ABSTRACT
Glioblastoma is an immunosuppressive, deadly brain tumor. IGFBP2, a circulating biomarker for cancer diagnosis and a potential immunotherapeutic target, is attracting more and more attention from oncologists and clinicians. Thus, it is urgent to thoroughly investigate the immune biological process of IGFBP2 to understand tumor immune complexity and provide potential evidence for anti-IGFBP2 therapy. Through authoritative public databases, we enrolled a total of 2447 glioma samples with gene expression profiles. Then, the clinical characteristics and immunosuppressive status of IGFBP2 in the glioma samples were analyzed. Immunohistochemical staining detected the expression of immunosuppressive biomarkers. We found that IGFBP2 expression was upregulated in high-grade glioma and GBM and downregulated in IDH mutant glioma. Increased IGFBP2 accompanied PTEN loss and EGFR amplification. Bioinformatic analysis revealed that IGFBP2 is related to immunological processes. We further selected specific immunologic related gene sets and found IGFBP2 predominated immunosuppressive activities in GBM. Furthermore, we explored the relationship between IGFBP2 and genes that were well-characterized glioma-mediated immunosuppressive molecules to investigate the potential effect of IGFBP2. We discovered that IGFBP2 was correlated with CHI3L1, TNFRSF1A, LGALS1, TIMP1, VEGFA, ANXA1 and LGALS3, which were classic immunosuppressive biomarkers. Higher IGFBP2 expression predicted unfavorable survival for patients with GBM. Our findings implied that IGFBP2 is involved in immunosuppressive activities and is an independent unfavorable prognostic biomarker for patients with GBM. IGFBP2 is a potential immunotherapeutic target for GBM in future clinical trials.
KEYWORDS: glioblastoma, IGFBP2, immune response, immunosuppressive activity, prognosis
Introduction
Glioblastoma is one of the most lethal tumors. Neurosurgical removal of the tumor accompanied with radiotherapy and adjuvant temozolomide1 is the conventional therapy for GBM. However, patients suffering from GBM just have a dismal prognosis with a median survival of less than one year.2 The discovery of the lymphatic system in the central nervous system gives inspiration to bring a novel theoretical foundation and new prospect for immunotherapy in brain tumors.3 Plenty of work has demonstrated the mutual effect between GBM and immunity.4–6 Multiple related biological processes influencing immune surveillance, such as the PI3K/Akt pathway, some chemokines, FAK, the IGF pathway, HIF-1α, IL-6, TGF-β, CTLA-4 and PD-1/PDL-1, could individually or collectively impact immunosurveillance.7–9 IGFBP2 is a member of the secreted IGFBP family that functions by interacting with circulating IGFs to modulate IGF-mediated signaling.10 As a secreted protein, IGFBP2 was reported to be a human tumor antigen that elicited T-cell and B-cell immunity in patients with some cancers.11 The circulating IGFBP2 antibodies may provide a potential approach for diagnosing early cancers in a broad population of patients.11,12 IGFBP2 peptide-specific T cells mediated an antitumor effect in a transgenic mouse model of breast cancer.13 A neutralizing antibody against IGFBP2 could impair downstream IGFBP2-mediated oncogenic signaling pathways and inhibit tumor cell spreading.10 Heretofore, there have been few reports comprehensively illustrating the immunosuppressive status and genomic alterations in glioma with different IGFBP2 expression. Thus, deeply investigating the immune biological process of IGFBP2 based on current genomic datasets may help to get a good idea of tumor immune complexity and guide potential anti-IGFBP2 therapy.
In the present study, we employed 2447 glioma specimens to further explore the IGFBP2 expression and clinical characteristics in glioma. IGFBP2 was upregulated in GBM and was an unfavorable prognostic biomarker for patients with GBM. Moreover, IGFBP2 was involved in the immunosuppressive response and synergistic with several immunosuppressive members, providing evidence for potential anti-IGFBP2 treatment in glioma immunotherapy.
Results
IGFBP2 expression was upregulated in high grade glioma, GBM and downregulated in IDH mutant glioma
IGFBP2 expression was analyzed according to the WHO grade system, histopathology and IDH mutation status (Supplementary Table 1). In the CGGA mRNA microarray dataset, the expression of IGFBP2 was highest in WHO IV glioma (Fig. 1A, P = 2.644E-37) and GBM (P = 7.691E-39). Furthermore, IDH wild type GBM expressed a higher level of IGFBP2 than IDH mutant GBM (P = 1.959E-08). In addition, we also validated that WHO IV glioma and GBM had higher IGFBP2 expression in the TCGA (Fig. 1B, P = 8.384E-113 and P = 4.929E-100, respectively), the GSE16011 (Fig. 1C, P = 3.177E-33 and P = 3.379E-25, respectively) and the Rembrandt (Fig. 1D, P = 3.791E-39 and P = 1.165E-41, respectively) datasets. In addition, we considered the age at diagnosis and gender as other conditions. We found that IGFBP2 was still correlated with WHO grades or histological types regardless of age at diagnosis or gender, and grade IV glioma or glioblastoma had the highest expression level of IGFBP2 in multiple datasets (Supplementary Table 2). GBM with wild type IDH presented a higher expression level of IGFBP2 in the TCGA and the GSE16011 datasets (P = 4.705E-09 and P = 6.297E-4, respectively). These findings further suggest that higher IGFBP2 expression accompanies higher malignancy in glioma.
Figure 1.
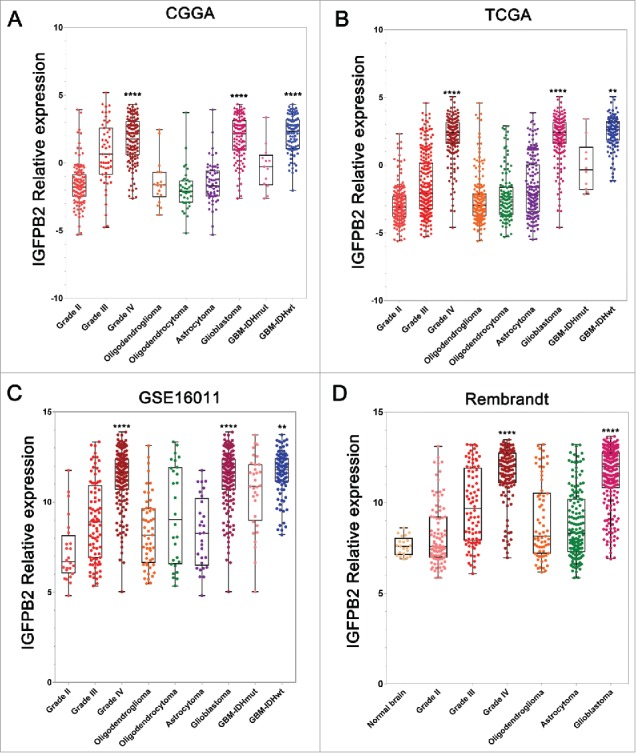
IGFBP2 expression in CGGA, TCGA, GSE16011 and Rembrandt datasets according to WHO grades, histopathologic classifications and IDH1 mutant status. (A-C) IGFBP2 was significantly increased in WHO IV, GBM and IDH wild type gliomas of CGGA, TCGA and GSE16011 datasets. (D). IGFBP2 was significantly increased in GBM (WHO IV) in the Rembrandt dataset. *, **, *** and **** indicate P < 0.05, P < 0.01, P < 0.001 and P < 0.0001, respectively.
Glioma containing different IGFBP2 expression profiles had distinct genomic and transcriptomic spectrums
To uncover the molecular characteristics related to the expression pattern of IGFBP2, we collected available mutation and CNV information. The occurrence of the 1p/19q codeletion, a genomic hallmark of oligodendroglioma,14 decreased along with increasing IGFBP2. While Chr7 amplification accompanied Chr10 loss, a representative event in GBM,15 was enriched in the high IGFBP2 expression group (Fig. 2A, B). Among patients with high IGFBP2 expression, the most frequently deleted genomic region was 10q23.3, which encompasses the PTEN locus (Fig. 2B, P = 4.239E-99). On the other hand, the most commonly amplified region associated with high expression level of IGFBP2 was 7p11.2, which contains EGFR (Fig. 2B, P = 1.832E-44). We conducted PCA to excavate the clinical (age at diagnosis and WHO grade or histological types) and molecular features such as EGFR and PETN alteration.15 PCA1 and PCA2 represented the top two dimensions showing a good separation between the high-IGFBP2 group and the low-IGFBP2 group in glioma specimens, which account for 45.32%, 36.21% in TCGA, 43.04%, 27.00% in CGGA database and 40.38%, 32.45% in Rembrandt database. Here, we observed a separation between high and low IGFBP2-expressing groups in the CGGA, TCGA and Rembrandt databases (Fig. 2C). PCA1 was correlated with clinical factors – age at diagnosis and WHO grade or histology types. And PCA2 mainly presented the molecular features – EGFR and PTEN alteration (Supplementary Table 3). Frequent mutations in IDH1, ATRX, CIC and NOTCH1 were more enriched in samples with a lower level of IGFBP2 than in samples with a higher level of IGFBP2 (Fig. 2D, P = 1.157E-16, P = 3.274E-11, P = 8.622E-63, P = 8.267E-06, respectively).
Figure 2.
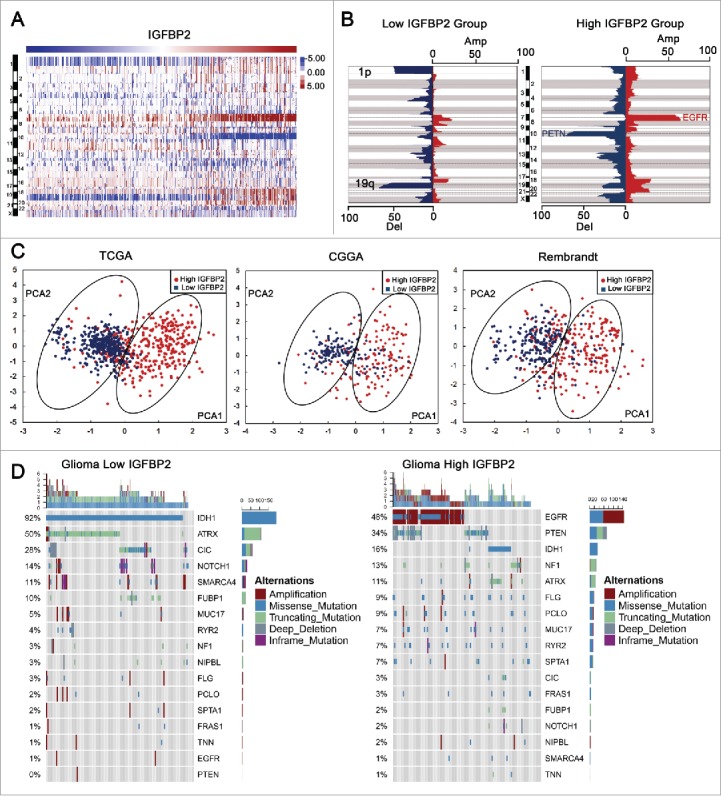
Gliomas with different IGFBP2 expression have distinct genomic and transcriptomic spectrums. (A) CNV spectrum with increasing order of the level of IGFBP2. (B) A distinct CNV spectrum was able to be observed between gliomas with low and high expression of IGFBP2. The incidence of deletion (blue) and amplification (red) in chromosomes were presented on the horizontal axis. (C) There was a separation between high and low IGFBP2 expressing groups in PCA analysis. PCA1 and PCA2 represented the top two dimensions of genes showing differential expression, respectively. (D) Distinct somatic mutation spectrums were visualized by comparing gliomas with low and high levels of IGFBP2.
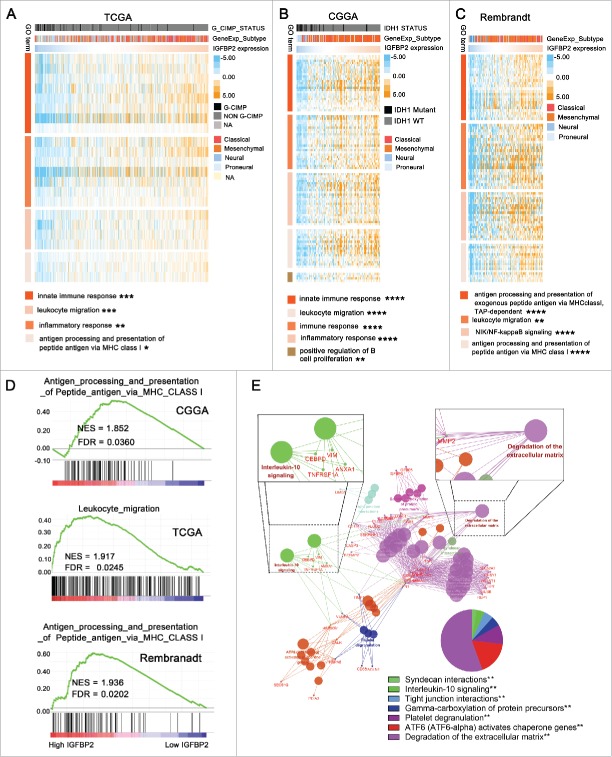
Functional enrichment analysis revealed that IGFBP2 positive-related genes were associated with immunologic events
To explore the biological features of GBM with different IGFBP2 expression, we selected the genes that strongly correlated with IGFBP2 expression (Pearson r > 0.4) in the CGGA, TCGA and Rembrandt databases. In the TCGA dataset, IGFBP2-positive-related genes were enriched in innate immune response (Fig. 3A, P = 3.011E-4, Benjamini = 0.0419), inflammatory response (Fig. 3A, P = 7.664E-4, Benjamini = 0.0458), leukocyte migration (Fig. 3A, P = 1.420E-3, Benjamini = 0.0487) and antigen processing and presentation of peptide antigen via MHC class I (Fig. 3A, P = 0.0262, Benjamini = 0.0498). We found that genes that were positively correlated with IGFBP2 expression were more involved in innate immune response (Fig. 3B, P = 2.509E-14, Benjamini = 1.704E-11), immune response (Fig. 3B, P = 1.451E-13, Benjamini = 6.568E-11), inflammatory response (Fig. 3B, P = 1.534E-13, Benjamini = 5.209E-11), leukocyte migration (Fig. 3B, P = 1.389E-15, Benjamini = 1.960E-12) and positive regulation of B cell proliferation (Fig. 3B, P = 3.381E-3, Benjamini = 0.0483) in CGGA cohort. IGFBP2-positive-related genes were relevant to antigen processing and presentation of exogenous peptide antigen via MHC class I, TAP-dependent (Fig. 3C, P = 6.760E-10, Benjamini = 3.280E-07), leukocyte migration (Fig. 3C, P = 1.906E-3, Benjamini = 0.0421), NIK/NF-kappaB signaling (Fig. 3C, P = 5.660E-6, Benjamini = 8.820E-04) and antigen processing and presentation of peptide antigen via MHC class I (Fig. 3C, P = 1.310E-8, Benjamini = 5.180E-06) in Rembrandt dataset. GSEA confirmed that IGFBP2 was related to immunologic processes in the CGGA (NES = 1.852, FDR = 0.0360), TCGA (NES = 1.917, FDR = 0.0245) and Rembrandt databases (Fig. 3D, NES = 1.936, FDR = 0.0202). We overlapped IGFBP2-positive-related genes of the CGGA, TCGA and Rembrandt datasets and obtained 127 genes (Supplementary Table 4). KEGG Pathway analysis revealed that these genes were mainly involved in immune related pathways, including the interleukin-10 (IL-10) signaling pathway (Fig. 3E, P = 6.321E-3), a recognized immunosuppressive pathway.16,17 These findings imply that IGFBP2 might play a role in immunologic biological processes in GBM.
Figure 3.
Functional enrichment analysis reveals that IGFBP2 positive-related genes are associated with immunologic events. (A-C) IGFBP2 associated with biological process by GO analysis in CGGA, TCGA and Rembrandt datasets. (D) GSEA indicated a significantly enhanced immunologic process in cases with high IGFBP2 expression. (E) A total of 127 genes that overlapped IGFBP2-positive-related genes in the CGGA, TCGA and Rembrandt datasets were analyzed by the pathway analysis tool, ClueGO. ** indicates P < 0.01.
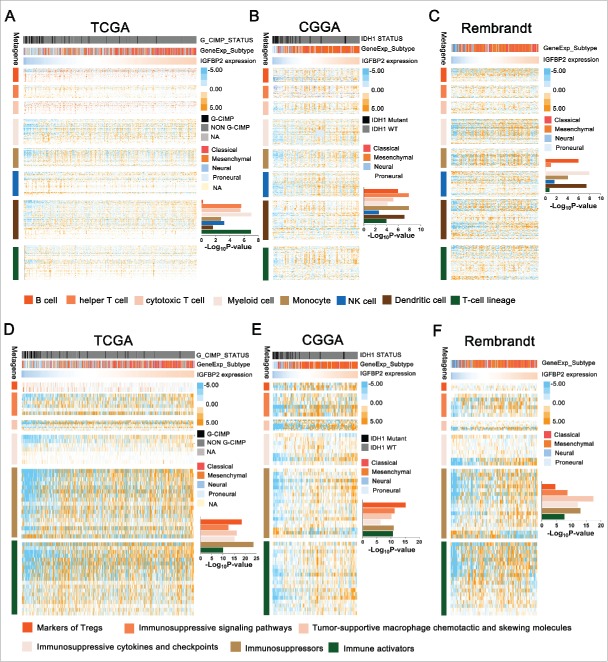
Immunosuppression is a predominate feature in GBMs with high IGFBP2
To get a further comprehensive study of IGFBP2-related immunologic biological processes in malignant brain tumors, we chose classifier gene sets for specific immune cell lineages6 and immunologic effectors18 which were subsequently defined as metagenes (Supplementary Table 5). Based on the single sample GSEA, we observed that IGFBP2 was positively associated with immune cell lineages, such as helper T cells (P = 2.57E-06), cytotoxic T cells (P = 3.01E-06), myeloid cells (P = 9.60E-08), monocytes (P = 1.77E-03), NK cells (P = 6.43E-04), dendritic cells (P = 2.39E-02) and T-cell lineage (P = 1.05E-07) in TCGA dataset (Fig. 4A). A similar pattern of GBM in the CGGA and Rembrandt datasets was observed (Fig. 4B, C, Supplementary Table 6). Next, we parsed these three datasets to show that the proportion of patients with high IGFBP2 had significantly higher mRNA expression of previously well-characterized glioma mediated immunosuppressors compared with immune activators (Fig. 4D-E, TCGA: r = 0.419, r = 0.275; CGGA: r = 0.556, r = 0.549; Rembrandt: r = 0.589, r = 0.459). These results indicate that IGFBP2-related immunologic biological processes might, through a series of immune cells, play an important role in the immunosuppressive response.
Figure 4.
Immunosuppression is a predominate feature in GBMs with high IGFBP2 expression. (A-C) IGFBP2 was related to immune cell lineages in the CGGA, TCGA and Rembrandt datasets. (B-D) IGFBP2 was related to well-recognized glioma-associated immunosuppressive activities.
IGFBP2 was synergistic with other immunosuppressive genes in glioblastoma
As revealed above, IGFBP2 played a key role of immunosuppression in GBM. Therefore, we would like to explore the key immunosuppressive effectors in GBM. We overlapped IGFBP2-positive-related genes of the CGGA, TCGA and Rembrandt datasets with immunosuppressive gene sets and got seven effectors (Fig. 5A). IGFBP2 was found to significantly correlate with CHI3L1 (CGGA, r = 0.642; TCGA, r = 0.552 and Rembrandt, r = 0.555), TNFRSF1A (CGGA, r = 0.590; TCGA, r = 0.463 and Rembrandt, r = 0.579), LGALS1 (CGGA, r = 0.643; TCGA, r = 0.458 and Rembrandt, r = 0.631), TIMP1 (CGGA, r = 0.748; TCGA, r = 0.609 and Rembrandt, r = 0.683), VEGFA (CGGA, r = 0.599; TCGA, r = 0.567 and Rembrandt, r = 0.734), ANXA1 (CGGA, r = 0.590; TCGA, r = 0.409 and Rembrandt, r = 0.641), and LGALS3 (CGGA, r = 0.569; TCGA, r = 0.485 and Rembrandt, r = 0.579) (Fig. 5B-D, Supplementary Table 7). IHC demonstrated that the protein level of IGFBP2 in GBM tissues was positively correlated with immunosuppressive molecules VEGFA (r = 06387), ANXA1 (r = 0.5693), LGALS3 (r = 0.5249), CHI3L1 (r = 0.4672), TNFRSF1A (r = 0.5789), LGALS1 (r = 0.5691) and TIMP1 (r = 0.5189) (Fig. 6, Supplementary Fig. 1).
Figure 5.

Correlation of immunosuppressive genes and IGFBP2 in GBM at transcriptome level. (A) A venn diagram showed the immunosuppressive genes among IGFBP2-positive-related genes of the CGGA, TCGA and Rembrandt datasets and immunosuppressive gene sets. (B-D) Correlation of IGFBP2 and immunosuppressive genes in GBM.
Figure 6.

Correlation of immunosuppressive molecules and IGFBP2 in GBM at the protein level. IHC staining showed the correlation of IGFBP2 and immunosuppressive molecules (AXAN1, VEGFA and LGALS3) in GBM tissues.
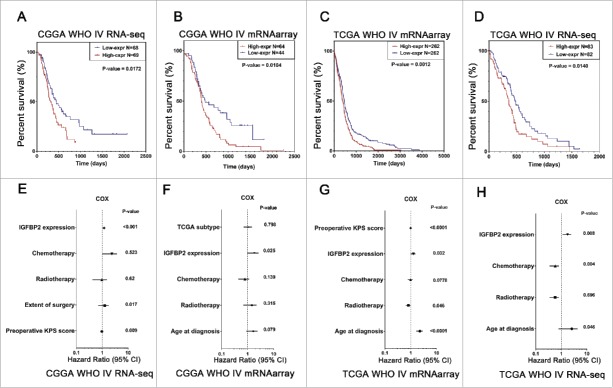
High expression of IGFBP2 predicted unfavorable survival in GBM
We employed dichotomization to separate cases for depicting the survival curves according to the median value and the best cutoff point. We evaluated the prognostic value of IGFBP2 in two databases. Patients with GBM containing higher IGFBP2 expression had a significantly shorter survival times than their counterparts (Fig. 7A, B, P = 0.0172, P = 0.0104) in the CGGA RNAseq and mRNA microarray data. We also observed that IGFBP2 was a dismal biomarker for patients with GBM in TCGA mRNA microarray data (P = 0.0012) and RNAseq (Fig. 7C, D, P = 0.0140). Next, we conducted cox regression analysis to evaluate the prognostic value of IGFBP2 expression and other prognostic factors. The results confirmed that the expression of IGFBP2 (P < 0.001) along with karnofsky performance score (KPS) (P = 0.009) and extent surgery (P = 0.017) were prognostic indicators in CGGA RNAseq data (Fig. 7E). In CGGA mRNA microarray data, the expression of IGFBP2 was still associated with survival (P = 0.025) after adjusting for other factors such as TCGA subtype, chemotherapy, radiotherapy and age at diagnosis (Fig. 7F). In TCGA mRNA microarray data, the expression of IGFBP2 (P = 0.032), along with KPS (P < 0.0001), radiotherapy (P = 0.046) and age at diagnosis (P < 0.0001), were prognostic indicators (Fig. 7G). The expression of IGFBP2 (P = 0.008) was still an independent prognostic indicator in TCGA RNAseq data (Fig. 7H). These results indicate that IGFBP2 is an unfavorable predictor for patients with GBM.
Figure 7.
IGFBP2 is a prognostic predictor in GBM patients. (A-D) Kaplan-Meier survival analysis revealed that a high level of IGFBP2 indicated an unfavorable outcome in GBM patients of the CGGA and TCGA datasets. (E-H) Cox regression analysis was used to assess the prognostic value of IGFBP2 expression among other factors in the CGGA and TCGA datasets.
Discussion
Glioblastoma is the most common malignant primary tumor of the central nervous system (CNS) in adults, contributing to approximately 50% of all gliomas.19 Despite advances in neurosurgery, along with the widely recognized chemotherapy and radiotherapy in newly diagnosed GBM, improvements in patient survival are very limited.20 Therefore, new treatment concepts and therapeutic approaches are urgently needed. Immune therapy is increasingly popular treatment method for GBM. Immune checkpoint blockades (e.g., PD-1, CTLA4)21 and chimeric antigen receptor T-Cell immunotherapy (CAR-T)22 have shown potential benefits for cancer treatment. Currently, patients suffering from melanoma and lung carcinoma benefit from novel immunotherapeutic treatment.23 GBM develops in a relatively immune-privileged CNS and thrives in an immunosuppressive microenvironment.3 In the present study, we would like to explore and describe the hidden relationship between glioma and immunosuppression through bioinformatic analysis.
Different glioma antigen molecules and immune related genes manifest differential expression patterns among GBM subtypes. The mesenchymal subtype, in which an enrichment of immunologic genes is involved, is sensitive to anti-tumor inflammatory responses, including immunosuppression.18 First, we enrolled the transcriptome data of more than 2000 glioma patients, including 447 gliomas from the CGGA, 475 gliomas from the Rembrandt database, and 1241 gliomas from the TCGA database. Through a deep analysis of the biological function of IGFBP2 in GBM, we found that IGFBP2 played an important role in immunologic processes of GBM, especially in immunosuppressive activities (e.g., immunosuppressive checkpoints, tumor-supportive macrophage chemotactic and skewing molecules) and immunosuppressive pathways (IL-10 signaling pathway). IL-10 limits intratumoral dendritic cell activation and production of IL-12, thereby inhibiting cytotoxic T cell responses during chemotherapy. Inflammatory responses are suppressed by IL-10 through inhibiting macrophage activation.17 The role of IGFBP2 in GBM was strongly synergistic with other immunosuppressive members such as CHI3L1, TNFRSF1A, LGALS1, TIMP1, VEGFA, ANXA1 and LGALS3 in both transcriptome and protein levels.
Tumor-associated macrophages (TAMs) are a lineage of immune cell population present in tumor tissues, which skew towards an M2-altered functional profile and play a crucial role in immune evasion within tumors.24 M2 macrophage-derived CHI3L1 specifically binds to the interleukin-13 receptor α2 chain (IL-13Rα2) of gastric and breast cancer cells, promoting cancer metastasis.25 Tumor necrosis factor (TNF) is a potent promoter of carcinogenesis and potentially important target for cancer prevention. Deletion of TNF or TNFRSF1A genes protects mice from 3-methylcholanthrene (MCA)-induced carcinogenesis and prevents MCA induction of cell-mediated suppression of NK-cell/DC crosstalk.27 LGALS1 is a member of a family of b-galactoside–binding lectins and has been reported that this represents the primary mechanism by which tumor-derived LGALS1 restains antitumor immunity.28,29 LGALS1 suppression in glioma could extend patient survival by recruiting NK immune surveillance which could eradicate glioma cells.31 Many studies demonstrate that elevated levels of TIMP-1 are associated with poor prognosis for a variety of cancers, including breast, colorectal cancer and lung carcinoma, or hematopoietic tumors.32 There is a linear relationship between TIMP-1 expression and Tregs in pancreatic cancer, suggesting that TIMP1 can promote immunosuppression.33 VEGFA can contribute to prevent the development of efficient antitumor immune responses by promoting local and systemic immunosuppression. VEGFA/VEGFR-targeting therapies may revert such an immunosuppressive state.34,35 Immune responses to glioma could be impaired by the tumor itself through expression of immunosuppressive cytokines such as TGF-β.36,37 In breast cancer, up-regulation of ANXA1 enchances TGF-β signaling.38 LGALS3 has been shown in vitro to possess several immunomodulatory functions, such as weakening the affinity of the T-cell receptor (TCR) for its cognate MHC I–peptide ligand by separating the TCR from its CD8+ coreceptor39 and inducing T-cell apoptosis.40 Inhibiting LGALS3 in conjunction with CD8+ T-cell–directed immunotherapies could enhance the tumor-specific immune response.41
The finding that IGFBP2 is associated with immunosuppressive molecules and implies that IGFBP2 plays a role in the immunosuppressive microenvironment in GBM. IGFBPs seems to increase the activity of IGFs to promote the IGF signaling pathway. These actions might involve in lengthening the half-life of IGF1 and IGF2, and delivering IGFs to IGF1R.48 The human mesenchymal stem cells (MSCs) culture supernatant induced CD4+FOXP3+ Tregs that expressed IGF-1R, and IGF-2R, showing antiproliferative activity against CD4+ T cells. The induction of Tregs by human MSC culture supernatant was enhanced by the addition of IGF and suppressed by the inhibition of IGF-1R.49 CD4+Foxp3+ Tregs could be play an important role in suppressing immune surveillance among tumor environment.50 IGFBP2 was confirmed to activate integrin β1 and downstream invasion pathways to induce cell motility and activate NF-κB signaling pathway.51 NF-κB is a major factor to induce immune responses and may be responsible for some cancers, inflammatory and autoimmune diseases.52 NF-κB is important in determining this balance between the protumour and antitumour properties of macrophages. Activated NF-κB signaling pathways were associated with TIMP-1 overexpression in tumor cells.53 Such regulation enabled TAMs to sustain the smouldering inflammatory microenvironment present in established metastatic neoplasia.52 Above all, IGFBP2 might potentially regulate the IGF and NF-κB signaling pathways to impact the immune response in the tumor tissue.
In addition, IGFBP2 expression was further confirmed to be significantly upregulated in highly malignant gliomas and predicted a worse outcome for patients. Next, distinct genomic and transcriptomic spectrums were analyzed. We found that Chr7 (EGFR) amplification accompanied with Chr10 loss (PTEN) occurred more frequent when IGFBP2 expression was elevated. On the other hand, most mutations of IDH1 and 1p/19q codeletion occurred in the relatively lower IGFBP2 group. All these findings also implicated IGFBP2 as a biomarker and potential therapeutic target for glioma immunotherapy.
In conclusion, we explored the clinical roles and immune biological processes of IGFBP2 in more than two thousand diffuse gliomas. IGFBP2 was involved in the immunosuppressive response and synergistic with several immunosuppressive members, acting as a potential therapeutic target. These findings extend our understanding of anti-cancer immunotherapy in glioma.
Methods and materials
Data collection
Four kinds of transcriptome data from patients who were diagnosed with glioma (WHO II-IV) were used. The datasets used were The Chinese Glioma Genome Atlas (CGGA) database (RNAseq, n = 137, microarray, n = 310) (http://www.cgga.org.cn), The Cancer Genome Atlas (TCGA) database (RNAseq, n = 702, microarray, n = 539) (http://cancergenome.nih.gov/), the GSE16011 database (n = 284) and the Rembrandt database (n = 475) (https://caintegrator.nci.nih.gov/rembrandt/). The copy number variation (CNV) profile and somatic mutation data were obtained from TCGA data portal (http://cancergenome.nih.gov/).
Immunohistochemistry
We obtained 25 paraffin-embedded GBM tissues from patients who provided informed consent under an Institutional Ethics Committee-approved study from the Second Affiliated Hospital of Harbin Medical University. IHC assay was described in our previous research.54 Briefly, the slices were incubated with primary antibody (IGFBP2, CST, 3922, 1:25; CHI3L1, Invitrogen, PA5-46996, 1:100; TNFRSF1A, Invitrogen, 710368, 1:100; LGALS1, CST, 13888, 1:250; TIMP1, Abcam, ab211926, 1:1000; VEGFA, Abcam, ab1316, 1:100; ANXA1, CST, 32934, 1:400 and LGALS3, CST, 87985, 1:600) for 12 h 4°C, then incubated with secondary antibody (ZSGB, 1:100) at 37℃ for 30 min. After washing with a phosphate-buffered solution (PBS) three times for 5 min, the slices were stained with Diaminobenzidine (DAB) for 2 min, rinsed in PBS and counterstained with hematoxylin. Quantitative evaluation was performed by examining each slice using at least three different high-power fields with the most abundant stained cells.
Statistical analysis
Differences in variables were assessed by Student's t-test for two groups or one-way analysis of variance (one-way ANOVA) for at least three groups (WHO grade and histological types as the basal levels, age at diagnosis and gender as other conditions). Comparisons of binary and categorical patient characteristics between subgroups were performed via the Chi-square test. The Kaplan–Meier survival curve and log-rank tests were used to describe survival distributions and assess statistical significance between two groups. The multiple variates cox proportional hazard model and the principal components analysis (PCA) were performed by using SPSS 22.0. GISTIC2.0 was used to assess CNV associated with IGFBP2 expression. GISTIC value less than −1 or more than 1 was defined as a deletion or amplification. Gene ontology (GO) analysis was performed when gene sets (Pearson r>0.4) were submitted to the DAVID website (http://david.abcc.ncifcrf.gov/home.jsp). ClueGO,55 a Cytoscape APP, was used to perform pathway analysis. R packages, such as circlize56 and ComplexHeatmap, were used to produce figures. Biological processes were further analyzed through gene set enrichment analysis (GSEA).57 Single-sample GSEA (ssGSEA)58 was used to calculates the enrichment score of every gene set for every sample. Heatmaps were constructed and produced by Gene Cluster 3.0 and Gene Tree View software. P values less than 0.05 were considered statistically significant.
Supplementary Material
Funding Statement
This study was supported by 1. The National Key Research and Development Plan (No. 2016YFC0902500); 2. The National Natural Science Foundation of China (No. 81702972, No. 81572701, No. 81772666, No. 81372700); 3. The Research Project of the Chinese Society of Neuro-oncology, CACA (CSNO-2016-MSD12); 4. The Research Project of the Health and Family Planning Commission of Heilongjiang Province (2017-201); and 5. The Harbin Medical University Scientific Research Innovation Fund (2017LCZX37).
Abbreviations
- ATRX
Alpha Thalassemia/Mental Retardation Syndrome X-Linked
- ANXA1
Annexin A1
- CGGA
Chinese Glioma Genome Atlas
- CHI3L1
Chitinase 3 Like 1
- CIC
Capicua Transcriptional Repressor
- CNS
Central Nervous System
- CNV
Copy Number Variation
- EGFR
Epidermal Growth Factor Receptor
- GBM
Glioblastoma
- GO
Gene Ontology
- GSEA
Gene Set Enrichment Analysis
- IDH1
Isocitrate Dehydrogenase (NADP(+)) 1
- IGFBP2
Insulin Like Growth Factor Binding Protein 2
- IHC
Immunohistochemistry
- IL-10
Interleukin 10
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- KPS
Karnofsky Performance Score
- LGALS1
Galectin 1
- LGALS3
Galectin 3
- MSCs
Mesenchymal Stem Cells
- NOCTH1
Translocation-Associated Notch Protein TAN-1
- PBS
Phosphate-buffered Solution
- PCA
Principal Components Analysis
- PTEN
Phosphatase And Tensin Homolog
- ssGSEA
Single-sample GSEA
- TCGA
The Cancer Genome Atlas
- TCR
T-cell Receptor
- TIMP1
Tissue Inhibitor Of Metalloproteinases 1
- TMZ
Temozolomide
- TNFRSF1A
TNF Receptor Superfamily Member 1A
- VEGFA
Vascular Endothelial Growth Factor A
- WHO
World Health Organization
Disclosure of potential conflicts of interest
The authors declare no potential conflicts of interest.
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al.. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. PMID:15758009. [DOI] [PubMed] [Google Scholar]
- 2.Jiang T, Mao Y, Ma W, Mao Q, You Y, Yang X, Jiang C, Kang C, Li X, Chen L, et al.. CGCG clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 2016;375:263–73. doi: 10.1016/j.canlet.2016.01.024. PMID:26966000. [DOI] [PubMed] [Google Scholar]
- 3.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, et al.. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–41. doi: 10.1038/nature14432. PMID:26030524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saha D, Martuza RL, Rabkin SD. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell. 2017;32:253–67 e5. doi: 10.1016/j.ccell.2017.07.006. PMID:28810147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rolle CE, Sengupta S, Lesniak MS. Mechanisms of immune evasion by gliomas. Adv Exp Med Biol. 2012;746:53–76. doi: 10.1007/978-1-4614-3146-6_5. PMID:22639159. [DOI] [PubMed] [Google Scholar]
- 6.Donson AM, Birks DK, Schittone SA, Kleinschmidt-DeMasters BK, Sun DY, Hemenway MF, Handler MH, Waziri AE, Wang M, Foreman NK. Increased immune gene expression and immune cell infiltration in high-grade astrocytoma distinguish long-term from short-term survivors. J Immunol. 2012;189:1920–7. doi: 10.4049/jimmunol.1103373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kravchenko J, Corsini E, Williams MA, Decker W, Manjili MH, Otsuki T, Singh N, Al-Mulla F, Al-Temaimi R, Amedei A, et al.. Chemical compounds from anthropogenic environment and immune evasion mechanisms: potential interactions. Carcinogenesis. 2015;36 Suppl 1:S111−27. doi: 10.1093/carcin/bgv033. PMID:26002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li G, Wang Z, Zhang C, Liu X, Cai J, Wang Z, Hu H, Wu F, Bao Z, Liu Y, et al.. Molecular and clinical characterization of TIM-3 in glioma through 1,024 samples. Oncoimmunology. 2017;6:e1328339. doi: 10.1080/2162402X.2017.1328339. PMID:28919992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z, Zhang C, Liu X, Wang Z, Sun L, Li G, Liang J, Hu H, Liu Y, Zhang W, et al.. Molecular and clinical characterization of PD-L1 expression at transcriptional level via 976 samples of brain glioma. Oncoimmunology. 2016;5:e1196310. doi: 10.1080/2162402X.2016.1196310. PMID:27999734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phillips LM, Zhou X, Cogdell DE, Chua CY, Huisinga A, K RH, Fuller GN, Zhang W. Glioma progression is mediated by an addiction to aberrant IGFBP2 expression and can be blocked using anti-IGFBP2 strategies. J Pathol. 2016;239:355–64. doi: 10.1002/path.4734. PMID:27125842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Jiang T, Zhang J, Zhang B, Yang W, You G, Xu K, Wu J, Luo C, Song SW. Elevated serum antibodies against insulin-like growth factor-binding protein-2 allow detecting early-stage cancers: evidences from glioma and colorectal carcinoma studies. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2012;23:2415–22. doi: 10.1093/annonc/mds007. [DOI] [PubMed] [Google Scholar]
- 12.Moore LM, Holmes KM, Smith SM, Wu Y, Tchougounova E, Uhrbom L, Sawaya R, Bruner JM, Fuller GN, Zhang W. IGFBP2 is a candidate biomarker for Ink4a-Arf status and a therapeutic target for high-grade gliomas. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:16675–9. doi: 10.1073/pnas.0900807106. PMID:19805356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park KH, Gad E, Goodell V, Dang Y, Wild T, Higgins D, Fintak P, Childs J, Dela Rosa C, Disis ML. Insulin-like growth factor-binding protein-2 is a target for the immunomodulation of breast cancer. Cancer Res. 2008;68:8400–9. doi: 10.1158/0008-5472.CAN-07-5891. PMID:18922913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML, Smirnov IV, et al.. Glioma Groups Based on 1p/19q, IDH, and TERT Promoter Mutations in Tumors. N Engl J Med. 2015;372:2499–508. doi: 10.1056/NEJMoa1407279. PMID:26061753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH, et al.. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77. doi: 10.1016/j.cell.2013.09.034. PMID:24120142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17:1006–18. doi: 10.1093/emboj/17.4.1006. PMID:9463379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mumm JB, Emmerich J, Zhang X, Chan I, Wu L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, et al.. IL-10 elicits IFNgamma-dependent tumor immune surveillance. Cancer cell. 2011;20:781–96. doi: 10.1016/j.ccr.2011.11.003. PMID:22172723. [DOI] [PubMed] [Google Scholar]
- 18.Doucette T, Rao G, Rao A, Shen L, Aldape K, Wei J, Dziurzynski K, Gilbert M, Heimberger AB. Immune heterogeneity of glioblastoma subtypes: extrapolation from the cancer genome atlas. Cancer Immunol Res. 2013;1:112–22. doi: 10.1158/2326-6066.CIR-13-0028. PMID:24409449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009–2013. Neuro-oncology. 2016;18:v1–v75. doi: 10.1093/neuonc/now207. PMID:28475809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woehrer A, Bauchet L, Barnholtz-Sloan JS. Glioblastoma survival: has it improved? Evidence from population-based studies. Current opinion in neurology. 2014;27:666–74. PMID:25364955. [DOI] [PubMed] [Google Scholar]
- 21.Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol. 2015;11:504–14. doi: 10.1038/nrneurol.2015.139. PMID:26260659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown CE, Badie B, Barish ME, Weng L, Ostberg JR, Chang WC, Naranjo A, Starr R, Wagner J, Wright C, et al.. Bioactivity and Safety of IL13Ralpha2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:4062–72. doi: 10.1158/1078-0432.CCR-15-0428. PMID:26059190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al.. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. PMID:22658127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. PMID:20371344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Zhang S, Wang Q, Zhang X. Tumor-recruited M2 macrophages promote gastric and breast cancer metastasis via M2 macrophage-secreted CHI3L1 protein. Journal of hematology & oncology. 2017;10:36. doi: 10.1186/s13045-017-0408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, Carson WF 4th, Cavassani KA, Li X, Lukacs NW, et al.. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114:3244–54. doi: 10.1182/blood-2009-04-217620. PMID:19567879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sobo-Vujanovic A, Vujanovic L, DeLeo AB, Concha-Benavente F, Ferris RL, Lin Y, Vujanovic NL. Inhibition of Soluble Tumor Necrosis Factor Prevents Chemically Induced Carcinogenesis in Mice. Cancer Immunol Research. 2016;4:441–51. doi: 10.1158/2326-6066.CIR-15-0104. PMID:26896171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, Mordoh J, Fainboim L, Podhajcer OL, Rabinovich GA. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–51. doi: 10.1016/S1535-6108(04)00024-8. PMID:15050916. [DOI] [PubMed] [Google Scholar]
- 29.Banh A, Zhang J, Cao H, Bouley DM, Kwok S, Kong C, Giaccia AJ, Koong AC, Le QT. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer Res. 2011;71:4423–31. doi: 10.1158/0008-5472.CAN-10-4157. PMID:21546572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lykken JM, Horikawa M, Minard-Colin V, Kamata M, Miyagaki T, Poe JC, Tedder TF. Galectin-1 drives lymphoma CD20 immunotherapy resistance: validation of a preclinical system to identify resistance mechanisms. Blood. 2016;127:1886–95. doi: 10.1182/blood-2015-11-681130. PMID:26888257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker GJ, Chockley P, Yadav VN, Doherty R, Ritt M, Sivaramakrishnan S, Castro MG, Lowenstein PR. Natural killer cells eradicate galectin-1-deficient glioma in the absence of adaptive immunity. Cancer research. 2014;74:5079–90. doi: 10.1158/0008-5472.CAN-14-1203. PMID:25038230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seubert B, Grunwald B, Kobuch J, Cui H, Schelter F, Schaten S, Siveke JT, Lim NH, Nagase H, Simonavicius N, et al.. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology. 2015;61:238–48. doi: 10.1002/hep.27378. PMID:25131778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.D'Costa Z, Jones K, Azad A, van Stiphout R, Lim SY, Gomes AL, Kinchesh P, Smart SC, Gillies McKenna W, Buffa FM, et al.. Gemcitabine-Induced TIMP1 Attenuates Therapy Response and Promotes Tumor Growth and Liver Metastasis in Pancreatic Cancer. Cancer Res. 2017;77:5952–62. doi: 10.1158/0008-5472.CAN-16-2833. PMID:28765154. [DOI] [PubMed] [Google Scholar]
- 34.Terme M, Tartour E, Taieb J. VEGFA/VEGFR2-targeted therapies prevent the VEGFA-induced proliferation of regulatory T cells in cancer. Oncoimmunology. 2013;2:e25156. doi: 10.4161/onci.25156. PMID:24083078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmittnaegel M, Rigamonti N, Kadioglu E, Cassara A, Wyser Rmili C, Kiialainen A, Kienast Y, Mueller HJ, Ooi CH, Laoui D, et al.. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med. 2017;9. doi: 10.1126/scitranslmed.aak9670. PMID:28404865. [DOI] [PubMed] [Google Scholar]
- 36.Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, Lin Y, Dietz AB, Forsyth PA, Yong VW, et al.. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro Oncol. 2010;12:351–65. doi: 10.1093/neuonc/nop023. PMID:20308313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol. 2011;2011:732413. doi: 10.1155/2011/732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Graauw M, van Miltenburg MH, Schmidt MK, Pont C, Lalai R, Kartopawiro J, Pardali E, Le Dévédec SE, Smit VT, van der Wal A, et al.. Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proc Natl Acad Sci U S A. 2010;107:6340–5. doi: 10.1073/pnas.0913360107. PMID:20308542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Demotte N, Wieers G, Van Der Smissen P, Moser M, Schmidt C, Thielemans K, Squifflet JL, Weynand B, Carrasco J, Lurquin C, et al.. A galectin-3 ligand corrects the impaired function of human CD4 and CD8 tumor-infiltrating lymphocytes and favors tumor rejection in mice. Cancer Res. 2010;70:7476–88. doi: 10.1158/0008-5472.CAN-10-0761. PMID:20719885. [DOI] [PubMed] [Google Scholar]
- 40.Fukumori T, Takenaka Y, Yoshii T, Kim HR, Hogan V, Inohara H, Kagawa S, Raz A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63:8302–11. PMID:14678989. [PubMed] [Google Scholar]
- 41.Kouo T, Huang L, Pucsek AB, Cao M, Solt S, Armstrong T, Jaffee E. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol Res. 2015;3:412–23. doi: 10.1158/2326-6066.CIR-14-0150. PMID:25691328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al.. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. PMID:20129251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang YH, Morand EF, Getting SJ, Paul-Clark M, Liu DL, Yona S, Hannon R, Buckingham JC, Perretti M, Flower RJ. Modulation of inflammation and response to dexamethasone by Annexin 1 in antigen-induced arthritis. Arthritis Rheumatism. 2004;50:976–84. doi: 10.1002/art.20201. PMID:15022342. [DOI] [PubMed] [Google Scholar]
- 44.Huang P, Zhou Y, Liu Z, Zhang P. Interaction between ANXA1 and GATA-3 in Immunosuppression of CD4+ T Cells. Mediators Inflamm. 2016;2016:1701059. doi: 10.1155/2016/1701059. PMID:27833268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016;19:20–7. doi: 10.1038/nn.4185. PMID:26713745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai J, Zhang W, Yang P, Wang Y, Li M, Zhang C, Wang Z, Hu H, Liu Y, Li Q, et al.. Identification of a 6-Cytokine Prognostic Signature in Patients with Primary Glioblastoma Harboring M2 Microglia/Macrophage Phenotype Relevance. PLoS One. 2015;10:e0126022. doi: 10.1371/journal.pone.0126022. PMID:25978454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biragyn A, Longo DL. Neoplastic "Black Ops": cancer's subversive tactics in overcoming host defenses. Semin Cancer Biol. 2012;22:50–9. doi: 10.1016/j.semcancer.2012.01.005. PMID:22257681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nature reviews Cancer. 2004;4:505–18. doi: 10.1038/nrc1387. PMID:15229476. [DOI] [PubMed] [Google Scholar]
- 49.Miyagawa I, Nakayamada S, Nakano K, Yamagata K, Sakata K, Yamaoka K, Tanaka Y. Induction of Regulatory T Cells and Its Regulation with Insulin-like Growth Factor/Insulin-like Growth Factor Binding Protein-4 by Human Mesenchymal Stem Cells. Journal of immunology. 2017;199:1616–25. doi: 10.4049/jimmunol.1600230. [DOI] [PubMed] [Google Scholar]
- 50.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, et al.. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–34. doi: 10.1016/j.ccr.2014.04.005. PMID:24856586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Holmes KM, Annala M, Chua CY, Dunlap SM, Liu Y, Hugen N, Moore LM, Cogdell D, Hu L, Nykter M, et al.. Insulin-like growth factor-binding protein 2-driven glioma progression is prevented by blocking a clinically significant integrin, integrin-linked kinase, and NF-kappaB network. Proc Natl Acad Sci U S A. 2012;109:3475–80. doi: 10.1073/pnas.1120375109. PMID:22345562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. PMID:18650914. [DOI] [PubMed] [Google Scholar]
- 53.Cheng G, Fan X, Hao M, Wang J, Zhou X, Sun X. Higher levels of TIMP-1 expression are associated with a poor prognosis in triple-negative breast cancer. Mol Cancer. 2016;15:30. doi: 10.1186/s12943-016-0515-5. PMID:27130446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei JW, Cai JQ, Fang C, Tan YL, Huang K, Yang C, Chen Q, Jiang CL, Kang CS. Signal Peptide Peptidase, Encoded by HM13, Contributes to Tumor Progression by Affecting EGFRvIII Secretion Profiles in Glioblastoma. CNS neuroscience & therapeutics. 2017;23:257–65. doi: 10.1111/cns.12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z, Galon J. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091–3. doi: 10.1093/bioinformatics/btp101. PMID:19237447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gu Z, Gu L, Eils R, Schlesner M, Brors B. circlize Implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2. doi: 10.1093/bioinformatics/btu393. PMID:24930139. [DOI] [PubMed] [Google Scholar]
- 57.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al.. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. PMID:16199517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verhaak RG, Tamayo P, Yang JY, Hubbard D, Zhang H, Creighton CJ, Fereday S, Lawrence M, Carter SL, Mermel CH, et al.. Prognostically relevant gene signatures of high-grade serous ovarian carcinoma. J Clin Invest. 2013;123:517–25. PMID:23257362. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.