Summary
Background
Visceral adipose tissue (VAT) generally demonstrates a stronger relationship with cardiometabolic risk factors than total body fat or subcutaneous adipose tissue.
Objectives
The purpose of this study was to compare VAT estimated in children by total volume dual energy X-ray absorptiometry (DXA) with a gold standard measurement, single slice (L4-L5) computed tomography (CT).
Methods
A total of 329 (152 females, 177 males) children ages 6–18 years (mean age 12.3 ±3.6) and with average body mass index percentile of 54.9% (3–99%) had their VAT estimated by both CT and DXA. Linear association between methods was measured using Pearson’s correlation. Multiple linear regressions compared the associations between cardiometabolic risk factors and both CT-VAT and DXA-VAT, respectively.
Results
In children, DXA-VAT was correlated significantly with CT-VAT, with a stronger relationship in overweight and obese children. Multiple regression analysis showed that both estimates of VAT were significantly associated with lipids and insulin sensitivity, measured by euglycemic-hyperinsulinaemic clamp. Additionally, DXA-VAT was associated with diastolic blood pressure, homeostasis model of insulin resistance and fasting insulin, but CT-VAT was not.
Conclusion
In children, total volume DXA-VAT and single slice CT-VAT are significantly correlated and each demonstrates similar associations with cardiometabolic risk factors. This suggests that DXA is a useful and valid method for estimation of VAT in children.
Keywords: Pediatrics, Obesity, Body Composition, Lipids, Insulin resistance
Introduction
Obesity continues to be a major public health issue in the United States and many industrialized nations 1 and has been linked to hypertension, insulin resistance, type 2 diabetes mellitus and coronary heart disease.2 It is well established in adults that visceral adipose tissue (VAT) has a stronger relationship with cardiometabolic risk factors and insulin resistance than other types of adipose tissue.3–5 In children, conflicting results in the association of VAT with lipid markers, insulin and blood pressure have been reported.6–14 Previous studies used either computed X-ray tomography (CT) or magnetic resonance imaging (MRI) to quantify VAT. Each has significant limitations for clinical research and practice because of cost, the need for manual analysis, extended scan times and a limitation to the maximum size of participants that can be examined. Additionally, CT emits ionizing radiation making its use for VAT especially problematic in children.15
Because of these limitations, most studies have used a single abdominal slice within the android region to estimate VAT, often at the umbilicus or L4–L5.3–14 However, studies in adults demonstrated high intrasubject variability16 depending on slice site, differences by sex and race17 and differences reported in the association between VAT and metabolic syndrome.18–20 Similar variations in regional adipose tissue distribution by sex and race have been shown in pre-pubertal children.21
Software advancements have made it possible for the dual-energy X-ray absorptiometry (DXA) to estimate the amount of VAT and subcutaneous adipose tissue within the android region. Recently, DXA for quantification of VAT has been validated in adults22 and a study in adults observed DXA-VAT to be reproducible and useful as a clinical marker of cardiometabolic risk.23 This new method is less expensive than CT or MRI, provides immediate quantification of regional body composition and scans participants weighing up to 450-lb (DXA; Lunar Prodigy, General Electric Medical Systems, Madison, WI, USA). In addition, the extremely low amount of radiation emitted by DXA is considered safe for use in children. To date, VAT estimation by DXA has not been examined in children. Furthermore, it has not been compared with current methods of single slice using CT or MRI in children. The purpose of this study was to compare total volume DXA-VAT with single slice CT-VAT in children. Given that DXA is currently the standard method used for measuring total body fat, confirmation of DXA as a useful estimate of visceral and subcutaneous adipose tissue would offer a valuable alternative to CT and MRI.
Methods
Study Design and Participants
The current study was a cross-sectional analysis of data obtained in 329 children ages 6–18 years. The range of adiposity ranged from lean to obese (body mass index [BMI] 13–45 kg m−2 and BMI percentile from 3% to 99% mean 55%). Data were combined from a community-based study evaluating cardiometabolic risk in children and a group of healthy siblings of childhood cancer survivors. Data were collected from 2007 to 2011 (n = 555). Only participants with VAT measured by CT and a full-body composition scan by DXA were included. The respective protocols were approved by the University of Minnesota Institutional Review Board and consent/assent was obtained from parents and participants, respectively.
Anthropometric and Blood Pressure Measurements
Testing was conducted at the University of Minnesota Clinical Translation Science Institute after participants had been fasting for a minimum of eight h. Tanner stage of sexual maturation was determined by trained paediatricians. Height and weight were measured on a calibrated stadiometer and electronic scale, respectively, while participants were wearing light clothes and without shoes. BMI was calculated as kg m−2. BMI percentile was calculated based on the Centers for Disease Control (CDC) growth charts using age and sex. Waist circumference was measured to the nearest 0.5 cm, taken in duplicate and the mean value reported. Blood pressure was measured in duplicate on the right arm after participants were sitting in a quiet room for at least five min using a digital blood pressure cuff and the average of the two values was reported.
Body Composition and Visceral Adipose Quantification
Total body composition was measured using DXA (Lunar Prodigy, General Electric Medical Systems) and analysed using its enCore™ software (platform version 13.6, GE Healthcare, Madison, WI, USA). Participants were scanned using standard imaging and positioning protocols while fasted. Estimates of abdominal visceral and subcutaneous adipose tissue were obtained using the method described previously for adults.22 Android fat was measured by a region of interest automatically defined with a caudal limit placed at the top of the iliac crest and its height set to 20% of the distance from the top of the iliac crest to the base of the skull. Subcutaneous fat and visceral fat were estimated within the android region. All scans were reviewed for accurate placement of the android box by the same technician.
Estimates of abdominal visceral and subcutaneous adipose tissue were obtained by CT using a Siemens Sensation 16 (Siemens Medical Solutions, Malvern, PA, USA) with two separate 10 mm slices obtained at the L4-L5 interspace. The two images were subdivided into 5 mm slices and the first and third 5 mm slices were combined and analysed for VAT. The upper limit of adipose tissue density was −30 Hounsfield units and the lower limit was −190 Hounsfield units. Image slices were individually analysed by one trained technician using a computer program (Fat Scan version 3.0; N2 System, Osaka, Japan). A lack of interrater and intrarater reliability may be a limitation; however, the technician is extremely experienced in CT imaging analyses. DXA and CT measurements were taken within 1 week of each other.
Measurement of Blood Markers
Insulin sensitivity was measured by the hyperinsulinaemic clamp as previously described.24 Insulin was infused at a constant rate of 1mU kg−1 min−1 for 3 h, and glucose was infused at a variable rate to maintain euglycemia. Insulin sensitivity (M) was expressed as the glucose infusion rate (mg kg−1 min−1 of glucose) during the last 40 minutes of the clamp, with adjustment for lean body mass (M/LBM). Low M/LBM represents insulin resistance. Fasting blood samples were collected for lipids, glucose and insulin and assays were conducted with standard procedures at the Fairview Diagnostic Laboratories, Fairview-University Medical Center (Minneapolis, MN, USA), a CDC-certified laboratory. Homeostasis model of insulin resistance (HOMA-IR) was calculated as described previously.25
Statistical Analysis
Unpaired t-tests were used to compare males and females for demographic and cardiometabolic characteristics and is presented in Table 1. Pearson’s correlation was used to assess the linear relationship between the two measures of VAT. Both CT and DXA-VAT were right skewed so data were log transformed before correlation was measured. As these two measures differed in measurement units, Kendall’s tau correlation coefficient was used to measure the concordance of the ranks between the two measures to assess their independence. Kendall’s tau correlation coefficient measures the probability of concordance of the rank values for each method. Multiple linear regression analysis for the whole sample, adjusted for age, sex, race, BMI percentile, Tanner stage and total fat mass (TFM) was used to evaluate associations of VAT with cardiometabolic risk factors. Sex, race and Tanner stage were factors with the reference levels set as male, Caucasian and Tanner stage 1, respectively. Interaction terms were included in the model and removed if they failed to show significance. Covariates were chosen based upon correlation analysis. Variables with a significant association (P< 0.05) with cardiometabolic risk factors were included within the model. Additionally, age and Tanner stage were used to control for pubertal status. Models were reduced if covariate variables were not significantly associated with the dependent variable. Variables were first removed at a modest level of significance (P>0.5). This cut-point decreased (P>0.3 and 0.1) after variables were removed. Akaike information criterion was calculated for each model to determine if the reduced, final model had the best fit. Separate analysis was completed for CT-VAT and DXA-VAT to compare the associations of each method with cardiometabolic risk factors. A variance inflation factor (VIF) was calculated for each model to monitor collinearity between covariates. Given the smaller variance explained by our models, conservative thresholds were set to monitor collinearity. Models were reexamined if variables of interest had a VIF greater than 2.5 or if covariates had a VIF greater than 4. The results for each regression are presented in Tables 2 and 3. The estimate, standard error, adjusted R2 for the final model and p-value for the estimate are reported with the individual R2 (proportion of explain variance for that variable) for DXA-VAT, CT-VAT and DXA-TFM. The covariates that remained in the final models are listed at the bottom of Tables 2 and 3. The complete results of each model including covariates are presented in Supporting Information Tables S1 and S2. All analyses were done using R (R Foundation for Statistical Computing, www.R-project.org).
Table 1. Demographics and Clinical measures table for females, males and the total sample.
Demographic and Clinical Measurements by Sex and Total (mean [±SE]) or geometric mean (x,x 95% confidence interval)
| Females (n=152) | Males (n=177) | p-value | Total (n=329) | |
|---|---|---|---|---|
|
| ||||
| Age (years) | 12.4(0.3) | 12.2(0.3) | 0.55 | 12.3(0.2) |
| Height (cm) | 150.2(1.0) | 153.4(1.1) | 0.14 | 151.9(1.1) |
| Weight (kg) | 50.2(1.2) | 52.5(1.4) | 0.37 | 51.4(1.3) |
| BMI Percentile (%) | 55.0(50,61) | 54.9(49,62) | 0.44 | 54.9(51,60) |
| Race (%) | ||||
| Non-Hispanic white | 108(71) | 127(72) | - | 235(72) |
| Non-Hispanic black | 29(19) | 28(16) | - | 57(17) |
| Other | 15(10) | 22(12) | - | 37(11) |
| Tanner Stage (%) | ||||
| 1 | 56(37) | 78(44) | - | 134(41) |
| 2 | 6(4) | 23(13) | - | 29(9) |
| 3 | 12(8) | 13(7) | - | 25(7) |
| 4 | 29(19) | 19(11) | - | 48(15) |
| 5 | 49(32) | 44(25) | - | 93(28) |
| SBP (mmHg) | 102*(0.8) | 106(0.8) | 0.002 | 104(0.6) |
| DBP (mmHg) | 59(0.6) | 60(0.7) | 0.39 | 60(0.5) |
| HDL-C (mmol/L) | 1.29(0.3) | 1.25(0.3) | 0.33 | 1.27(0.3) |
| LDL-C (mmol/L) | 2.24(0.6) | 2.25(0.6) | 0.90 | 2.24(0.6) |
| Triglycerides (mmol/L) | 0.77(0.7,0.8) | 0.76(0.7,0.8) | 0.90 | 0.77(0.7,0.8) |
| Insulin (pmol/L) | 43.8(38,51) | 41.8(37,47) | 0.55 | 42.8 (39,47) |
| Glucose (mmol/L) | 4.4*(0.7) | 4.6(0.8) | 0.01 | 4.5(0.8) |
| M/LBM (mg/kg/min) | 12.8(0.3) | 13.2(0.4) | 0.48 | 13.0(0.3) |
| HOMA-IR | 1.2(1.0,1.4) | 1.2(1.0,1.4) | 0.73 | 1.2(1.1,1.3) |
| CT-VAT (cm2) | 15.9(15,17) | 15.5(14,17) | 0.80 | 15.7(15,17) |
| DXA-VAT (cm3) | 80.5*(66, 98) | 120.9(103,142) | 0.002 | 113(100,129) |
| DXA-TFM (kg) | 11.7*(10,13) | 8.9(8,10) | 0.003 | 10.1(9,11) |
Significantly different from males at P=0.05.
BMI = Body Mass Index, SBP = systolic blood pressure, DB= diastolic blood pressure, HDL-C = High density lipoprotein cholesterol, LDL-C = Low Density lipoprotein cholesterol, Mlbm = glucose utilization per minute per kg of lean body mass, CT VAT = Computed Tomography derived visceral adipose tissue, DXA VAT = Dual X-ray absorptiometry derived visceral adipose tissue, TFM = total fat mass
Table 2. Comparison of regression results between CT VAT and DXA VAT for cardiovascular risk variables.
Regression analysis for blood lipid variables and blood pressure
| DXA | CT | ||||
|---|---|---|---|---|---|
|
| |||||
| Variables | Estimate (±SE) | p-value | Variables | Estimate (±SE) | p-value |
| log TG (model adjusted R2 = 0.187) | log TG (Model adjusted R2 = 0.1984) | ||||
| Independent Variables | Independent Variables | ||||
| log DXA VAT (R2 = 0.107) | 0.11(.02) | <0.001 | log CT VAT (R2 = 0.198) | 0.35(0.04) | <0.001 |
| log DXA TFM (R2 = 0.086) | 0.13(.04) | <0.001 | |||
| HDL-C (model adjusted R2 = 0.1416) | HDL-C (model adjusted R2 = 0.1181) | ||||
| Independent Variables | Independent Variables | ||||
| log DXA VAT (R2 = 0.082) | −2.39(0.6) | <0.001 | log CT VAT (R2 = 0.118) | −7.0(1.1) | <0.001 |
| log DXA TFM (R2 = 0.063) | −2.66(0.9) | <0.001 | |||
| LDL-C (model* adjusted R2 =0.1103) | LDL-C (model* adjusted R2 = 0.1185) | ||||
| Independent Variables | Independent Variables | ||||
| log DXA VAT (R2 = 0.025) | 3.0(1.4) | 0.027 | log CT VAT (R2 = 0.038) | 13.4(2.7) | <0.001 |
| log DXA TFM (R2 = 0.020) | 7.0(2.4) | 0.003 | |||
| SBP (model* adjusted R2 = 0.3671) | SBP (model* adjusted R2 = 0.3633) | ||||
| Independent Variables | Independent Variables | ||||
| log DXA VAT (R2 = 0.031) | 0.84(.5) | 0.107 | log CT VAT (R2 = 0.082) | 3.1(1.7) | 0.077 |
| log DXA TFM (R2 = 0.139) | 3.4(.9) | <0.001 | |||
| DBP (model* adjusted R2 = 0.073) | DBP (model* adjusted R2 = 0.074) | ||||
| Independent Variables | Independent Variables | ||||
| log DXA VAT (R2 = 0.026) | 1.0(0.4) | 0.022 | log CT VAT (R2 = 0.009) | −0.09(1.8) | 0.96 |
TG=triglycerides, HDL-C= high density lipoproteins cholesterol, LDL-C= low density lipoproteins cholesterol, SBP=systolic blood pressure
DBP= diastolic blood pressure, ref.= reference, DXA VAT= dual x-ray absorptiometry visceral adipose tissue
TFM = Total fat mass, CT VAT = computed tomography visceral adipose tissue
other covariates included within this final model are not presented
DXA models: LDL-C = Tanner stage; SBP = Tanner stage, Sex, Race; DBP = Tanner stage, Age
CT models: LDL-C = Race, Age; SBP = Tanner stage, Age, Sex, Race; DBP = Tanner stage, Age
Table 3. Comparison of regression results between CT VAT and DXA VAT for glucose metabolism variables.
Regression analysis for glucose metabolism variables.
| DXA | CT | ||||
|---|---|---|---|---|---|
|
| |||||
| Variables | Estimate (±SE) | p-value | Variables | Estimate (±SE) | p-value |
| Glucose (model* adjusted R2 = 0.4361) | Glucose (model* adjusted R2 = 0.4418) | ||||
| Independent Variables | Independent Variables | ||||
| log DXA VAT (R2 = 0.014) | 0.5(0.7) | 0.467 | log CT VAT (R2 = 0.035) | 0.8(2.1) | 0.71 |
| log DXA TFM (R2 = 0.244) | 4.3(1.1) | <0.001 | log DXA TFM (R2 = 0.244) | 4.4(1.7) | 0.008 |
| log HOMA-IR (model* adjusted R2 = 0.5329) | log HOMA-IR (model* adjusted R2 = 0.5234) | ||||
| Independent Variables | Independent Variables | ||||
| log DXA VAT (R2 = 0.068) | 0.1(0.04) | 0.023 | log CT VAT (R2 = 0.077) | 0.14(0.1) | 0.334 |
| log DXA TFM (R2 = 0.267) | 0.55(0.1) | <0.001 | log DXA TFM (R2 = 0.257) | 0.56(0.1) | <0.001 |
| log Insulin (model* adjusted R2 = 0.5137) | log Insulin (model* adjusted R2 = 0.5014) | ||||
| Independent Variables | Independent Variables | ||||
| log DXA VAT (R2= 0.067) | 0.11(0.04) | 0.002 | log CT VAT (R2= 0.062) | 0.1(0.1) | 0.43 |
| log DXA TFM (R2 = 0.286) | 0.47(0.1) | <0.001 | log DXA TFM (R2 = 0.287) | 0.52(0.1) | <0.001 |
| Mlbm (model* adjusted R2=0.1801) | Mlbm (model* adjusted R2 = 0.1543) | ||||
| Independent Variables | Independent Variables | ||||
| log DXA VAT (R2 = 0.068) | −0.87(0.3) | <0.001 | log CT VAT (R2 = 0.077) | −1.4(0.6) | 0.03 |
HOMA-IR = homeostasis model for insulin resistance, Mlbm= insulin sensitivity m-value per lean body mass
DXA VAT = dual x-ray absorptimetry visceral adipose tissue, TFM = total fat mass
CT VAT = computed tomography visceral adipose tissue
other covariates included within this final model are not presented
DXA models: Gluc = Tanner stage, Age, Sex, Race; HOMA = Tanner stage; INS = Tanner stage; Mlbm = Tanner stage
CT models: Gluc = Tanner stage, Age, Sex, Race; HOMA = Tanner stage; INS = Tanner stage; Mlbm = Tanner stage, Race
Results
Data from 329 children ages 6–18 years old (152 females, 177 males) were included. Demographic data and clinical measures for females, males and the total sample are presented in Table 3 as mean ± standard error. Based on CDC classifications, the number of participants classified in each category are: 5 (<5% - underweight); 207 (5–84.9% - normal weight); 64 (85–94.9% - overweight); 53 (>94.9% - obese). Log transformation was performed for CT-VAT, DXA-VAT, DXA-TFM, triglycerides, insulin, BMI percentile and HOMA-IR because of the lack of normal distribution as determined by Anderson-Darling test. These results are presented as the geometric mean and 95% confidence interval (CI). Systolic blood pressure, glucose, DXA-VAT and DXAk-TFM were significantly different between males and females. CT-VAT was not significantly different between sexes.
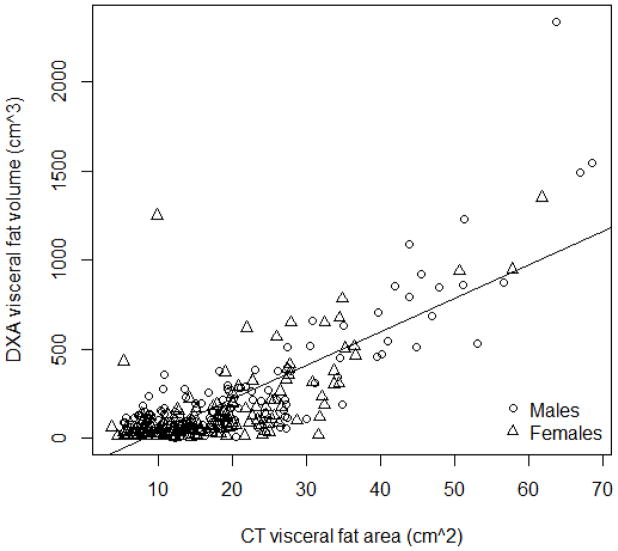
Figure 1 presents the scatterplot of DXA-VAT volume and CT-VAT area. The association does not appear to be directly linear. The Pearson’s correlation for CT and DXA (log transformed) within the total sample was 0.626 (95% CI = 0.554, 0.689). Due to the wide age range, we measured the correlations for younger (age 6–11 years) and older (age 12–18 years) children. The Pearson’s correlations for these groups were 0.55 (95% CI = 0.43, 0.65) and 0.63 (95% CI = 0.53, 0.71), respectively. This demonstrates similar significant moderate linear relationships between CT-VAT and DXA-VAT across each age subgroup. Additionally, we calculated the correlations based on BMI percentile, above or below the 85th percentile. The Pearson’s correlation for these groups were 0.859 (95% CI = 0.802, 0.900) and 0.226 (95% CI = 0.092, 0.353), respectively. This demonstrates a strong positive relationship between each method in the heavier participants. The correlations between DXA-VAT and TFM, waist circumference and BMI are 0.564, 0.652, and 0.656, respectively. The correlations between CT-VAT and the same measures are 0.863, 0.795 and 0.786, respectively. The Kendall’s tau correlation coefficient for the total sample was 0.451 (P<0.001). This suggests that VAT values for each participant would be ranked similarly by each method (the highest VAT by DXA is the highest VAT value by CT).
Figure 1.
Scatter plot of CT versus DXA visceral fat estimation in the sample population
Table 1 presents the regression analysis for lipid and blood pressure variables for both CT-VAT and DXA-VAT. Regression analysis for glucose metabolism variables for both CT-VAT and DXA-VAT is presented in Table 2. The R2 value represents the final model which may have included some of the covariate variables. These final models always produced the lowest Akaike information criterion values.
Regression analysis for computed tomography-visceral adipose tissue
CT-VAT was associated with adverse lipid levels and insulin resistance. There was a positive association with triglycerides and low-density lipoproteins and an inverse association with high-density lipoproteins and M/LBM (all below P<0.05). No significant association was observed between CT VAT and systolic blood pressure, diastolic blood pressure, fasting glucose, fasting insulin or HOMA-IR.
Regression analysis for dual-energy X-ray absorptiometry-visceral adipose tissue
DXA-VAT was also associated with adverse lipid levels, insulin resistance, and additionally diastolic blood pressure. A positive association was observed with triglycerides, diastolic blood pressure, HOMA-IR and fasting insulin; and an inverse association is observed with high density lipoprotein cholesterol and M/LBM (all below P<0.05). No associations were observed between DXA-VAT, systolic blood pressure and fasting glucose.
DXA-TFM and Tanner stage were consistently included within the final models for DXA-VAT. Tanner stage, sex, race and age, remained in several final models for CT-VAT. Tanner stage was significantly associated with all glucose metabolism variables (fasting glucose, fasting insulin, HOMA-IR and M/LBM) in models for both CT-VAT and DXA-VAT. The full results for each final model are presented in Supporting Information Tables S1 and S2.
Discussion
To our knowledge, this is the first study to use DXA to estimate VAT in children. Because of this, we used the current gold standard, single slice CT-VAT estimation, to compare the total volume DXA method. The purpose of this study was not to demonstrate that these methods are interchangeable, but rather to determine if DXA-VAT provides an accurate estimate of VAT that demonstrates an association to cardiometabolic risk factors in children.
This study compared the linear relationship and concordance of each method. The log transformed sample correlation provided evidence of a significant linear relationship between CT-VAT and DXA-VAT estimates. This relationship was consistent across all ages. While the correlation between the two measures was lower than expected; the strength of this relationship increases in the largest children, which is important given the increased risk in heavier children, suggesting that the relationship is dependent on total adiposity. The correlation observed in overweight/obese children is consistent with correlations observed in adults between L4-L5 and total volume.19,20 To measure the concordance between each method, we ranked both VAT estimates for each participant. We observed that the rankings were similar to one another based on the Kendall’s tau correlation which compares the rankings for concordance. This suggests that these two methods for estimating VAT classify participants similarly despite the differences in measurement units.
While the results of the Kendall’s tau suggest that the measurements are not independent from each other, the plot of the ranks suggests that this relationship may be influenced by the largest participants. This is expected given the increased association demonstrated in heavier participants in this study. A leaner individual may accumulate VAT in different areas, which could lead to discordant relationship between a single slice and total volume measurement, suggesting that a single slice at L4-L5 may not be representative of total VAT volume in lean children. This is consistent with research in adults that observed a stronger association between single slice and total volume 5–10 cm above L4-L5.26 This may explain the differences observed between the relationships of each method with certain cardiometabolic risk factors.
To our knowledge, this is the first study in children utilizing concurrent measurement of VAT by both CT and DXA. This allowed the associations of each method with several cardiometabolic risk factors to be indirectly compared. While the two measurement units are different, significant associations were observed between cardiometabolic risk factors for both DXA-VAT and CT-VAT. These results suggest that VAT quantified by DXA provides similar information about the relationship between VAT and measures of cardiometabolic risk. Each method resulted in a similar proportion of explained variance with metabolic risk factors (individual R2). Furthermore, DXA-VAT demonstrates a significant association with additional variables (diastolic blood pressure, fasting insulin, HOMA-IR) for which CT shows no significant association. This may be due to the slice site used in this study; L4-L5 is not the site with the strongest association with cardiometabolic risk factors in adults.19,20 While we believe this is the first study to use DXA-derived VAT to measure the association with risk factors in children, a previous study observed that total fat measured at L1-L4 and regional fat at other depots, using DXA, provided the best predictive measure of insulin resistance and other cardiovascular risk factors independent of total body mass in children.27,28
The gender differences in VAT observed in this study were apparent by DXA-VAT but not with CT-VAT. This is similar to a recent study which observed ethnic and sex differences by total volume MRI in children.29 Adolescence is a time where adipose tissue accumulation differences develop between the sexes, in part because of introduction of sex-specific hormones; puberty results in shifts in accumulation of adipose tissue to specific depot.30 Females, generally, store more adipose tissue in the gynoid region and males, generally, store more adipose tissue in the android region. In this study, and many others, CT-VAT estimates VAT in a single slice at L4-L5, near the umbilicus. This location is near the inferior border of the android region. It is possible that accumulation of adipose tissue in the visceral region starts higher than L4-L5 and thus sex-specific differences cannot be identified by a single slice at this region. The fact that with obesity more storage of adipose tissue is likely to accumulate through the whole android region, including the lower regions, would explain the stronger linear relationship in the heavier participants. Thus, for most children who have relatively small amounts of VAT, total volume of VAT by DXA may provide important information that is missed in the single slice approach.
DXA offers several advantages over CT in the pediatric population. DXA is less expensive and associated with significantly lower radiation exposure than CT. In addition to VAT, DXA provides additional useful information including bone mineral density, total and regional body composition. A third method, MRI does not involve exposure to radiation but the cost and feasibility concerns are similar to CT in this population. Also, neither CT nor MRI provides a measure of total body composition. The results of this study suggest that DXA-VAT is significantly associated with CT-VAT, especially in overweight and obese children. Furthermore, DXA-VAT was associated with several measures of cardiometabolic risk. This demonstrates that DXA-VAT, estimated in children, maintains the independent relationship with cardiometabolic risk factors observed previously using single slice VAT estimation.6–14 These results provide evidence that DXA is an acceptable method for quantification of VAT in children.
Limitations
A limitation of this study is that it was an analysis of previously collected data. This did not allow for comparison of equal-sized VAT regions, or multiple slice sites. Additionally, there is limited evidence as to the best slice site in children; however, the evidence in adults suggests a slice higher than L4-L5 may provide a stronger association to total volume and metabolic risk factors.
Supplementary Material
Acknowledgments
JS, ARS and AM developed and carried out the experiments. TAB analysed the data and wrote the manuscript. All authors were involved in editing the manuscript and had final approval of the submitted and published versions. We would also like to acknowledge Derran Bedward and Joanna Liu for their support in conversion of data.
Research reported in this publication was supported by the National Institutes of Health: NCI/NIDDK: RO1CA113930-01A1 (JS), NIH/NIDDK R01DK072124 (JS), the GCRC: M01-RR00400, General Clinical Research Center Program, NCRR/NIH, and CTSI NIH/NCATS UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- VAT
visceral adipose tissue
- CT
computed tomography
- MRI
magnetic resonance imaging
- DXA
dual energy X-ray absorptiometry
- DXA-VAT
visceral adipose tissue estimated by DXA
- CT-VAT
visceral adipose tissue estimated by CT
- BMI
body mass index
- M
glucose utilization
- M/LBM
glucose utilization per kg of lean body mass
- HOMA-IR
homeostasis model of insulin resistance
- DXA-TFM
total fat mass measured by DXA
Footnotes
Conflict of Interest: The authors do not have any disclosures.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. J Am Med Assoc. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tobisch B, Blatniczky L, Barkai L. Cardiometabolic risk factors and insulin resistance in obese children and adolescents: relation to puberty. Pediatr Obes. 2013 doi: 10.1111/j.2047-6310.2013.00202x. [DOI] [PubMed] [Google Scholar]
- 3.Després JP, Lemieux I, Prud’homme D. Treatment of obesity: need to focus on high risk abdominally obese patients. Br Med J. 2001;322:716–720. doi: 10.1136/bmj.322.7288.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakamura T, Tokunaga K, Shimomura I, et al. Contribution of visceral fat accumulation to the development of coronary artery disease in non-obese men. Atherosclerosis. 1993;107:239–246. doi: 10.1016/0021-9150(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 5.Neeland IJ, Turer AT, Ayers CR, et al. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308:1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrington DM, Staiano AE, Broyles ST, Gupta AK, Katzmarzyk PT. Waist circumference measurement site does not affect relationships with visceral adiposity and cardiometabolic risk factors in children. Pediatr Obes. 2013;8:199–206. doi: 10.1111/j.2047-6310.2012.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owens S, Gutin B, Ferguson M, Allison J, Karp W, Le NA. Visceral adipose tissue and cardiovascular risk factors in obese children. J Pediatr. 1998;133:41–45. doi: 10.1016/s0022-3476(98)70175-1. [DOI] [PubMed] [Google Scholar]
- 8.Ali O, Cerjak D, Kent JW, Jr, James R, Blangero J, Zhang Y. Obesity, central adiposity and cardiometabolic risk factors in children and adolescents: a family-based study. Pediatr Obes. 2014;9(3):358–362. doi: 10.1111/j.2047-6310.2014.218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–1521. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 10.Rasmussen-Torvik LJ, Pankow JS, Jacobs DR, Jr, Steinberger J, Moran A, Sinaiko AR. Development of associations among central adiposity, adiponectin and insulin sensitivity from adolescence to young adulthood. Diabet Med. 2012;29:1153–1158. doi: 10.1111/j.1464-5491.2012.03726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taksali SE, Caprio S, Dziura J, et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes. 2008;57:367–371. doi: 10.2337/db07-0932. [DOI] [PubMed] [Google Scholar]
- 12.Caprio S, Hyman LD, Limb C, et al. Central adiposity and its metabolic correlates in obese adolescent girls. Am J Physiol. 1995;269:E118–E126. doi: 10.1152/ajpendo.1995.269.1.E118. [DOI] [PubMed] [Google Scholar]
- 13.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and Syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–2540. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]
- 14.Syme C, Abrahamowicz M, Leonard GT, et al. Intra-abdominal adiposity and individual components of the metabolic syndrome in adolescence. Arch Pediatr Adolesc Med. 2008;162:453–461. doi: 10.1001/archpedi.162.5.453. [DOI] [PubMed] [Google Scholar]
- 15.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167:700–707. doi: 10.1001/jamapediatrics.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenfield JR, Samaras K, Chisholm DJ, Campbell LV. Regional intra-subject variability in abdominal adiposity limits usefulness of computed tomography. Obes Res. 2002;10:260–265. doi: 10.1038/oby.2002.35. [DOI] [PubMed] [Google Scholar]
- 17.Demerath EW, Sun SS, Rogers N, et al. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity (Silver Spring) 2007;15:2984–2993. doi: 10.1038/oby.2007.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuk JL, Blair SN, Church TS, Ross R. Does measurement site for visceral and abdominal subcutaneous adipose tissue alter associations with metabolic syndrome. Diabetes Care. 2006;29:679–684. doi: 10.2337/diacare.29.03.06.dc05-1500. [DOI] [PubMed] [Google Scholar]
- 19.Shen W, Punyanitya M, Chen J, et al. Visceral adipose tissue: relationships between single slice areas at different locations and obesity-related health risks. Int J Obes. 2007;31:763–769. doi: 10.1038/sj.ijo.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irlbeck T, Massaro JM, Bamberg F, O’Donnell CJ, Hoffman U, Fox CS. Association between single-slice measurements of visceral and abdominal subcutaneous adipose tissue with volumetric measurement: the Framingham Heart Study. Int J Obes. 2010;34:781–787. doi: 10.1038/ijo.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Q, Horlick M, Thornton J, et al. Sex and race differences in fat distribution among Asian, African–American and Caucasian prepubertal children. J Clin Endocrinol Metab. 2002;87:2164–2170. doi: 10.1210/jcem.87.5.8452. [DOI] [PubMed] [Google Scholar]
- 22.Kaul S, Rothney MP, Peters DM, et al. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 2012;20:1313–1318. doi: 10.1038/oby.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katzmarzyk PT, Greenway FL, Heymsfield SB, Bouchard C. Clinical utility and reproducibility of visceral adipose tissue measurements derived from dual-energy X-ray absorptiometry in white and African American adults. Obesity (Silver Spring) 2013;21:2221–2224. doi: 10.1002/oby.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinaiko AR, Jacobs DR, Jr, Steinberger J, et al. Insulin resistance syndrome in childhood: associations of the euglycemic insulin clamp and fasting insulin with fatness and other risk factors. J Pediatr. 2001;139:700–707. doi: 10.1067/mpd.2001.118535. [DOI] [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Shen W, Punyanitya M, Wang Z, et al. Visceral adipose tissue: relations between single-slice areas and total volume. Am J Clin Nutr. 2004;80:271–278. doi: 10.1093/ajcn/80.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teixeira PJ, Sardinha LB, Going SB, Lohman TG. Total and regional fat and serum cardiovascular disease risk factors in lean and obese children and adolescents. Obes Res. 2001;9:432–442. doi: 10.1038/oby.2001.57. [DOI] [PubMed] [Google Scholar]
- 28.Daniels SR, Morrision JA, Sprecher DL, Khoury P, Kimball TR. Association of body fat distribution and cardiovascular risk factors in children and adolescents. Circulation. 1999;99:541–545. doi: 10.1161/01.cir.99.4.541. [DOI] [PubMed] [Google Scholar]
- 29.Staiano AE, Broyles ST, Gupta AK, Katzmarzyk PT. Ethnic and sex differences in visceral, subcutaneous and total body fat in children and adolescents. Obesity (Silver Spring) 2013;21:1251–1255. doi: 10.1002/oby.20210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo SS, Chumlea WMC, Roche AF, Siervogel RM. Age- and maturity-related changes in body composition during adolescence into adulthood: the Fels Longitudinal study. Appl Radiat Isot. 1998;49:581–585. doi: 10.1016/s0969-8043(97)00190-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.