Abstract
Setting
Inaccurate diagnosis and care inaccessibility undercut the effectiveness of high-quality tuberculosis (TB) treatment and select for resistance. Rapid diagnosis systems (e.g., GeneXpert) for TB diagnosis and drug-susceptibility testing (DST), and programs that provide high-quality DOTS TB treatment to patients in the unregulated private sector (pubic private mix, or PPM), may help address these challenges, albeit at increased cost.
Objective/design
We extend a microsimulation model of TB in India calibrated to demographic, epidemiologic, and care trends to evaluate: (1) replacing DST with GeneXpert; (2) replacing microscopy and culture with GeneXpert to diagnose non-MDR and MDR TB; (3) implementing nationwide PPM; and combinations of (3) with (1) or (2).
Results
PPM (assuming costs of $38/person) and GeneXpert improve health and increase costs relative to the status quo. PPM alone or with GeneXpert cost less than one GDP/capita per QALY gained relative to the next-best intervention and dominated GeneXpert interventions absent PPM.
Conclusions
While both PPM and GeneXpert are promising tools for combating TB in India, PPM should be prioritized over GeneXpert, as private sector engagement is more cost-effective than GeneXpert alone and, if sufficient resources are available, would substantially increase the value of GeneXpert if both interventions are implemented together.
Keywords: PPM, Xpert MTB/RIF, simulation
INTRODUCTION
Despite the longstanding availability of effective treatment regimens, tuberculosis (TB) remains a challenge in many resource-poor settings. Effective treatment delivery in India, a country with one of the world’s largest TB burdens, is compromised by low-sensitivity diagnostics and an unregulated private TB treatment sector that often provides substandard care, resulting in low cure rates and potential selection for multidrug resistance (MDR), defined as strains of TB resistant to at least isoniazid and rifampin, two first line anti-TB medications.1
Recent innovations in diagnostic technologies could improve care at increased costs. We focus on Xpert MTB/RIF, based on the Cepheid GeneXpert diagnostic system (“GeneXpert” hereafter), a platform for rapid and accurate diagnosis of TB and drug resistance. Sputum-smear microscopy has lower test sensitivity than GeneXpert, and improving sensitivity with culture may require months to complete, compared to hours with GeneXpert. Conventional drug susceptibility testing (DST) for MDR identification further delays accurate diagnosis, and patients infected with MDR TB may not receive appropriate treatment while awaiting their results.
Yet, prevailing patterns of TB care-seeking may diminish much of the potential value of technologies like GeneXpert. As many as 46% of TB patients in India seek care outside the Revised National TB Control Program (RNTCP) public clinic system in smaller, low-cost private clinics whose TB care practices are unregulated and where the high price of GeneXpert may limit its availability. Such patients can spend months before–eventually if ever – accessing TB care in public clinics where GeneXpert may first become available,2,3 limiting the benefits of GeneXpert rollout in public clinics.
Therefore approaches to quickly reroute private clinic patients to effective public TB care using directly observed treatment-short course (DOTS) may be required to unlock the value of new technologies implemented in the public sector. These approaches may also be cost-effective in their own right if they can prevent costly and difficult-to-cure MDR TB cases. Current national TB control goals call for extending RNTCP services,4 and pilot studies of “public-private mix” (PPM) programs that extend high-quality care to private sector patients have found PPM to be effective in shifting patients towards DOTS programs.4–7 We therefore consider PPM programs similar to these, or with more limited success, at national scales.
Our study contributes to prior studies of GeneXpert and PPM programs for TB by evaluating the cost-effectiveness of these innovations used alone or in combination and estimating public expenditure increases required to implement these strategies. 6–8 While prior studies have looked at short term effects of both strategies, no study has evaluated both lifetime costs and health benefits or evaluated these in combination policies. Quantifying lifetime cost-effectiveness addresses an important need in formulating national policy.
METHODS
We evaluate the cost-effectiveness of GeneXpert and PPM delivered alone or in combination from 2015–2025 using a previously published dynamic transmission microsimulation model of TB calibrated to Indian demography and TB epidemiology.9 The model is implemented in MATLAB (2014b, The Mathworks, Natick, MA).
Model
The model follows individuals from birth to death, through potential latent infection, active pulmonary disease, and treatment episodes in either public or private sectors. Individuals’ disease and treatment status and history influence the risk of subsequent events, reflecting India’s demographically dependent TB dynamics.10 We previously published the model calibration and validation to multiple demographic and epidemiological measures over time, including TB case notification rates, MDR incidence, and MDR prevalence.9 Additional model description and calibration results appear in the supplemental information (SI). There were no participants in this study and no Ethics Approval Statement applies.
Modeled public sector TB screening and diagnosis follow published government norms.11 Patients are initially diagnosed via sputum smear microscopy (SSM) and may be additionally screened for MDR TB during their treatment through culture and DST, as determined by their SSM status and treatment duration.11 Incomplete treatment may lead to MDR TB acquisition (Table 1 and 2). We also model private clinic care seeking and treatment. Since the quality of care in the private sector may be low and patients commonly transition between private clinics,3 there is a higher risk of exposure to TB medications for insufficient durations with consequent lower cure rates and potential for MDR acquisition (Table 1).3,12 Modeled private treatment uptake and duration are consistent with the existing literature.3,8,12
Table 1.
Selected Model Parameters
| Description | Value | Source | ||
|---|---|---|---|---|
| Test Characteristics | ||||
|
| ||||
| Test characteristics for 3 sputum smears combined for active pulmonary TB | 28 | |||
| Sensitivity | All Ages | 60% | ||
| Specificity | All Ages | 100% | ||
| DST using Löwenstein-Jensen culture | ~* | |||
| Sensitivity | All Ages | 100% | ||
| Specificity | All Ages | 100% | ||
| Time required before patient is notified of DST results | All Ages | 6 months | 13 | |
|
| ||||
| GeneXpert Test Characteristics | ||||
|
| ||||
| Sensitivity non-MDR TB | All Ages | 90% | 14 | |
| Specificity non-MDR TB | All Ages | 98% | 14 | |
| Sensitivity for rifampin resistance | All Ages | 94% | 14 | |
| Specificity for rifampin resistance | All Ages | 97% | 14 | |
| Time before patient is notified of DST results | All Ages | 0 months (2 hours) | ||
| Public-Private Mix | ||||
| Proportion of private patients within PPM system | All Ages | 0.7 | 5 | |
| Proportion of patients referred to public system | All Ages | 0.6 | 5 | |
|
| ||||
| Treatment Uptake | ||||
|
| ||||
| Probability of being tested for TB, no prior treatment | All Ages | 0.0009 | Calibrated | |
| Probability of being tested for TB, prior treatment | All Ages | 0.0013 | Calibrated | |
| Overall monthly probability of receiving RNTCP treatment for treatment naïve active TB case, given treatment is available | Age | Males | Females | Calibrated, 16,2 |
| 0 | 0.0968 | 0.0402 | ||
| 20 | 0.1254 | 0.0477 | ||
| 30 | 0.1800 | 0.0861 | ||
| 40 | 0.3743 | 0.1033 | ||
| 50 | 0.4505 | 0.1244 | ||
| 60 | 0.6000 | 0.1158 | ||
| 70 | 0.3346 | 0.0326 | ||
| Overall monthly probability of receiving RNTCP treatment if treatment experienced active TB case, given treatment is available | 0 | 0.2178 | 0.0905 | Calibrated, 16,2 |
| 20 | 0.2821 | 0.1073 | ||
| 30 | 0.4051 | 0.1939 | ||
| 40 | 0.6000 | 0.2325 | ||
| 50 | 0.6000 | 0.2799 | ||
| 60 | 0.6000 | 0.2606 | ||
| 70 | 0.6000 | 0.0734 | ||
|
| ||||
| Public Treatment | ||||
|
| ||||
| Category I/III treatment | Male | Female | 16,12,29 | |
| Probability of death**** | All Ages | 0.0101 | 0.0101 | |
| Default probability, given not dead**** | 0 | 0.02383 | 0.02421 | |
| 20 | 0.02337 | 0.0178 | ||
| 30 | 0.02145 | 0.01767 | ||
| 40 | 0.01663 | 0.01532 | ||
| 50 | 0.02278 | 0.01246 | ||
| 60 | 0.02222 | 0.01151 | ||
| 70+ | 0.02256 | 0.02038 | ||
| Probability of successful treatment, given patient has been in treatment for required time and not died nor defaulted**** | All Ages | 0.98 | 0.98 | |
| Category II treatment | Male | Female | 16,12 | |
| Probability of death**** | All Ages | 0.026 | 0.026 | |
| Default probability, given not dead**** | 0 | 0.05804 | 0.05895 | |
| 20 | 0.0569 | 0.04335 | ||
| 30 | 0.05223 | 0.04303 | ||
| 40 | 0.04051 | 0.0373 | ||
| 50 | 0.05547 | 0.03034 | ||
| 60 | 0.05411 | 0.02803 | ||
| 70+ | 0.05493 | 0.04963 | ||
| Probability of successful treatment, given patient has been in treatment for required time and not died nor defaulted**** | All Ages | 0.94 | 0.94 | |
| Probability of testing SS+ at 4 months and receiving DST (per RNTCP protocol) | All Ages | 0.57 | 10 | |
| Probability of developing MDR TB if default from treatment | All Ages | 0.242 | 30 | |
| Probability of developing MDR TB if fail from treatment | All Ages | 0.187 | 30 | |
| Probability of having latent TB after treatment | All Ages | 0.197 | 30 | |
| Category IV treatment | ||||
| Probability of death in category IV treatment | All Ages | 0.01689 | 31 | |
| Default probability, given not dead | All Ages | 0.01749 | ||
| Probability of successful treatment, given patient has been in treatment for required time and not died nor defaulted | All Ages | 0.7375 | ||
|
| ||||
| Private Treatment Parameters | ||||
|
| ||||
| Probability of cure in one private clinic treatment episode | All Ages | 0.0211 | * | |
| Probability of MDR acquisition in private clinic treatment episode | All Ages | 0.0055 | ** | |
| Maximum number of private clinic treatment episodes per active TB case | All Ages | 7 | 3 | |
| Duration (in months) of one private clinic treatment episode | All Ages | 1 | 3 | |
|
| ||||
| Self Cure | ||||
|
| ||||
| Monthly Probability of Self Cure | All Ages | 0.0064 | *** | |
The remaining model parameters may be found in Suen 2012 9, where the model was described in detail.
* Presumptive Gold Standard
Equivalent to 15% over 8 months, the length of DOTS first line treatment
Equivalent to probability of default and acquiring MDR TB in public system
Equivalent to 20% over 3 years.
Cumulative probabilities of death, default, failure, and success for a complete treatment regimen is given in Table 2
Table 2.
Treatment Outcome Probabilities for a Course of Treatment These are the proportion of patients who start category 1, 2, or 4 treatment who will die while on treatment, or will default, fail, or successfully complete that course of treatment.
| Treatment Category | Age | Success | Default | Failure | Death | ||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | Males | Females | ||
| 1 | 0 | 80.20% | 80.30% | 12.60% | 12.50% | 1.60% | 1.60% | 5.60% | 5.60% |
| 20 | 80.60% | 82.10% | 12.20% | 10.60% | 1.60% | 1.70% | 5.60% | 5.60% | |
| 30 | 80.70% | 83.00% | 12.00% | 9.60% | 1.70% | 1.70% | 5.60% | 5.70% | |
| 40 | 80.90% | 84.00% | 11.80% | 8.60% | 1.70% | 1.70% | 5.60% | 5.70% | |
| 50 | 81.10% | 84.90% | 11.70% | 7.60% | 1.70% | 1.70% | 5.60% | 5.70% | |
| 60 | 81.30% | 85.90% | 11.50% | 6.60% | 1.70% | 1.80% | 5.60% | 5.70% | |
| 70+ | 81.40% | 86.90% | 11.30% | 5.60% | 1.70% | 1.80% | 5.60% | 5.80% | |
| 2 | 0 | 48.10% | 48.10% | 33.10% | 33.00% | 3.10% | 3.10% | 15.80% | 15.80% |
| 20 | 48.80% | 51.90% | 32.20% | 28.50% | 3.10% | 3.30% | 15.90% | 16.30% | |
| 30 | 49.10% | 53.90% | 31.80% | 26.10% | 3.10% | 3.40% | 15.90% | 16.50% | |
| 40 | 49.50% | 56.00% | 31.40% | 23.70% | 3.20% | 3.60% | 16.00% | 16.80% | |
| 50 | 49.80% | 58.20% | 31.00% | 21.10% | 3.20% | 3.70% | 16.00% | 17.00% | |
| 60 | 50.20% | 60.40% | 30.60% | 18.50% | 3.20% | 3.90% | 16.10% | 17.30% | |
| 70+ | 50.50% | 62.60% | 30.20% | 15.80% | 3.20% | 4.00% | 16.10% | 17.50% | |
| 4 | all | 32.10% | 32.10% | 28.50% | 28.50% | 11.40% | 11.40% | 28.00% | 28.00% |
Interventions
We evaluate six strategies: 1) the status quo; 2) GeneXpert for DST; 3) GeneXpert for initial diagnoses and DST in public clinics; 4) PPM; 5) PPM combined with GeneXpert for DST; and 6) PPM combined with GeneXpert for initial diagnoses and DST in public clinics. While interventions to engage the private sector are heterogeneous, in this study we consider a PPM intervention where some patients entering private clinics may be referred to public clinics, assuming a cost of $38 per referral. All interventions are described briefly below and detailed in the SI.
GeneXpert for DST continues to use the triple-sputum smear method for initial TB diagnosis but uses GeneXpert instead of culture for MDR TB testing. We assume GeneXpert reduces the time from initial DST to starting TB strain-appropriate treatment from 6 months to 2 hours (duration varied in sensitivity analyses). GeneXpert strategies only use GeneXpert for public system diagnosis, although downstream effects on cure and transmission for those patients may affect individuals outside the public system.
GeneXpert for all diagnosis (initial diagnoses and DST) uses GeneXpert for initial TB diagnosis and DST instead of SSM and culture in public clinics. More TB suspects are accurately diagnosed given the higher sensitivity than SSM (Table 1),13,14 and because patients with MDR are detected from the outset, they experience fewer delays in initiating appropriate treatment. However, screening costs increase because GeneXpert is used for more patients.
The PPM intervention increases engagement of the private sector by referring 70% of suspected TB patients entering private treatment to public clinics, incurring a $38 referral cost; however, only 60% of those referred actually transfer to DOTS treatment (with PPM, 42% of private patients enter public clinic care). We estimate these rates by comparing increases in case notifications with estimates of patient volumes in private care in PPM studies. Patients who successfully transfer receive DOTS care (have a 48–86% probability of being cured over 6–8 months, depending on whether they are in category I or II treatmenta, age, and sex, versus an effective success probability of 15% over 8 months in the private sector) and incur public system costs (Table 3). To examine outcomes with less-effective PPM, in sensitivity analyses we reduce referral rates (8% of all private clinic patients enter DOTS) and increase costs (double per-patient PPM costs to $68).
Table 3.
QALY Weights and Costs
| Mean | Source | |
|---|---|---|
| QALY weights | ||
|
| ||
| Healthy | 1 | 22 |
| Healthy, with past treatment (non-MDR or MDR) | 0.942 | 16 |
| Latent DS TB | 1 | 22 |
| Latent MDR TB | 1 | 22 |
| Latent with past treatment (non-MDR or MDR) | 0.942 | 16 |
| Active DS TB | 0.663 | 17 |
| Active DS TB in RNTCP Cat I | 0.843 | 32 |
| Active DS TB in RNTCP Cat II | 0.843 | 32 |
| Active MDR TB | 0.663 | 17 |
| Active MDR TB in RNTCP Cat I | 0.663 | 17 |
| Active MDR TB in RNTCP Cat II | 0.663 | 17 |
| Active MDR TB in RNTCP Cat IV | 0.753 | * |
| Dead | 0 | |
|
| ||
| Costs, in 2013 USD | ||
| Treatment Costs** | ||
|
| ||
| Public System | ||
| Nonmedical Public Clinic Patient Costs, Monthly | 6.20 | 8 |
| Fixed Costs | ||
| Sputum Smear Cost | 4.89 | 18 |
| DST cost | 26.04 | 18 |
| CAT I/III Monthly Costs | ||
| Drugs | 3.53 | 11,19,20 |
| Other Clinic Costs | 31.08 | 33 |
| CAT II Monthly Costs | ||
| Drugs | 8.01 | 11,19,20 |
| Other Clinic Costs | 31.08 | 33 |
| CAT IV Monthly Costs | ||
| Drugs, IP (first 6 months) | 84.92 | 11,19,20 |
| Drugs, CP (following 18 months) | 67.50 | 11,19,20 |
| Other Clinic Costs | 31.08 | 33 |
| Private System | ||
| Private Treatment Direct Costs, Monthly | 68.18 | 8 |
|
| ||
| Intervention Costs | ||
|
| ||
| PPM referral (includes per-person program & monitoring costs) | 34.03 | 34 |
| GeneXpert | 18.30 | 18 |
|
| ||
| Non-TB Health Related Costs | ||
|
| ||
| Monthly Per Person, Non-TB Health Expenses | 23 | |
| Age | Males | |
| 0–9 | 0.20 | |
| 10–19 | 0.17 | |
| 20–29 | 1.20 | |
| 30–39 | 1.59 | |
| 40–49 | 1.62 | |
| 50–59 | 2.48 | |
| 60–69 | 3.25 | |
| 70+ | 4.66 | |
Average of no treatment and CAT I (effective treatment for MDR but toxic)
A full public clinic treatment regimen includes two sputum smears, one DST, and monthly drug and other clinic costs (sputum smears are replaced by DST in Cat IV) for total costs of 183.33, 264,97, and 3649.58 for category I, II, and IV treatment respectively. See Appendix Table S3 for details.
Combination strategies
Strategies that combine PPM and GeneXpert allow patients transferred from private to public sector care to benefit from GeneXpert’s speed and accuracy but incur its increased costs.
Outcomes
Main outcomes include TB prevalence, incidence, and cost-effectiveness measures over 2015–2025. We adopt a societal perspective, considering costs and benefits over a lifetime horizon and discount both at 3% annually.15 While the analysis period is 2015–2025, a lifetime horizon implies that costs and benefits for individuals alive at the end of 2025 are also counted. Post-2025 costs and health outcomes are computed using age-, sex-, disease status-, treatment status-, and strategy-specific lifetime costs and quality-adjusted life expectancy (see SI). We ensure that all outputs were robust to Monte Carlo noise by repeating simulations (see SI).
Benefits and Costs
Health benefits are expressed in quality adjusted life years (QALYs) to combine intervention effects on length and quality of life.15 QALY weights are assigned per person per month, based on health and treatment status (Table 3).15–18
In addition to costs of delivering PPM and GeneXpert, we include costs related to TB disease and treatment in private and public systems as well as background medical costs.15 Diagnostic costs vary by strategy (SSM vs. GeneXpert).18 Indian studies inform PPM cost estimates.6,19 Total costs for public sector treatment include patient, facility, personnel, and drug costs consistent with prior studies10,20–22 which depend on treatment category (see SI).18,22,23 Average monthly patient costs for private treatment were taken from the literature (Table 3).8 We estimate age- and sex-specific background medical expenditures from National Sample Survey data using a constrained linear regression model (see SI).24 All costs are in 2013 U.S. dollars, inflation adjusted and converted from Indian Rupees as required (see SI). Costs and QALYs are discounted at 3% annually.
Cost-Effectiveness and Affordability
We compare strategies using incremental cost-effectiveness ratios (ICERs) that represent the additional cost of a strategy for each additional unit of health benefit compared to the next best alternative.15 Using the convention set by the World Health Organization, an intervention is deemed cost-effective if its cost per QALY gained is less than per-capita GDP.25 We also quantify public budget expenditure requirements for each intervention to estimate affordability.
Sensitivity Analyses
To assess the sensitivity of our findings to alternative plausible assumptions and to uncertainty, we conduct univariate, multivariate, and scenario sensitivity analyses, focusing on GeneXpert and PPM attributes since these may influence cost-effectiveness. Additionally, while it is not computationally feasible to conduct a probabilistic sensitivity analysis of all inputs, we conduct probabilistic analysis on the simultaneous effect of uncertainty about the quality of life lost due to TB and the costs of care. Details of this analysis are provided in the SI.
RESULTS
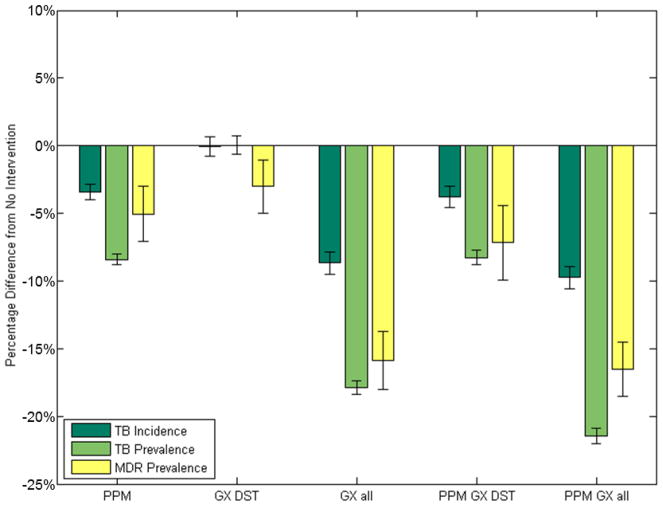
All strategies reduce future TB prevalence and incidence relative to the status quo, in which MDR prevalence and incidence rise through 2025. GeneXpert with PPM produces the largest reductions in TB incidence (10% [95% CI: 8%–11%]) and prevalence (21% [95% CI: 20%– 22%]) by 2025 (Figure 1), although even with the interventions considered, MDR incidence continues to grow, albeit more slowly. As expected, GeneXpert for DST has the smallest overall effect on TB as it targets MDR TB, a minority of TB cases. The modeled reductions are similar to published estimates for comparable interventions.26
Figure 1. Percentage Reductions in Overall and MDR TB Incidence and Prevalence.
All strategies considered reduce future TB prevalence and incidence over the 10-year analysis period relative to no intervention, although MDR TB prevalence grows in absolute size in all cases. PPM with GeneXpert for all diagnosis produces the largest percentage decreases in TB incidence and prevalence relative to the status quo, and GeneXpert for DST has the smallest effect on the overall TB as it intentionally targets MDR which affects a relatively small number of TB cases. GX = GeneXpert.
Reducing TB burden by increasing effective care, either through GeneXpert or PPM, both reduces the TB death rate and increases the quality of life for those who are cured increasing overall QALYs (Table 4).
Table 4.
Resource Use, Costs, Health Benefits, and Cost-Effectiveness
| Strategy | RNTCP Expenditures Needed Relative to Current Expenditures | Case Notifications* | Avg Lifetime Costs | s.e. | Avg Lifetime QALYs | s.e. | ICER at Mean |
|---|---|---|---|---|---|---|---|
| No intervention | 100% | 131 | 506.87 | 0.09 | 24.740 | 0.00225 | NA |
| GeneXpert for DST | 104% | 131 | 507.27 | 0.12 | 24.743 | 0.00226 | dominated |
| PPM | 109% | 138 | 507.87 | 0.11 | 24.754 | 0.00228 | 72.06 |
| PPM + GeneXpert for DST | 112% | 211 | 508.12 | 0.10 | 24.756 | 0.00213 | 144.52 |
| GX for all diagnosis | 384% | 137 | 523.61 | 0.13 | 24.764 | 0.00207 | dominated |
| PPM + GeneXpert for all diagnosis | 394% | 243 | 524.57 | 0.12 | 24.771 | 0.00236 | 1103.58 |
Annual case notifications per 100,000 for first-line public sector treatment over 2015–2025
Strategies alter current patterns of care within the public and private systems to achieve these health benefits, increasing healthcare resource use and consequent costs. PPM resulted in an average annual case notification rate of 138 per 100,000 over the analysis period, compared to 131 in the status quo (a 5% increase, comparable to the 2–26% increases observed in empirical studies5). GeneXpert for all diagnosis resulted in an average case notification rate of 211 per 100,000, as its high sensitivity allowed diagnosis of more of the prevalent TB cases than current methods. The costs of treating these additional patients, acquiring diagnostic systems, establishing infrastructure, transferring patients, maintaining a larger trained workforce, and quality monitoring and maintenance are considerable. The average discounted lifetime cost under the status quo was $507, for PPM $508, for GeneXpert for all diagnosis $524, and the combination of the two was $525 (see Table 4 for total expenditures relative to current RNTCP expenditures).
While GeneXpert for all diagnosis and PPM increase case notifications to similar values when implemented alone, PPM does so at a lower cost since it targets patients already identified by the private system, therefore dominating GeneXpert despite its other advantage in triaging MDR patients to appropriate treatment. However, while the total cost outlay for the combination strategy would be large, it would be cost-effective given its large additional health benefits gained from increasing the number of patients receiving effective TB and MDR-TB care.
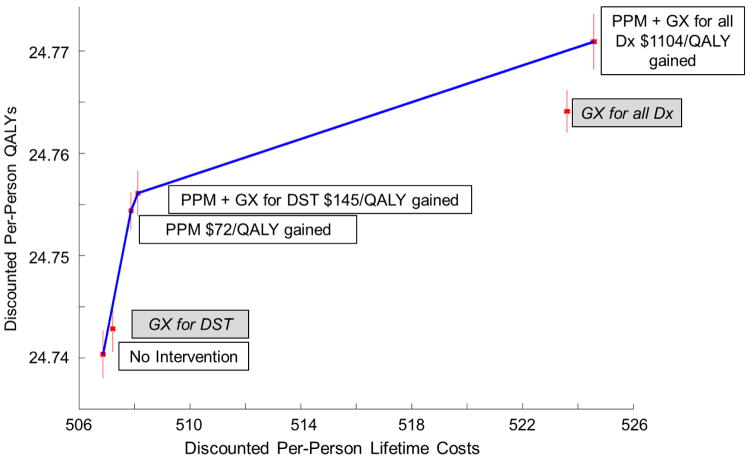
Figure 2 shows cost-effectiveness results. Employing a willingness-to-pay threshold of India’s per-capita GDP ($1,450),27 the preferred strategy is PPM with GeneXpert for all diagnosis at a cost per QALY gained of $1,103 compared to PPM with GeneXpert for DST. These estimates are robust to simulation sampling noise (with PPM with GeneXpert for all diagnosis preferred 99% of the time [see SI]).
Figure 2. Cost-Effectiveness Frontier.
The efficient frontier shows strategies that are potentially cost-effective (those labeled in white boxes with incremental cost-effectiveness ratios). Dominated strategies are shown off the efficient frontier and labeled with gray boxes. Monte Carlo simulation sampling uncertainty for the costs and QALYs of each strategy is depicted as red 95% confidence intervals. PPM with GeneXpert is cost-effective (with an expected cost of $1103.58 per QALY gained), and even with sampling noise, this finding occurs with 99% probability at a willingness to pay of India’s per-capita GDP ($1,450). GX = GeneXpert and Dx = diagnosis.
While the interventions may represent reasonable value for money, implementation expenses may stress public budgets for TB (e.g., RNTCP). For each strategy, we estimate the annual per-capita public expenditure on TB care provision between 2015 and 2025. If the status quo continued, expenditures would be $0.37 per person per year ($444 million for a population of 1.2 billion). The cost-effective strategies increased this to $0.40 per person (PPM) and $1.45 (PPM with GeneXpert for all diagnosis). The feasibility of a 400% increase in India’s governmental TB care budget, relative to the current expenditures of $444 million, is unclear.
Sensitivity Analyses
Given the findings of our main analysis, we conduct sensitivity analyses focusing on circumstances where PPM, GeneXpert, or both might be expected to deliver less additional health benefit or incur higher costs. In general, our main findings remain robust and unchanged. We highlight key analyses here and provide detail on all analyses in the SI.
Natural history and treatment-related uncertainties: In reality, the sensitivity and specificity of culture is not perfect, a simplifying assumption used in the model. Reduction in the sensitivity and specificity of culture (both to 90%) did not change our findings. Reducing private treatment system costs until they were similar to public category I treatment costs, or doubling category IV treatment costs, did not change our results. Our results remained robust when we reduced or eliminated coverage of category IV treatment or access to culture, reduced diagnostic delay from culture to 2 months, assumed equal public clinic uptake across age and sex. Likewise, they remained robust when we incorporated a 6% initial default rate after immediately after diagnosis, higher MDR TB acquisition rates for failures in public clinics (60%), reducing MDR TB acquisition rates for defaulters (5%), or increasing the self-cure rate to 50% over 3 years.
PPM-related uncertainties: Varying the rate of MDR acquisition by 2.5-fold did not alter our primary findings, although then PPM would reduce MDR prevalence by 1.2 per 100,000, and likewise, if rates are lower (equal to those in the public sector in our main analysis), then PPM reduces MDR prevalence by 0.2 per 100,000. Similarly, independently increasing private clinic cure rates, or public sector treatment failure, default, or death rates by 2-fold did not alter our main results.
Reducing private clinic care use did not change our main findings unless uptake was less than 10% of our main analysis. In contrast, higher private clinic use rates would increase the number of patients affected by PPM and strengthen our main results. Additionally, our results remain unchanged even if only 8.4% all private clinic patients transfer to public sector care (20% of the main analysis) or if per-patient PPM costs were doubled.
GeneXpert-related uncertainties: Our findings remain unchanged even when we increase the delay between testing and the start of strain-appropriate treatment to two months. When we vary GeneXpert’s sensitivity and specificity simultaneously for non-MDR and rifampicin-resistant TB to their lower 95% confidence bounds, our findings remain unchanged, although public expenditures would increase by 8% ($1.56 per person) for PPM with GeneXpert for all diagnosis with decreased TB specificity. Cost-effectiveness results also remain unchanged if GeneXpert costs decrease to a volume-discounted price of $14.94.18
Simultaneous Variation of Treatment Characteristics: PPM may seem less attractive in settings where more effective private clinics target select patients in areas with low public clinic presence. In a scenario where public clinic death, default, failure rates and private clinic cure rates are simultaneously 20% higher and private clinic treatment uptake rates, private clinic MDR acquisition rates, and PPM referral success rates are simultaneously 20% lower, the efficient frontier includes PPM alone ($80 per QALY gained relative to the status quo), PPM with GeneXpert for DST ($262 per QALY gained), and GeneXpert for all diagnosis ($1,072 per QALY gained) (see SI). Hence, relative differences in public and private sector treatment quality and uptake can be important determinants of the value of PPM and GeneXpert for all diagnosis, implying that they should be evaluated carefully within the context of Indian regional differences.
In a probabilistic sensitivity analysis of uncertainties in costs and QALY (ranges in Appendix), we find that at a willingness to pay of India’s per-capita GDP, PPM with GeneXpert for all diagnosis is cost-effective nearly 100% of the time.
DISCUSSION AND CONCLUSION
In India, health systems innovations such as engaging the private TB sector through PPM programs may be a valuable investment due to their potential for unlocking the benefits of technical innovations implemented in the public sector. A national-scale program that combined PPM and GeneXpert for both initial TB diagnosis and DST for MDR is projected to cost $1103 per QALY gained relative to PPM programs that make less use of GeneXpert. Furthermore, programs that include PPM dominate those that use GeneXpert without engagement of the private sector. Challenges facing such programs include affordability, as national-scale programs might require 400% of RNTCP’s current TB care budget, as well as ensuring that both health system and technical innovations maintain their efficacy and unit costs at scale.
Our results illustrate that there is no silver bullet for combating the TB epidemic -- introducing rapid and accurate diagnostic systems, either for initial diagnosis or DST, will have limited ability to control the epidemic and, in a context where PPM is available, is not cost-effective if implemented without substantial effort to bring the fragmented public and private treatment systems together. PPM should be prioritized over GeneXpert, as private sector engagement is more cost-effective than GeneXpert alone and, if sufficient resources are available, would substantially increase the value of GeneXpert if both interventions are implemented together.
The combination of PPM and GeneXpert for all diagnosis including MDR diagnosis would have the additional benefit of reducing the prevalence of MDR TB, which is an important issue in ongoing epidemic control. PPM programs alone may not be able to significantly impact the MDR TB epidemic, as only GeneXpert strategies would be able to quickly provide DST and consequent strain-appropriate care -- GeneXpert used for initial diagnosis and DST at the outset of treatment may generate 15% declines in MDR TB prevalence over the next ten years (Figure 1).
This study has several limitations. Our model does not account for the fact that smear and Xpert preferentially diagnose the most infectious cases as it is unclear to what extent patient infectivity declines as more cases are diagnosed. It is unclear how and to what extent, assuming no change in overall infectivity, this would alter our results, as both PPM and GeneXpert interventions would diagnose relatively lower-infectivity patients. Our model also does not consider the impacts of other diseases and policies on India’s TB epidemic such as HIV or diabetes. However, including comorbidities in the analysis may imply that the strategies we identify are even more beneficial, if other diseases can be addressed incidentally as more individuals receive better healthcare and avoid the serious financial hardships accompanying TB. It is also important to recognize the relative scarcity of data on the TB epidemic in India, as our results rely on estimates of private and public clinic use, treatment effectiveness, and PPM program effectiveness from studies that may not be population representative. To mitigate this uncertainty, we perform extensive sensitivity analyses, which indicate that our results are generally robust. We also identify situations where one might prefer to implement technical innovations like GeneXpert without also implementing PPM health system programs. This occurs in settings where the public system provides only marginally better quality care than the private system and private clinic usage is low. To improve the accuracy of cost-effectiveness estimates for TB in India, increasing both epidemiological and health systems data collection, particularly about the private sector, is a research priority.
While there is substantial focus and excitement about technological innovations to address health challenges in the developing world, our findings show that health systems innovations are important complements to technical innovations. Our results indicate that PPM could deliver substantial value in its own right, and when combined with GeneXpert, could unlock substantial additional value by expanding the pool of TB patients in India accessing this new technology.
Supplementary Material
Acknowledgments
Sources of funding: SS is supported by the National Science Foundation Graduate Research Fellowship under Grant No. DGE-114747 (http://www.nsfgrfp.org/). EB is supported by the National Institute of Allergy and Infectious Diseases (K01-AI084582)(http://www.niaid.nih.gov/) and the Doris Duke Charitable Foundation (http://www.ddcf.org/). JGF is supported in part by the National Institute for Aging (K01-AG037593-01A1)(http://www.nia.nih.gov/), Stanford’s Global Development and Poverty Research Initiative, and Stanford’s Freeman Spogli Institute’s Underdevelopment Action Fund. Sections of this work supported in part by the National Institute on Drug Abuse Grant (R01-DA15612).
The authors would like to thank Dr. Kimberly Babiarz for her work on the National Sample Survey data which made estimating background medical costs possible.
Abbreviations
- TB
Tuberculosis
- MDR
multi-drug resistant
- SSM
sputum smear microscopy
- DST
drug susceptibility testing
- PPM
public-private mix
- GeneXpert or GX
Xpert MTB/RIF using Cepheid GeneXpert diagnostic system
- DOTS
directly observed treatment-short course
- QALYs
quality adjusted life years
Footnotes
As defined by the RNTCP, the category I/III treatment regimen is for treatment-naïve patients and is 6 months; category II treatment is 8 months long and for treatment-experienced patients. Both use DOTS and are for treating non-MDR TB cases. Category IV treatment is MDR TB treatment that uses a 24-month regimen. Drugs and treatment protocols are listed in the Appendix.
Declaration of Interests: No conflicts of interests exist.
Author contributions statement: SS, EB, and JGF contributed to the conception of the work, study design, and data acquisition. SS implemented the simulation. All authors contributed to the analysis, manuscript drafting, and revision.
References
- 1.Vandan N, Ali M, Prasad R, Kuroiwa C. Assessment of doctors’ knowledge regarding tuberculosis management in Lucknow, India: a public-private sector comparison. Public Health. 2009 Jul;123(7):484–9. doi: 10.1016/j.puhe.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Satyanarayana S, Nair SA, Chadha SS, Shivashankar R, Sharma G, Yadav S, et al. From where are tuberculosis patients accessing treatment in India? Results from a cross-sectional community based survey of 30 districts. PLoS One. 2011 Jan;6(9):e24160. doi: 10.1371/journal.pone.0024160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dye C. The potential impact of new diagnostic tests on tuberculosis epidemics. Indian J Med Res. 2012 May;135(5):737–44. [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Cost and cost-effectiveness of public-private mix dots: evidence from two pilot projects in India [Internet] Geneva, Switzerland: 2004. Available from: http://apps.who.int/iris/bitstream/10665/68620/1/WHO_HTM_TB_2004.337.pdf?ua=1. [Google Scholar]
- 5.Dewan PK, Lal SS, Lonnroth K, Wares F, Uplekar M, Sahu S, et al. Improving tuberculosis control through public-private collaboration in India: literature review. BMJ [Internet] 2006 Mar 11;332(7541):574–8. doi: 10.1136/bmj.38738.473252.7C. [cited 2014 Oct 16] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1397734&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pantoja A, Lönnroth K, Lal SS, Chauhan LS, Uplekar M, Padma MR, et al. Economic evaluation of public-private mix for tuberculosis care and control, India. Part II. Cost and cost-effectiveness. Int J Tuberc Lung Dis. 2009 Jan;13:705–12. [PubMed] [Google Scholar]
- 7.Acuna-Villaorduna C, Vassall A, Henostroza G, Seas C, Guerra H, Vasquez L, et al. Cost-Effectiveness Analysis Of Introduction Of Rapid, Alternative Methods To Identify Multidrug-Resistant Tuberculosis In Middle-Income Countries. Clin Infect Dis [Internet] 2008 Aug 15;47(4):487–95. doi: 10.1086/590010. [cited 2012 Mar 9] Available from: http://www.ncbi.nlm.nih.gov/pubmed/18636955. [DOI] [PubMed] [Google Scholar]
- 8.Pantoja A, Floyd K, Unnikrishnan KP, Jitendra R, Padma MR, Lal SS, et al. Economic evaluation of public-private mix for tuberculosis care and control, India. Part I. Socioeconomic profile and costs among tuberculosis patients. Int J Tuberc Lung Dis. 2009 Dec;13:698–704. 2008. [PubMed] [Google Scholar]
- 9.Suen S, Bendavid E, Goldhaber-Fiebert JD. Disease control implications of India’s changing multi-drug resistant tuberculosis epidemic. PLoS One [Internet] 2014 Jan;9(3):e89822. doi: 10.1371/journal.pone.0089822. [cited 2014 Oct 16] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3946521&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santha T, Garg R, Frieden TR, Chandrasekaran V, Subramani R, Gopi PG, et al. Risk factors associated with default, failure and death among tuberculosis patients treated in a DOTS programme in Tiruvallur district, south India, 2000. Int J Tuberc Lung Dis [Internet] 2002 Sep;6(9):780–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12234133. [PubMed] [Google Scholar]
- 11.Revised National Tuberculosis Control Programme (RNTCP) Training Module for Medical Practitioners. Journal of the Indian Medical Association. 2010 Dec; [PubMed] [Google Scholar]
- 12.Central TB Division Directorate General of Health Services and Ministry of Health and Family Welfare. RNTCP performance report, India: Third quarter, 2010 [Internet] 2010 Available from: http://www.tbcindia.org.
- 13.Singla R, Sarin R, Khalid UK, Mathuria K, Singla N, Jaiswal A, et al. Seven-year DOTS-Plus pilot experience in India: results, constraints and issues. Int J Tuberc Lung Dis. 2009 Aug;13(8):976–81. [PubMed] [Google Scholar]
- 14.Chang K, Lu W, Wang J, Zhang K, Jia S, Li F, et al. Rapid and effective diagnosis of tuberculosis and rifampicin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect. 2012 Jun;64(6):580–8. doi: 10.1016/j.jinf.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford University Press; 1996. p. 1456. [Google Scholar]
- 16.Collaborations for Health System Improvement and Impact Evaluation in India. Cohesive India [Internet] 2010 Available from: Http://www.cohesiveindia.org/research-projects.html#best.
- 17.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2010:2129–43. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vassall A, van Kampen S, Sohn H, Michael JS, John KR, den Boon S, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med [Internet] 2011 Nov;8(11):e1001120. doi: 10.1371/journal.pmed.1001120. [cited 2014 Jul 9] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3210757&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stop TB Partnership. Global drug facility product catalogue [Internet] Geneva, Switzerland: 2013. Available from: www.stoptb.org/gdf. [Google Scholar]
- 20.Global Drug Facility, Stop TB Partnership. Buying quality, affordable tuberculosis drugs through the Global Drug Facility: A guide to the Direct Procurement Service for donors, non-governmental organizations and programme managers. 2013 [Google Scholar]
- 21.Resch SC, Salomon Ja, Murray M, Weinstein MC. Cost-effectiveness of treating multidrug-resistant tuberculosis. PLoS Med [Internet] 2006 Jul;3(7):e241. doi: 10.1371/journal.pmed.0030241. [cited 2012 Mar 9] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1483913&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winetsky DE, Negoescu DM, DeMarchis EH, Almukhamedova O, Dooronbekova A, Pulatov D, et al. Screening and rapid molecular diagnosis of tuberculosis in prisons in Russia and Eastern Europe: a cost-effectiveness analysis. PLoS Med [Internet] 2012 Jan;9(11):e1001348. doi: 10.1371/journal.pmed.1001348. [cited 2014 Oct 14] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3507963&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Indian Ministry of Statistics and Programme Implementation. NSS Survey Reports [Internet] 2014 Available from: http://mospi.nic.in/Mospi_New/site/inner.aspx?status=3&menu_id=31.
- 24.Goldhaber-Fiebert JD, Suen S, Babiarz K. Society for Medical Decision Making [Internet] Miami, Florida: Society for Medical Decision Making; 2014. Estimating individual medical expenditures from household surveys in the context of urbanization and economic development: India, 1995–2010. Available from: https://smdm.confex.com/smdm/2014fl/webprogram/Paper8347.html. [Google Scholar]
- 25.World Health Organization. Cost effectiveness and strategic planning (WHO-CHOICE): Cost-Effectiveness Thresholds [Internet] 2014:2014. Available from: http://www.who.int/choice/costs/CER_thresholds/en/
- 26.Salje H, Andrews JR, Deo S, Satyanarayana S, Sun AY, Pai M, et al. The importance of implementation strategy in scaling up Xpert MTB/RIF for diagnosis of tuberculosis in the Indian health-care system: a transmission model. PLoS Med [Internet] 2014 Jul;11(7):e1001674. doi: 10.1371/journal.pmed.1001674. [cited 2014 Jul 21] Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4098913&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The World Bank. GDP Per Capita [Internet] 2014:1–12. Available from: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD.
- 28.Srikanth P, Kamesh S, Daley P. Bleach optimization of sputum smear microscopy for pulmonary tuberculosis. Indian J Tuberc. 2009;56:174–84. [PubMed] [Google Scholar]
- 29.Babiarz KS, Suen S, Goldhaber-Fiebert JD. Tuberculosis treatment discontinuation and symptom persistence: an observational study of Bihar, India’s public care system covering > 100,000,000 inhabitants. BMC Public Health. 2014;14(418):1–13. doi: 10.1186/1471-2458-14-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma SK, Kumar S, Saha PK, George N, Arora SK, Gupta D, et al. Prevalence of multidrug-resistant tuberculosis among Category II pulmonary tuberculosis patients. Indian J Med Res [Internet] 2011 Mar;Mar;133:312–5. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3103157&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 31.Sachdeva KS. Overview of drug resistant TB in India and national scale up of MDR TB diagnosis and treatment TB burden in India. Facing the Reality Of Multidrug-Resistant Tuberculosis: Challenges and Potential Solutions in India; Apr 18–19; New Delhi, India: Indian National Science Academy; 2011. [Google Scholar]
- 32.Dhingra V, Rajpal S. Health related quality of life (HRQL) scoring in tuberculosis. Indian J Tuberc [Internet] 2003:99–104. [cited 2014 Oct 17] Available from: http://medind.nic.in/ibr/t03/i2/ibrt03i2p99.pdf.
- 33.World Health Organization. Cost effectiveness and strategic planning (WHO-CHOICE) Tables of Costs and Prices used in WHO-CHOICE Analysis [Internet] 2014 Available from: http://www.who.int/choice/costs/en/
- 34.Ferroussier O, Kumar MKA, Dewan PK, Nair PKJ, Sahu S, Wares DF, et al. Cost and cost-effectiveness of a public-private mix project in Kannur District, Kerala, India, 2001–2002. Int J Tuberc Lung Dis. 2007 Jul;11:755–61. 2006. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.