]
- 10.Song JE, Um YJ, Kim CS, Choi SH, Cho KS, et al. CK Thickness of posterior palatal masticatory mucosa: the use of computerized tomography. J periodontol. 2008;79(3):406–412. doi: 10.1902/jop.2008.070302. https://doi.org/10.1902/jop.2008.070302. PMid:18315422. [DOI] [PubMed] [Google Scholar]
- 11.Lima RS, Peruzzo DC, Napimoga MH, Saba-Chujfi E, Santos-Pereira SA, Martinez EF. Evaluation of the biological behavior of Mucograft®in human gingival fibroblasts: an in vitro study. Brazilian dental journal. 2015;26(6):602–6. doi: 10.1590/0103-6440201300238. https://doi.org/10.1590/0103-6440201300238. PMid:26963203. [DOI] [PubMed] [Google Scholar]
- 12.Rothamel D, Schwarz F, Sager M, Herten M, Sculean A, Becker J. Biodegradation of differentlycross-linked collagen membranes: an experimental study inthe rat Clin. Oral Implants Res. 2005;16:369–78. doi: 10.1111/j.1600-0501.2005.01108.x. https://doi.org/10.1111/j.1600-0501.2005.01108.x. PMid:15877758. [DOI] [PubMed] [Google Scholar]
- 13.Rothamel D, Schwarz F, Sculean A, Herten M, Scherbaum W, Becker J. Biocompatibility of various collagenmembranes in cultures of human PDL fibroblasts andhuman osteoblast-like cells Clin. Oral Implants Res. 2004;15:443–9. doi: 10.1111/j.1600-0501.2004.01039.x. https://doi.org/10.1111/j.1600-0501.2004.01039.x. PMid:15248879. [DOI] [PubMed] [Google Scholar]
- 14.Harris RJ, Harris LE, Harris CR, Harris AJ. Evaluation of root coverage with two connective tissue grafts obtained from the same location. Int J Periodontol Rest Dent. 2007;27(4):333–339. [PubMed] [Google Scholar]
- 15.Schmitt CM, Tudor C, Kiener K, Wehrhan F, Schmitt J, Eitner S, Agaimy A, Schlegel KA. Vestibuloplasty: porcine collagen matrix versus free gingival graft: a clinical and histologic study. Journal of periodontology. 2013;84(7):914–23. doi: 10.1902/jop.2012.120084. https://doi.org/10.1902/jop.2012.120084. PMid:23030237. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt CM, Moest T, Lutz R, Wehrhan F, Neukam FW, Schlegel KA. Long?term outcomes after vestibuloplasty with a porcine collagen matrix (Mucograft®) versus the free gingival graft: a comparative prospective clinical trial. Clinical oral implants research. 2016;27(11) doi: 10.1111/clr.12575. https://doi.org/10.1111/clr.12575. [DOI] [PubMed] [Google Scholar]
- 17.McGuire MK, Scheyer ET. Xenogeneic collagen matrix with coronally advanced flap compared to connective tissue with coronally advanced flap for the treatment of dehiscence-type recession defects. Journal of Periodontology. 2010;81(8):1108–17. doi: 10.1902/jop.2010.090698. https://doi.org/10.1902/jop.2010.090698. PMid:20350159. [DOI] [PubMed] [Google Scholar]


