Introduction
Each year, over 1 million women worldwide are newly diagnosed with gynecologic malignancy, and almost 500,000 women will die from gynecologic cancer [1]. The last decade of research in the treatment of gynecologic cancers has seen the development of multimodal options, including anti-angiogenic biologics, like bevacizumab, and targeted therapies, like olaparib, a PARPi recently approved for the fourth-line treatment of BRCA-deficient ovarian cancers. Olaparib is the first PARPi approved by the Food and Drug Administration (FDA) for clinical use in the treatment of cancer in the USA, based only on phase II efficacy and safety data. PARP inhibitors have the benefit of being an oral medication which minimizes the impact on quality of life for patients with recurrent cancer. The significant clinical impact of PARP inhibition, a manifestation of sound translational rationale behind therapeutic development, can be attributed to the exploitation of synthetic lethality as a mechanism that selectively targets a specific population of cancer cells, those deficient in tumor suppressor genes BRCA 1 and BRCA 2. The purpose of this article is to provide a background on synthetic lethality, an overview of clinical trials investigating the use of PARPi in gynecologic cancers, highlighting the approval of olaparib in the treatment of recurrent BRCA-deficient ovarian cancer, and identification of patients with a homologous recombination-deficient (HRD) profile to better tailor treatment of women with gynecologic cancers in the future (Table 1).
Table 1.
Important phase II trials of parp inhibitors and ovarian cancer
| Trial (year) | Study Design | N | Eligibility | Drug dose | ORR | PFS | Grade 3/4 AEs |
|---|---|---|---|---|---|---|---|
| Lederman et al. (2013) Study 19 [36••] | Randomized phase II | 265 | · Recurrent HGSOC
|
|
12 % versus 4 % | 8.8 versus 4.8 months | Nausea, fatigue, emesis, anemia |
| Coleman et al. (2014) [56•] | Phase II | 52 | · Recurrent HGSOC
|
Veliparib 400 mg BID x 28d for 6 cycles | 26% | 8.1 months | Nausea, emesis, neutropenia, TCP |
| OS: 19 months | |||||||
| Liu et al. (2014) [54••] | Randomized phase II | 90 | · Recurrent disease
|
|
48 % versus 80 % | 9.0 versus 17.7 months | Fatigue, diarrhea, hypertension |
| Cediranib 30 mg daily | OS at 2 years: | ||||||
| 65 % versus 85 % | |||||||
| Oza et al. (2015) Study 41 [48] | Randomized phase II | 162 | · Recurrent HGSOC |
|
64 % versus 58 % | 12.2 versus 9.6 months | Neutropenia, anemia |
|
|
||||||
| OS: | |||||||
| 33.8 versus | |||||||
| 37.6 months | |||||||
| McNeish et al. (2015) ARIEL2 [20] | Phase II | 204 | · Recurrent HGSOC
|
Rucaparib 600 mg BID | +BRCA: 82 % | 9.4 months | Anemia, transaminitis, fatigue |
| BRCA-like: 45 % | 7.1 months | ||||||
| - Biomarker: 21 % | 3.7 months | ||||||
| Kaufman et al. (2015) Study 42 [37] | Phase II | 298 | · Recurrent disease, ovarian cancer (N = 193)
|
Olaparib 400 mg BID | Ovarian cancer: 31 % | 7 months | Anemia, abdominal pain, fatigue |
| OS: 16.6 months |
AE adverse events. BID twice daily. gBRCA germline breast cancer gene. HGSOC high-grade serous ovarian cancer. ORR response rate. OS overall survival. PFS progression-free survival. TCP thrombocytopenia
Synthetic Lethality
During the early twentieth century, the American geneticist Calvin Bridges (1889–1938) (Fig. 1) noted that when crossing the fruit fly, Drosophila melanogaster, certain non-allelic genes were lethal only in combination even when homozygous parents were viable [2]. Twenty years later, the term “synthetic lethality” was coined by Bridges’ colleague, Theodosius Dobzhansky, who reported the same observations in Drosophila pseudoobscura [3]. The ancient Greek meaning of synthetic is the combination of two entities to form something new [4••]. Thus, synthetic lethality occurs when a genetic defect or defective protein is compatible with cell viability but is lethal when combined (i.e., synthesized) with another genetic/protein defect. By way of contrast, genetic combinations resulting in non-lethal growth impairment are synthetic sick.
Fig. 1.
American geneticist, Calvin Blackman Bridges in 1927. Photograph courtesy of Cold Spring Harbor Laboratory Archives, used with permission.
In their description of induced essentiality, Tischler et al. provided a hypothesis to account for synthetic lethal and oncogene addiction effects in nature [5]. Lord et al. note that as tumor cells acquire more mutations, significant deleterious effects may be minimized through molecular networks within a cell to facilitate compensatory alterations that permit cell survival via escape or functional buffering [3]. As an example, oncogenes leading to increased cell proliferation induce a state of replicative stress which results in slowing or stalling at the replication forks, ultimately leading to DNA damage deleterious to cancer cells. To minimize this effect, oncogene activation is often associated with compensatory molecular changes mediated by the ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related (ATR) protein kinases.
Exploitation of synthetic lethality to eradicate cancer was initially suggested by Hartwell et al. who outlined strategies through which drugs could be profiled for their ability to selectively kill cells in a molecular context that matches those found in malignant neoplasms [6]. Kaelin advanced this idea in noting that because targeting a gene that is synthetic lethal to a cancer-relevant mutation should kill only cancer cells and spare normal cells, synthetic lethality provides a conceptual framework for the development of cancer-specific agents [7]. Theoretically, the development of synthetic lethal resistance occurs not through modulation of the drug target but rather through modulation of the synthetic lethal partner. The most robust demonstration of the principle of harnessing synthetic lethality comes from the treatment of cancers resulting from loss of BRCA gene function.
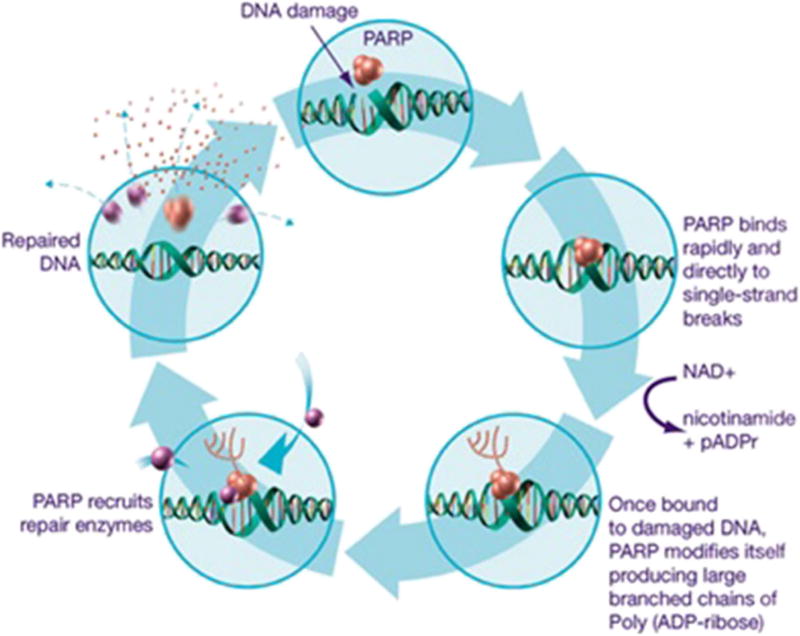
The development of PARPi as therapeutic options for cancer treatment capitalizes on the role of PARP in DNA repair and the cancers already deficient in homologous recombination, like BRCA-related breast and ovarian cancers (Fig. 2, [8]). DNA undergoes constant damaging sequence alterations due to toxic byproducts of the cell cycle, environmental insult, and errors in replication. Several mechanisms have evolved to repair these errors, including (1) nucleotide excision repair, (2) base excision repair (BER), (3) homologous recombination (HR), and (4) non-homologous end-joining (NHEJ).
Fig. 2.
PARP inhibition mechanism of action—blockade of the base excision pathway. Poly(ADP-ribose) polymerase (PARP) recognizes and binds to sites of DNA damage through its zinc-finger domains and recruits proteins involved in DNA repair through polyADP-ribose catalyzation. PARP inhibitors function by trapping PARP to sites of DNA damage and blocking the enzymatic transformation required for polyADP-ribosylation. Adapted from Tewari KS, Monk BJ, BTranslational Science,” In: The 21st Century Handbook of Clinical Ovarian Cancer, page 80. © Springer International Publishing Switzerland 2015, with permission of Springer.
PARP enzymes are found in the cellular nucleus where they are activated by DNA damage to identify DNA single-strand breaks (SSB) [9]. PARP is thought to have four domains: an N-terminal DNA-binding domain comprised of two zinc finger motifs, a C-terminal catalytic domain, a central auto-modification domain, and a caspase-cleaved domain [10]. After binding to a site of SSB, PARP undergoes a conformational change allowing for the C-terminal catalytic domain to transfer ADP-ribose moieties from nicotinamide-adenine-dinucleotide (NAD+) to form PAR chains that recruit other DNA repair proteins (e.g., DNA ligase III, DNA polymerase beta) to form the BER multiplex, ultimately repairing SSB (Fig. 2). The formation of PAR chains also appears to play a role in multiple other cellular tasks, like gene expression and signal response [11–13], as well as double-stranded DNA repair [14–16]. PARPi interrupts the DNA repair process by impairing two specific mechanisms of PARP: (1) binding sites of SSB with its own zinc finger domains thereby directly blocking DNA access by PARP and (2) preventing the transfer of ADP-ribose to form PAR chains thereby blocking the formation of the BER multiplex. The summation of these actions leads to the progression of SSB to DSBs at replication forks, and in cells without intact homologous repair, chromosomic integrity is destroyed, the cell cycle arrests, and apoptosis results.
The loss of high-fidelity homologous recombination is partly due to an inability to localize DNA polymerase RAD51 [3]. Preclinical studies showed that treatment of BRCA-deficient cells with PARP inhibition induced the presence of nuclear RAD51 foci, an indication of double-strand DNA repair [17]. Indeed, subsequent in vitro studies demonstrated that cells with BRCA mutations are 1000 times more sensitive to PARPi compared to wild-type cells [18, 19]. These observations provided the translational impetus to begin phase I and II clinical trials with PARPi in breast, ovarian, and prostate cancers. In the most recent gynecologic cancer clinical trials of PARPi, specifically in the ARIEL2 trial, tumors with deficiencies in RAD51 demonstrated a BRCA-like HRD phenotype with high genomic loss of heterozygosity (LOH) and increased response to rucaparib [20••].
While the focus of PARPi has been in the treatment of BRCA-related ovarian cancer, their therapeutic use in other gynecologic cancers is under investigation. Up to 80 % of sporadic endometrial cancers have been associated with activation of the phosphatidylinositide 3-kinase (PI3-kinase) pathway via mutations in phosphatase and tensin homologue (PTEN) [21, 22], and early studies in mouse embryonic fibroblasts showed that PTEN inactivation induced genomic instability due to defective RAD51 -mediated HR DNA repair [23]. Two in vitro studies followed demonstrating sensitivity of PTEN-deficient cells to PARP inhibition [24, 25]. Compared to the work done in ovarian cancer, the basic science support is less robust; therefore, only a handful of phase I and phase II clinical trials are active in uterine cancer. A phase 0 trial, the Preoperative Olaparib Endometrial Carcinoma Study (POLEN, NCT 02506816) will be recruiting patients to assess the biological impact of PARP inhibition during the period of time between diagnosis and surgery.
The role and application of PARP inhibition in malignancies of the cervix, vagina, and vulva has yet to be clearly determined. To date, no clinical trials have been conducted in the treatment of vaginal and vulvar cancers using PARP inhibition. There is some preclinical evidence of the mediation of PARP activity by HPV infection [26–28]. Specifically in a series of head and neck squamous cell carcinomas, repair of DNA DSB was significantly delayed in HPV+ tumors, which correlated with increased in vitro sensitivity to veliparib [28]. Veliparib is currently under study in a phase I and phase II trial in advanced cervical cancer (see below). Olaparib is being investigated in a phase I trial in recurrent/refractory cervical cancer (NCT01237067), which seeks to determine the safety and efficacy of combined carboplatin and olaparib on different doses and schedules in women with recurrent/refractory cervical cancer, as well as uterine, ovarian, and breast cancer and in men with metastatic breast cancer and BRCA mutation.
Olaparib
Olaparib (AZD2281) is an oral PARP-1 and PARP-2 inhibitor manufactured by AstraZeneca that was approved for fourth-line treatment of recurrent BRCA-related ovarian cancer in December 2014. In the original dose-escalation phase I clinical trial published in 2009, Fong et al. reported a 47 % overall response rate (ORR) and a 64 % clinical benefit rate (CBR—tumor marker or radiologic response or stable disease) of ≥4 months in a total of 60 heavily pretreated patients with refractory breast, ovarian, or prostate cancer, 22 of whom had BRCA1 and BRCA 2 germline mutations [29•]. Additionally, a supplemental expansion cohort of 50 patients with BRCA1/2 mutations was later recruited into the study. The maximum-tolerated dose (MTD) of olaparib was 400-mg BID after reversible dose-limiting toxicity was seen in one of eight patients receiving 400-mg BID dosing (grade 3 mood alterations and fatigue) and two of five patients receiving 600-mg BID dosing (grade 4 thrombocytopenia, grade 3 somnolence). The study also demonstrated that CBR was associated with platinum sensitivity (23 % in platinum refractory, 45 % in platinum resistant versus 69 % in platinum sensitive patients) [30].
After these initial findings, two phase II proof-of-concept trials were initiated [31, 32], followed by phase II trials of olaparib for maintenance therapy and in combination with cytotoxic chemotherapy agents. The phase II proof of concept trial of olaparib in gynecologic cancer patients found a dose-response to olaparib in 57 women with BRCA-related recurrent ovarian cancer (ORR 33 % with 400 mg BID vs 13 % with 100 mg BID) [32]. Grade 3/4 toxicities in the higher dose group were two cases of nausea and one case each of fatigue and anemia. At 16 weeks of treatment, prevalence of progression was higher in the lower dose group (65 % vs 33 %). In the first phase II trial confirming olaparib efficacy in sporadic ovarian cancers, Gelmon et al. reported an ORR in seven of 17 cases (41 %) of BRCA-mutated ovarian cancer, compared to 11 of 46 cases of sporadic cases (24 %) [33]. Platinum sensitivity also correlated with response in this study: among sensitive patients, 60 % of patients with mutations and 50 % of patients without mutations responded while only 4 % of patients with platinum resistant and non-BRCA-associated cancer had a response.
Olaparib monotherapy has been tested in two randomized phase II studies, directly against pegylated liposomal doxorubicin (PLD) in the recurrent germline BRCA mutation population and against placebo in the maintenance setting in the platinum-sensitive population [34, 35]. The first study showed comparable PFS of PLD (50 mg/m2 IV q28 days) compared to two doses of olaparib (200-mg BID or 400-mg BID continuously) in the recurrent BRCA-deficient ovarian cancer. Though the differences were not statistically significant, ORR also differed between the three groups (18, 25, and 31 %, respectively). In the maintenance study (also known as Study 19), patients received placebo or olaparib 400-mg BID after platinum-based chemotherapy. Compared to placebo, olaparib conferred a 3.6-month advantage in PFS (8.4 versus 4.8 months), and among BRCA mutation carriers, this benefit in PFS was the greatest (11.2 months compared to 4.3 months) [36••]. Patients with sporadic cancers had a more modest improvement in PFS of 7.4 months versus 5.5 months. There was no benefit in overall survival (OS).
The multicenter phase II study that led to the FDA approval of olaparib as fourth-line therapy for women with BRCA-related ovarian cancer is known as Study 42 by Kaufman et al. [37••]. The trial enrolled patients with a germline BRCA1/2 mutation and recurrent cancer with three or more prior lines of chemotherapy and administered olaparib 400-mg BID; their primary endpoint was tumor response rate. A total of 298 patients received treatment, including 193 women with ovarian cancer who achieved an ORR of 34 % and a median response duration of 8 months. The study further strengthened the utility of olaparib among this heavily pretreated population. On December 19, 2014, the FDA granted accelerated approval to olaparib as fourth-line therapy for women with recurrent ovarian cancer and germline BRCA mutations as determined by the FDA-approved companion diagnostic BRCAnalysis test by Myriad, the first approval of its kind for a laboratory-developed test [38].
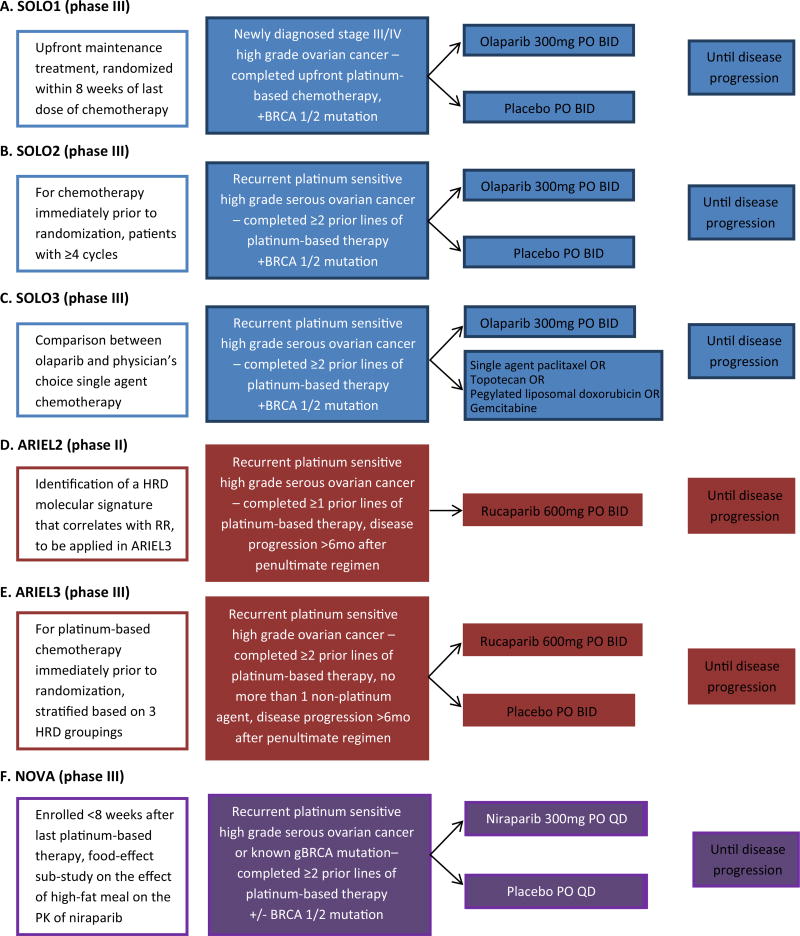
Given the efficacy and safety of olaparib in phase II trials, several phase III clinical trials of olaparib in ovarian cancer are underway (Fig. 3). The study of Olaparib in ovarian cancer (SOLO) studies are all in patients with BRCA-deficient cancers. SOLO1 (NCT01844986), the follow-up to Study 19, is the first double-blind, placebo-controlled phase III trial in newly diagnosed ovarian cancer of olaparib maintenance post-platinum-based chemotherapy. With an estimated enrollment of 344, it randomizes patients 2:1 to olaparib to placebo, and its primary endpoint is PFS. SOLO2 (NCT01874353) is the follow-up study to Study 41 investigating maintenance olaparib versus placebo in recurrent platinum-sensitive disease. In a similar fashion, patients will be randomized 2:1 to olaparib versus placebo with an estimated enrollment of 264. Primary endpoint is PFS. SOLO3 (NCT02282020) will assess olaparib monotherapy versus physician’s choice single agent cytotoxic chemotherapy in recurrent platinum-sensitive disease. Its estimated enrollment is 411, and primary endpoint is PFS. Notably, the FDA approval of olaparib for use in this population is contingent upon the results of SOLO3.
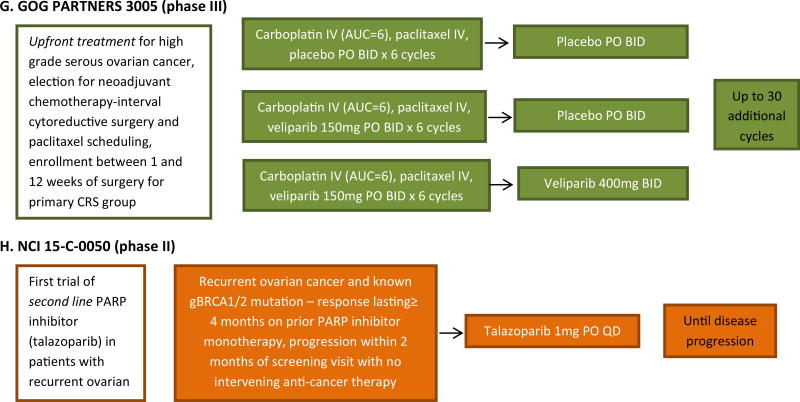
Fig. 3.
Schema of phase II and phase III trials of PARP inhibitors in patients with ovarian cancer. ARIEL Assessment of Rucaparib in ovarian cancEr triaL. BRCA1 breast cancer 1 gene. BRCA2 breast cancer 2 gene. GOG gynecologic oncology group. HRD homologous recombination deficicency. NOVA niraparib ovarian trial. PARP, poly(ADP-ribose) polymerase. SOLO study of OLaparib in ovarian cancer.
Olaparib has been studied in combination with chemotherapeutic agents, and a number of preclinical studies have demonstrated synergy between PARPi and platinum-containing compounds [39–41]. However, the overlapping myelosuppressive toxicities of olaparib and chemotherapeutics have limited the achievement of full-dose chemotherapy in these phase I trials [42–47]. Phase II studies reinforced the activity of PARPi in patients with BRCA mutations. Lee et al. studied olaparib in combination with carboplatin for up to 8 cycles of therapy, followed by single-agent maintenance olaparib until progression, in 45 cases of either breast or ovarian cancer and germline BRCA mutation [47]. For women with ovarian cancer, the ORR among the platinum-sensitive cases was 71 %, platinum-resistant cases was 25 %, with an overall ORR of 44 %; an additional 41 % had stable disease from 3 to 25+ months. Olaparib was studied in a randomized phase II trial, known as Study 41, in combination with carboplatin/paclitaxel compared to chemotherapy alone in platinum-sensitive recurrent ovarian cancer [48•]. PFS was significant for the combination arm (12.2 versus 9.6 months) with the greatest benefit for those with known BRCA mutations. When studied in combination with carboplatin and metronomic paclitaxel in 12 patients with at least three prior therapies, four had complete response, four had partial response or stable disease, and two had progression [49]. A planned expansion of this phase Ib study will recruit an additional 40 patients.
Olaparib has also been combined with anti-angiogenic agents. Homologous recombination can be suppressed by hypoxia through the regulation of hypoxia-inducible factor 1α and nuclear factor κB (NF-κB) [50]. Bevacizumab (10 mg/kg IV q14 days) was combined with dose escalations of continuous olaparib in the phase I setting with 12 ovarian cancer patients. The MTD of olaparib was 400 mg BID, and toxicities were mild, limited to grade 1/2 nausea and fatigue [51]. Trials of olaparib and cerdiranib, an oral vascular endothelial growth factor receptor 2 inhibitor, have shown encouraging results. The initial phase I trial established the MTD of olaparib to be 200-mg BID in combination with cediranib 30 mg daily [52•, 53]. This dose was tested in a randomized phase II study of 90 women with recurrent platinum-sensitive high-grade serous or endometrioid ovarian cancer [54••]. Combination therapy was associated with significantly improved PFS (9 months versus 17.7 months) and ORR (48 % versus 80 %). OS data is not mature, but at 24 months, 81 % of combination patients are alive compared to 65 % of olaparib only patients. Grade 3/4 toxicities more common in the combination group were fatigue, diarrhea, and hypertension. These promising results have led to the development of two phase III trials using combination olaparib and cediranib therapy: a direct comparison to standard chemotherapy in the platinum-resistant or -refractory setting (NCT02502266) and as compared to olaparib monotherapy or standard platinum-based chemotherapy in the platinum-sensitive setting (NCT02446600).
Veliparib
Veliparib (ABT-888) is also an oral PARP-1 and PARP-2 inhibitor made by AbbVie that has been extensively studied as a single agent as well as in combination with chemotherapy in gynecologic cancers. Mechanistically, it appears to have an inferior ability to trap PARP-1 and PARP-2 at the site of SSB compared to both olaparib and niraparib [55]. The toxicity profile of veliparib is similar to olaparib, with nausea, fatigue, and myelosuppression seen most commonly with monotherapy [56•]. Velaparib has been studied in combination with topotecan, doxorubicin, and cyclophosphamide with variable response [57–59].
In GOG 280, a phase II study on veliparib monotherapy in the treatment of persistent or recurrent BRCA-related ovarian cancer, patients were enrolled to receive veliparib 400 mg BID [56•]. Fifty patients with three or fewer prior regimens were enrolled; ORR was 26 % with median PFS of 8.2 months. When stratified on platinum sensitivity, platinum-resistant patients had ORR of 20 % compared to 35 % in platinum-sensitive disease. Grade 3/4 toxicities were limited to one case of grade 4 thrombocytopenia and the following grade 3 events: fatigue (n = 3), nausea (2), leukopenia (1), neutropenia (1), dehydration (1), and increased ALT (1). Almost 50 % of patient experienced grade 2 nausea and 25 % had grade 2 fatigue.
Veliparib is currently undergoing investigation in the phase III arena through GOG PARTNERS 3005 (NCT02470585, Fig. 3f). In a trial schema identical to GOG 218 with bevacizumab [60, 61], veliparib will be studied in the primary setting, in a 1:1:1 randomized, double-blinded trial given concurrently with standard carboplatin/paclitaxel chemotherapy with and without veliparib maintenance therapy. The trial began accrual in October 2015. The primary endpoint is PFS, which was be explored in the general population as well as those with BRCA mutations.
As one of the few PARPi currently under investigation in cervical cancer, veliparib is being used in combination with topotecan in persistent or recurrent cervical cancer (NCT01266447), as well as a phase 1/2 trial in combination with cisplatin and paclitaxel in advanced or recurrent disease (NCT01281852).
Niraparib
Niraparib (MK4827) is also an oral inhibitor of PARP-1 and PARP-2, the first whose pharmacokinetics allows for once daily dosing. Niraparib entered clinical studies in 2008 with a phase I study of advanced solid tumors, including high-grade serous ovarian cancer, with enrichment for those with BRCA mutations [62]. A modified 3 + 3 design was utilized for dose escalation in 100 patients to determine the 300-mg/day MTD. The most common toxicities were predominantly grade 1/2 anemia (48 %), nausea (42), fatigue (42 %), thrombocytopenia (35 %), anorexia (26 %), neutropenia (24 %), and vomiting (20 %). The ORR of BRCA-related ovarian cancer cases (n = 20) was 40 %, with a median response duration of 387 days. Among the BRCA-deficient ovarian cancer cases, 10 had platinum sensitive disease, and these patients had an ORR of 50 % and median response duration of 431 days. Currently, there are no completed phase II studies of this drug in ovarian cancer.
Tesaro, Inc. is presently recruiting for a phase II trial for women with recurrent high-grade serous ovarian cancer who have completed at least three previous chemotherapy regimens (NCT02354586). It also recently completed accrual for the NOVA trial, a phase III double-blind, placebo-controlled, 2:1 randomized trial of maintenance niraparib versus placebo in patients with recurrent platinum-sensitive high-grade serous ovarian cancer or known to have a germline BRCA mutation (NCT01847274, Fig. 3e) [63•]. NOVA enrolled 490 participants, and its primary objective is PFS. Additionally, the trial is evaluating the effect of a high-fat meal on the pharmacokinetics of a single 300-mg dose of niraparib in patients with ovarian cancer [64]. A 2-treatment (fed versus fasting), 2-way crossover design was used to evaluate the effect of food on PK parameters. Sixteen subjects were enrolled in the food effect cohort, and each subject received two separate 300-mg doses of niraparib, one each in a fasting and a fed state. Data from the NOVA are expected in 2016.
Rucaparib
Rucaparib (CO338, AGO14699, and PF01367338) is another PARP-1 and PARP-2 oral inhibitor that has entered into clinical trial testing [65, 66]. The phase I study of oral rucaparib tested doses of from 40 to 840-mg BID and recommended the phase II dose of single-agent rucaparib to be 600-mg BID. Rucaparib has demonstrated durable responses greater than 6 months in both platinum-sensitive and platinum-resistant ovarian cancer [66]. Preliminary results of the phase II trial in 17 women with germline-BRCA mutations and ovarian cancer reported at the 2014 European Society of Medical Oncology meeting showed an ORR of 82 % and a disease control rate of 93 % at 12 weeks [67]. The most common treatment-related toxicity occurring in >15 % of patients were nausea, asthenia, vomiting, transient transaminitis, and anemia. Rucaparib was granted US FDA Breakthrough Therapy designation on April 6, 2015, based on the interim results of the Kristeleit phase II trial and the ARIEL2 study from the Assessment of Rucaparib in Ovarian Cancer Trial (ARIEL group) by Clovis Oncology, Inc.
The ARIEL2 study evaluated rucaparib in a pivotal phase II prospective biomarker trial in high-grade ovarian cancer focused on identification of a molecular signature to predict clinical benefit for patients with platinum-sensitive disease with at least one prior regimen (NCT01891344, Fig. 3c) [20••]. Interim results were presented at the 2015 Annual Meeting on Women’s Cancer of the Society of Gynecologic Oncology [68] with final results available at the 2015 Annual Meeting of American Society of Clinical Oncology [20••]. The primary objective of this single-arm, open-label study, was to identify a molecular HRD signature in ovarian cancer associated with clinical benefit from rucaparib treatment. Known germline BRCA-related ovarian cancers were capped in this study with the 208 patients divided into the following distribution of homologous repair-deficient molecular subgroups: BRCA-mutated 20 %, BRCA-like 40 %, biomarker negative 34 %, and unclassified 6 %. Tumors with RAD51C genetic alterations had high genomic LOH and demonstrated a BRCA-like phenotype. Overall response rate was highest in the BRCA-mutated (82 %), followed by the BRCA-like group (45 %), with 21 % of the biomarker negative group showing response based on RECIST+CA-125. Median PFS was 9.4, 7.1, and 3.7 months, respectively. Grade 3/4 toxicity was mostly limited to anemia (16 %) and transient transaminitis (11 %). An expansion cohort is now currently recruiting in ARIEL2 Part 2 to include patients with three or more prior chemotherapy regimens. The results of ARIEL2 are potentially practice changing as the data provide proof of concept for the development of biomarker assays for HRD, which are now under development (see below). Using the predictive HRD assay prospectively determined in ARIEL2, the follow-up study, ARIEL3, a randomized, phase III trial, will stratify patients who have received two or more prior platinum regimens with platinum-sensitive disease into the three HRD groupings and investigate the use of rucaparib as maintenance versus placebo (NCT01968213, Fig. 3d).
Talazoparib (BMN 673)
Talazoparib is an oral PARP-1 and PARP-2 inhibitor [69] manufactured by BioMarin that has undergone phase I testing in an open-label study of once-daily treatment in patients with advanced or recurrent solid tumors [70]. The MTD of 1000 µg/day was determined in this study of 39 patients. Dose-limiting thrombocytopenia occurred in one out of six patients and two out of five patients at the 900 and 1100 µg/day, respectively. Grade 3/4 adverse events included anemia, neutropenia, and thrombocytopenia. Twenty-three patients were enrolled with either ovarian or primary peritoneal cancer, and 17 of these patients had a germline BRCA mutation. RECIST and/or CA-125 responses occurred at doses ≥ 100 µg/day in 11 out of 17 BRCA-related ovarian or peritoneal cancers. Currently, there is a phase III study testing Talazoparib in patients with metastatic breast cancer and a phase II trial sponsored by the National Cancer Institute for women with deleterious BRCA 1/2 mutation-associated ovarian cancer who had had prior PARP inhibitor treatment (NCT02326844).
Talazoparib is also being studied in uterine cancer in the PARP inhibitor for Inoperable Advanced eNDometrial cAncer phase II trial (PANDA, NCT02127151). This study is not yet open for recruitment. Patients with inoperative advanced or recurrent endometrial cancer with no more than one prior line of systemic cancer therapy will be given daily BMN 673 to determine primary outcome measure PFS.
Future Directions: Development of a Homologous Recombination Deficiency Assay
The role homologous recombination assays will continue to play a role in the treatment of women with gynecologic malignancies, especially in those with ovarian cancer because of the therapeutic advantage generated by PARP inhibition in tumors with HRD. The ARIEL2 trial incorporated the translation piece through studying LOH, a marker of HRD and measurable using next-generation sequencing. The investigators developed an algorithm for LOH and confirmed correlation with response rates and PFS. Other HRD assays are in development. At the 2014 EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapies Meeting, Haluska et al. presented a HRD score obtained from performing HRD analysis on 106 high-grade ovarian tumors with known responses to niraparib [71]. Using genome-wide SNP data, tumor xenografts were analyzed using three algorithms: LOH, telomeric allelic imbalance (TAI), and large-scale state transitions (LST). The xenografts were again treated with niraparib, and the HRD score was calculated as the sum of the LOH+ TAI + LST scores. High HRD scores were correlated with in vivo response to niraparib and BRCA deficiency, and low HRD scores were associated with niraparib resistance or lack of in vivo efficacy. Both ARIEL3 and the NOVA study are developing HRD assays as part of the phase III clinical trials.
Genetic sequencing technology through the use of microarrays and next-generation sequencing, as well the data from The Cancer Genome Project, has made these analyses possible. They provide methods to comprehensively capture the diverse ways HRD may manifest itself outside of traditional BRCA 1/2 mutation analyses to incorporate copy number variability to LOH, TAI, and LST scores [72–75]. Others have suggested a diagnostic assay integrating a genomic scar-based biomarker with a marker of PARPi resistance because tumors can undergo events that restore HR [72]. Once HR is restored, the tumors can then be misclassified as HRD and thereby inaccurately sensitive to PARP inhibitors and other anti-cancer therapies. There are limitations of these scores, however, as our understanding of genomics and targetable deficiencies in DNA repair mechanisms has been only recently bolstered by advancements in single nucleotide polymorphism microarrays and high-throughout sequencing technology.
The incorporation of prospective assay data into clinical trials is the first step towards utilizing the HRD phenotype in personalized treatment plans for patients. Furthermore, the FDA has recently begun requiring that all new drug applications be accompanied to the market by a biomarker that predicts its effectiveness. Validation studies will undoubtedly be required and forthcoming; however, this represents an exciting area of research with significant practice changing potential.
Conclusion
PARP inhibitors represent an exciting new targeted treatment option in the management of BRCA-related ovarian cancer and may have significant role in the treatment of other gynecologic cancers. The approval of olaparib as fourth-line therapy for women with germline BRCA mutations and ovarian cancer is a perfect example of applying translational research to drive forward another method by which patients may potentially achieve a durable emission from disease. For patients with BRCA mutations especially, ovarian cancer can become a life-long chronic disease that will require multiple different treatment regimens. The genomic science used in studies like ARI-EL2 also provides a window into the future of research in personalized cancer care and may eventually determine how clinicians will triage patients to therapy. PARP inhibitors have already demonstrated impressive responses in phase II clinical trials and provide a necessary option for patients with recurrent disease and could also possibly serve as a viable option for newly diagnosed disease.
Opinion statement.
Inhibitors of poly (ADP-ribose) polymerase (PARP) have emerged as a new class of anti-cancer drugs, specifically for malignancies bearing aberrations of the homologous recombination pathway, like those with mutations in the BRCA 1 and BRCA 2 genes. Olaparib, a potent PARP1 and PARP2 inhibitor, has been shown to significantly increase progression-free survival (PFS) in women with recurrent ovarian cancer related to a germline BRCA mutation and is currently approved fourth-line treatment in these patients. PARP inhibitors (PARPi) target the genetic phenomenon known as synthetic lethality to exploit faulty DNA repair mechanisms. While ovarian cancer is enriched with a population of tumors with known homologous recombination defects, investigations are underway to help identify pathways in other gynecologic cancers that may demonstrate susceptibility to PARPi through synthetically lethal mechanisms. The ARIEL2 trial prospectively determined a predictive assay to identify patients with HRD. The future of cancer therapeutics will likely incorporate these HRD assays to determine the best treatment plan for patients. While the role of PARPi is less clear in non-ovarian gynecologic cancers, the discovery of a predictive assay for HRD may open the door for clinical trials in these other gynecologic cancers enriched with patients with HRD. Identification of patients with tumors deficient in homologous repair or have HRD-like behavior moves cancer treatment towards individualized therapies in order to maximize treatment effect and quality of life for women living with gynecologic cancers.
Footnotes
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
• • Of major importance
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Bridges CB. The origin of variation. The American Naturalist. 1922:13. [Google Scholar]
- 3.Lord CJ, Ashworth A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat Med. 2013;19:1381–8. doi: 10.1038/nm.3369. [DOI] [PubMed] [Google Scholar]
- 4••.Dobzhansky T. Genetics of natural populations Xiii. Recombination and variability in populations of dro-sophila pseudoobscura. Genetics. 1946;31:269–90. doi: 10.1093/genetics/31.3.269. The first description in the literature of the concept of synthetic lethality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tischler J, Lehner B, Fraser AG. Evolutionary plasticity of genetic interaction networks. Nat Genet. 2008;40:390–1. doi: 10.1038/ng.114. [DOI] [PubMed] [Google Scholar]
- 6.Hartwell LH, Szankasi P, Roberts CJ, Murray AW, Friend SH. Integrating genetic approaches into the discovery of anticancer drugs. Science. 1997;278:1064–8. doi: 10.1126/science.278.5340.1064. [DOI] [PubMed] [Google Scholar]
- 7.Kaelin WG. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–98. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- 8.Tewari KS, Monk BJ. The 21st century handbook of clinical ovarian cancer. Cham: Adis. 2015 [Google Scholar]
- 9.Amé JC, Rolli V, Schreiber V, Niedergang C, Apiou F, Decker P, et al. PARP-2, a novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J Biol Chem. 1999;274:17860–8. doi: 10.1074/jbc.274.25.17860. [DOI] [PubMed] [Google Scholar]
- 10.Dantzer F, Schreiber V, Niedergang C, Trucco C, Flatter E, De La Rubia G, et al. Involvement of poly(ADP-ribose) polymerase in base excision repair. Biochimie. 1999;81:69–75. doi: 10.1016/s0300-9084(99)80040-6. [DOI] [PubMed] [Google Scholar]
- 11.Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ri-bose) and PARPs. doi: 10.1038/nrm3376. [DOI] [PubMed] [Google Scholar]
- 12.Leung AKL. Poly(ADP-ribose): an organizer of cellular architecture. J Cell Biol. 2014;205:613–9. doi: 10.1083/jcb.201402114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isabelle M, Moreel X, Gagné JP, Rouleau M, Ethier C, Gagné P, et al. Investigation of PARP-1, PARP-2, and PARG interactomes by affinity-purification mass spectrometry. Proteome Sci. 2010;8:22. doi: 10.1186/1477-5956-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Wu W, Rosidi B, Zhang L, Wang H, Iliakis G. PARP-1 and Ku compete for repair of DNA double strand breaks by distinct NHEJ pathways. Nucleic Acids Res. 2006;34:6170–82. doi: 10.1093/nar/gkl840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haince JF, McDonald D, Rodrigue A, Déry U, Masson JY, Hendzel MJ, et al. PARP1-dependent kinetics of recruitment of MRE11 and NBS1 proteins to multiple DNA damage sites. J Biol Chem. 2008;283:1197–208. doi: 10.1074/jbc.M706734200. [DOI] [PubMed] [Google Scholar]
- 16.Hirschhorn R. In vivo reversion to normal of inherited mutations in humans. J Med Genet. 2003;40:721–8. doi: 10.1136/jmg.40.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz N, Lopez E, Saleh-Gohari N, Helleday T. Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res. 2003;31:4959–64. doi: 10.1093/nar/gkg703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 19.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 20••.McNeish IA, Oza AM, Coleman RL, Scott CL, Konecny GE, Tinker A, et al. ASCO Annual Meeting. Vol. 33. Chicago, Illinois: J Clin Oncol; 2015. Results of ARIEL2: A Phase 2 trial to prospectively identify ovarian cancer patients likely to respond to rucaparib using tumor genetic analysis. (suppl; abstr 5508); 2015. The first prospectively determined HRD assay in a PARPi clinical trial and confirmed with tumor reponse to rucaparib. [Google Scholar]
- 21.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Senechal K, Neshat MS, Whang YE, Sawyers CL. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1998;95:15587–91. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, et al. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell. 2007;128:157–70. doi: 10.1016/j.cell.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 24.Mendes-Pereira AM, Martin SA, Brough R, McCarthy A, Taylor JR, Kim JS, et al. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1:315–22. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dedes KJ, Wetterskog D, Mendes-Pereira AM, Natrajan R, Lambros MB, Geyer FC, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med. 2010;2:53ra75. doi: 10.1126/scitranslmed.3001538. [DOI] [PubMed] [Google Scholar]
- 26.Hegan DC, Lu Y, Stachelek GC, Crosby ME, Bindra RS, Glazer PM. Inhibition of poly(ADP-ribose) polymerase down-regulates BRCA1 and RAD51 in a pathway mediated by E2F4 and p1 30. Proc Natl Acad Sci USA. 2010;107:2201–6. doi: 10.1073/pnas.0904783107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassumi-Fukasawa MK, Miranda-Camargo FA, Zanetti BR, Galano DF, Ribeiro-Silva A, Soares EG. Expression of BAG-1 and PARP-1 in precursor lesions and invasive cervical cancer associated with human papillomavirus (HPV) Pathol Oncol Res. 2012;18:929–37. doi: 10.1007/s12253-012-9523-y. [DOI] [PubMed] [Google Scholar]
- 28.Weaver AN, Cooper TS, Rodriguez M, Trummell HQ, Bonner JA, Rosenthal EL, et al. DNA double strand break repair defect and sensitivity to poly ADP-ribose polymerase (PARP) inhibition in human papillomavirus 16-positive head and neck squamous cell carcinoma. Oncotarget. 2015;6:26995–7007. doi: 10.18632/oncotarget.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29•.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of polyDADP-ribose] polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. Original phase I trial that determined MTD of olaparib 400mg twice daily and confirmed that its clinical benefit rate was associated with platinum sensitivity. [DOI] [PubMed] [Google Scholar]
- 30.Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J Clin Oncol. 2010;28:2512–9. doi: 10.1200/JCO.2009.26.9589. [DOI] [PubMed] [Google Scholar]
- 31.Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–44. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 32.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–51. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 33.Yang D, Khan S, Sun Y, Hess K, Shmulevich I, Sood AK, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306:1557–65. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–92. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 35.Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30:372–9. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 36••.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–61. doi: 10.1016/S1470-2045(14)70228-1. Randomized phase II trial, known as Study 19, demonstrated the PFS benefit of olaparib in the maintenance setting, especially in BRCA mutation carriers. It serves as the precursor for the SOLO-1 trial. [DOI] [PubMed] [Google Scholar]
- 37••.Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–50. doi: 10.1200/JCO.2014.56.2728. Multicenter phase II trial, known as Study 42, that lead to the FDA approval of olaparib as fourth line therapy for women with BRCA-related ovarian cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Food and Drug Administration. [Access date: September 10, 2015];FDA approves Lynparza to treat advanced ovarian cancer. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm427554.htm.
- 39.Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA. 2008;105:17079–84. doi: 10.1073/pnas.0806092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evers B, Drost R, Schut E, de Bruin M, van der Burg E, Derksen PW, et al. Selective inhibition of BRCA2-deficient mammary tumor cell growth by AZD2281 and cisplatin. Clin Cancer Res. 2008;14:3916–25. doi: 10.1158/1078-0432.CCR-07-4953. [DOI] [PubMed] [Google Scholar]
- 41.Hay T, Matthews JR, Pietzka L, Lau A, Cranston A, Nygren AO, et al. Poly(ADP-ribose) polymerase-1 inhibitor treatment regresses autochthonous Brca2/p53-mutant mammary tumors in vivo and delays tumor relapse in combination with carboplatin. Cancer Res. 2009;69:3850–5. doi: 10.1158/0008-5472.CAN-08-2388. [DOI] [PubMed] [Google Scholar]
- 42.Joenje H, Patel KJ. The emerging genetic and molecular basis of fanconi anaemia. Nat Rev Genet. 2001;2:446–57. doi: 10.1038/35076590. [DOI] [PubMed] [Google Scholar]
- 43.Grompe M, DAndrea A. Fanconi anemia and DNA repair. Hum Mol Genet. 2001;10:2253–9. doi: 10.1093/hmg/10.20.2253. [DOI] [PubMed] [Google Scholar]
- 44.DAndrea AD, Grompe M. The fanconi anaemia/BRCA pathway. Nat Rev Cancer. 2003;3:23–34. doi: 10.1038/nrc970. [DOI] [PubMed] [Google Scholar]
- 45.Pothuri B. BRCA1- and BRCA2-related mutations: therapeutic implications in ovarian cancer. Ann Oncol. 2013;(Suppl 8):viii22–7. doi: 10.1093/annonc/mdt307. [DOI] [PubMed] [Google Scholar]
- 46.Del Conte G, Sessa C, von Moos R, Viganò L, Digena T, Locatelli A, et al. Phase I study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br J Cancer. 2014;111:651–9. doi: 10.1038/bjc.2014.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JM, Hays JL, Annunziata CM, Noonan AM, Minasian L, Zujewski JA, et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with bio-marker analyses. J Natl Cancer Inst. 2014;106:dju089. doi: 10.1093/jnci/dju089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Oza AM, Cibula D, Benzaquen AO, Poole C, Mathijssen RH, Sonke GS, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16:87–97. doi: 10.1016/S1470-2045(14)71135-0. Randomized phase II trial, known as Study 41, that demonstrated improved PFS of olaparib with chemotherapy compared to carboplatin/paclitaxel alone. [DOI] [PubMed] [Google Scholar]
- 49.Rivkin SE, Iriarte D, Sloan H, Wiseman C, Moon J. ASCO Annual Meeting. Vol. 32. Chicago: J Clin Oncol; 2014. Phase Ib/II with expansion of patients at the MTD study of olaparib plus weekly (metronomic) carboplatin and paclitaxel in relapsed ovarian cancer patients; p. 5s. (suppl; abstr 5527) [Google Scholar]
- 50.Giansanti V, Donà F, Tillhon M, Scovassi AI. PARP inhibitors: new tools to protect from inflammation. Biochem Pharmacol. 2010;80:1869–77. doi: 10.1016/j.bcp.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 51.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–54. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 52•.Liu JF, Tolaney SM, Birrer M, Fleming GF, Buss MK, Dahlberg SE, et al. A Phase 1 trial of the polyDADP-ribose] polymerase inhibitor olaparib DAZD2281] in combination with the anti-angiogenic cediranib DAZD2171] in recurrent epithelial ovarian or triple-negative breast cancer. Eur J Cancer. 2013;49:2972–8. doi: 10.1016/j.ejca.2013.05.020. The initial phase I trial that established the MTD of olaparib to be 200mg BID in combination with cedrianib 30mg daily. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009;27:5601–6. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54••.Liu JF, Barry WT, Birrer M, Lee JM, Buckanovich RJ, Fleming GF, et al. Combination cediranib and olaparib versus olaparib alone for women with recurrent platinum-sensitive ovarian cancer: a randomised phase 2 study. Lancet Oncol. 2014;15:1207–14. doi: 10.1016/S1470-2045(14)70391-2. Randomized phase II trial demonstrating significantly improved PFS of combination cediranib and olaparib compared to olaparib alone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner LM. Profile of veliparib and its potential in the treatment of solid tumors. Oncol Targets Ther. 2015;8:1931–9. doi: 10.2147/OTT.S69935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Coleman RL, Sill MW, Bell-McGuinn K, Aghajanian C, Gray HJ, Tewari KS, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation - An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;137:386–91. doi: 10.1016/j.ygyno.2015.03.042. Phase II trial demonstrating activity of veliparib in heavily pretreated patients with BRCA-related ovarian cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kummar S, Ji J, Morgan R, Lenz HJ, Puhalla SL, Belani CP, et al. A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res. 2012;18:1726–34. doi: 10.1158/1078-0432.CCR-11-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kummar S, Chen A, Ji J, Zhang Y, Reid JM, Ames M, et al. Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res. 2011;71:5626–34. doi: 10.1158/0008-5472.CAN-11-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan AR, Toppmeyer D, Stein MN, Moss RA, Gounder M, Lindquist DC, et al. ASCO Annual Meeting. Vol. 29. Chicago, Illinois: J Clin Oncol; 2011. Phase I trial of veliparib, (ABT-888), a poly(ADP-ribose) polymerase (PARP) inhibitor, in combination with doxorubicin and cyclophosphamide in breast cancer and other solid tumors. (suppl; abstr 3041) [Google Scholar]
- 60.Liu FW, Cripe J, Tewari KS. Anti-angiogenesis therapy in gynecologic malignancies. Oncology (Williston Park) 2015;29:350–60. [PubMed] [Google Scholar]
- 61.Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, Huang H, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 62.Sandhu SK, Schelman WR, Wilding G, Moreno V, Baird RD, Miranda S, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14:882–92. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 63•.Matulonis U, Mahner S, Wenham RM, Ledermann JA, Monk BJ, Del Campo JM, et al. ASCO Annual Meeting. Vol. 2. Chicago, Illinois: J Clin Oncol; 2014. A phase 3 randomized double-blind trial of maintenance with niraparib versus placebo in patients with platinum-sensitive ovarian cancer (ENGOT-OV16/NOVA trial) p. 5s. (suppl; abstr TPS5625); 2014. A phase III double-blind, placebo-controlled, 2:1 randomized trial of maintenance niraparib versus placebo in patients with recurrent platinum sensitive high grade serous ovarian cancer or known to have a germline BRCA mutation. [Google Scholar]
- 64.Moore KN, Zhang Z-Y, Agarwal S, Patel MR, Burris HA, Martell RE, et al. ASCO Annual Meeting. Vol. 32. Chicago, Illinois: J Clin Oncol; 2014. Food effect substudy of a phase 3 randomized double-blind trial of maintenance with niraparib (MK4827), a poly(ADP)ribose polymerase (PARP) inhibitor versus placebo in patients with platinum-sensitive ovarian cancer. (suppl; abstr e16531) [Google Scholar]
- 65.Ihnen M, zu Eulenburg C, Kolarova T, Qi JW, Manivong K, Chalukya M, et al. Therapeutic potential of the poly(ADP-ribose) polymerase inhibitor rucaparib for the treatment of sporadic human ovarian cancer. Mol Cancer Ther. 2013;12:1002–15. doi: 10.1158/1535-7163.MCT-12-0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shapiro G, Kristeleit R, Middleton M, Burris H, Rhoda Molife L, Jeff E, et al. Pharmacokinetics of orally administered rucaparib in patients with advanced solid tumors. Mol Cancer Ther. 2013;12:A218. Proceedings of AACR/NCI/EORTC targeted therapies meeting. [Google Scholar]
- 67.Kristeleit R, Shapira-Frommer R, Burris H, Patel MR, Lorusso P, Oza AM, et al. Phase 1/2 study of oral rucaparib: updated phase 1 and preliminary phase 2 results. Ann Oncol; ESMO Annual Meeting; Madrid, Spain. 2014. pp. iv305–26. [DOI] [Google Scholar]
- 68.Swisher EM, Oza AM, Coleman RL, Scott C, Lin K, Dominy E, et al. Tumor BRCA mutation or high genomic LOH identify ovarian cancer patients likely to respond to rucaparib: interim results for ARIEL2 clinical trial; Chicago. Annual Meeting on Women’s Cancer.2015. [Google Scholar]
- 69.Shen Y, Rehman FL, Feng Y, Boshuizen J, Bajrami I, Elliott R, et al. BMN 673, a novel and highly potent PARP 1/2 inhibitor for the treatment of human cancers with DNA repair deficiency. Clin Cancer Res. 2013;19:5003–15. doi: 10.1158/1078-0432.CCR-13-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Bono JS, Mina LA, Gonzalez M, Curtin NJ, Wang E, Henshaw JW, et al. ASCO Annual Meeting. Vol. 31. Chicago, Illinois: J Clin Oncol; 2013. First-in-human trial of novel oral PARP inhibitor BMN 673inpatients with solid tumors. (suppl; abstr 2580) [Google Scholar]
- 71.Haluska P, Timms KM, AlHilli M, Wang Y, Hartman AM, Jones J, et al. Homologous recombination deficiency DHRD] score and niraparib efficacy in high grade ovarian cancer. Eur J Cancer. 2014;50 (supp 6 abst 214):72. 26th EORTC–NCI–AACR Symposium on Molecular Targets and Cancer Therapies. Madrid, Spain. [Google Scholar]
- 72.Watkins JA, Irshad S, Grigoriadis A, Tutt AN. Genomic scars as biomarkers of homologous recombination deficiency and drug response in breast and ovarian cancers. Breast Cancer Res. 2014;16:211. doi: 10.1186/bcr3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Birkbak NJ, Wang ZC, Kim JY, Eklund AC, Li Q, Tian R, et al. Telomeric allelic imbalance indicates defective DNA repair and sensitivity to DNA-damaging agents. Cancer Discov. 2012;2:366–75. doi: 10.1158/2159-8290.CD-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mulligan JM, Hill LA, Deharo S, Irwin G, Boyle D, Keating KE, et al. Identification and validation of an anthracycline/cyclophosphamide-based chemotherapy response assay in breast cancer. J Natl Cancer Inst. 2014;106:djt335. doi: 10.1093/jnci/djt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Telli ML, Jensen KC, Vinayak S, Kurian AW, Lipson JA, Flaherty PJ, et al. Phase II study of gemcitabine, carboplatin, and iniparib as neoadjuvant therapy for triple-negative and BRCA1/2 mutation-associated breast cancer with assessment of a tumor-based measure of genomic instability: PrECOG 0105. J Clin Oncol. 2015;33:1895–901. doi: 10.1200/JCO.2014.57.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]