Abstract
Purpose of Review
The Institutional Development Award (IDeA) program was created to increase the competitiveness of investigators in states with historically low success rates for National Institutes of Health (NIH) research funding applications. IDeA states have high numbers of rural and medically underserved residents with disproportionately high rates of infant mortality, obesity, and poverty. This program supports the development and expansion of research infrastructure and research activities in these states. The IDeA States Pediatric Clinical Trials Network (ISPCTN) is part of the Environmental Influences on Child Health Outcomes (ECHO) program. Its purpose is to build research capacity within IDeA states and provide opportunities for children in IDeA states to participate in clinical trials. This review describes the current and future activities of the network.
Recent findings
In its initial year, the ISPCTN created an online series on clinical trials, initiated participation in a study conducted by the Pediatric Trials Network, and proposed two novel clinical trials for obese children. Capacity building and clinical trial implementation will continue in future years.
Summary
The ISPCTN is uniquely poised to establish and support new pediatric clinical research programs in underserved populations, producing both short- and long-term gains in the understanding of child health.
Keywords: Rural and underserved children, capacity building and professional development for clinical trial implementation, multicenter pediatric clinical trials
Introduction
Multicenter clinical trials and clinical research networks play a crucial role in advancing the diagnosis, treatment, and prevention of illness, particularly for children [1–5]. Clinical research networks increase capacity and establish the infrastructure necessary to support multiple multicenter clinical trials over time [4, 6, 7]. Clinical research networks can also help develop the capacity of individuals to conduct research through access to collaborators and mentors [8]. The study of multiple, diverse groups of subjects allows clinicians to identify strategies that are beneficial to a wider range of child populations [1–3]. Additionally, multicenter clinical trials provide participants with access to state-of-the-art care [1, 2].
Children are often underrepresented in clinical research [9–12]. There are fewer clinical trials published with pediatric patients, particularly randomized controlled trials and multicenter studies [10, 12–14]. This state of affairs has a negative impact on the ability to provide evidence-based medical care for children, as age-based differences in pediatric pathophysiology and drug metabolism, among other factors, can affect treatment options [9, 14]. There is also a mismatch between disease prevalence and sites of pediatric studies, highlighting the need to expand the geographic reach of clinical trials networks. The populations studied need to more accurately reflect disease incidence and pathophysiology [13].
Racial and ethnic minorities, rural populations, and patients of low socioeconomic status are particularly underrepresented in clinical trials, even more so among pediatric studies [1, 3, 15]. Expanding the capacity to perform high-quality, multicenter pediatric clinical trials that include underrepresented populations is essential to increasing the understanding of child health in general and the applicability of clinical trials data to broader populations. Such studies will also give children greater access to cutting-edge medical care [1]. The Institutional Development Award (IDeA) States Pediatric Clinical Trials Network (ISPCTN) aligns institutions throughout the country to begin to address these gaps.
The IDeA program is aimed at increasing the competitiveness of investigators in states that have historically low success rates for receiving NIH research funding. The program supports the development of faculty and research infrastructure through four components: Center of Biomedical Research Excellence (COBRE), targeted at faculty research capacity; IDeA Networks of Biomedical Research Excellence (INBRE), targeted at undergraduate research development; IDeA Program Infrastructure for Clinical and Translational Research (IDeA-CTR), which supports broad clinical and translational research; and IDeA Co-funding, which provides limited support to NIH R15 or R01 applicants whose proposal received excellent rating through the peer review process but fell short of the Institute’s or Center’s pay line. The IDeA program includes 24 states and territories: Alaska, Arkansas, Delaware, Hawaii, Idaho, Kansas, Kentucky, Louisiana, Maine, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Mexico, North Dakota, Oklahoma, Puerto Rico, Rhode Island, South Carolina, South Dakota, Vermont, West Virginia, and Wyoming.
Children in IDeA states have higher risks than the United States (US) average for several conditions that comprise the focus areas of ECHO: asthma; preterm birth; infant mortality; overweight and obesity; and emotional, behavioral or neurodevelopment conditions (Table 1). While 31% of children between 10 and 17 years of age are overweight or obese nationally, over 35% of children in 5 IDeA states are overweight or obese [16]. Infant mortality rates over 9.0 per 1,000 live births were reported in IDeA states compared to 5.9 per 1,000 live births in the US as a whole [16]. Additionally, limited or lack of access to health care due to poverty and geographic distance from medical facilities increases these health risks. Over 30% of the population in the IDeA states lives in a rural area compared to 12% in non-IDeA states [17]. Seven of the 10 states with the highest poverty rates are also IDeA states [18]. Thus, children in IDeA states are at higher risk of several health conditions and experience greater barriers to both routine health care and participation in clinical trials [19].
Table 1.
Number of IDeA states in the highest quartile for negative health indicator
| Health indicator | Number of IDeA states |
|---|---|
| Preterm birth | 8 |
| Infant mortality | 8 |
| Overweight or obese 10–17 year olds | 8 |
| Asthma | 5 |
| Emotional, behavioral, or neurodevelopmental conditions | 10 |
| Children living in poverty (100% poverty) | 8 |
Data from [16]
While clinical trials are widely viewed as essential to evaluating new treatments, prevention strategies, and other approaches to improve health, children are underrepresented in clinical trials literature [11, 20]. Even more concerning, children in IDeA states are underrepresented in clinical trials. For example, among the 22 pediatric sites in the AsthmaNet [21], a clinical research network formed by the National Heart, Lung and Blood Institute, only one is in an IDeA state. Similarly, only 2 IDeA states are represented among the 15 clinical centers in the Neonatal Research Network, which evaluates treatment and management strategies for newborns and is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Challenges to clinical trial participation among rural and other historically underrepresented residents include cultural barriers, perceptions of researchers as outsiders, lack of knowledge about clinical trials, and geographic distance to research sites [22, 23]. Successful recruitment of geographically, ethnically, and socioeconomically diverse pediatric populations will require a multifaceted approach that incorporates engaging communities, enhancing awareness about clinical trials, and instituting approaches to reduce the burden of child/parent participation.
The ISPCTN was formed as part of the NIH Environmental Influences on Child Health Outcomes (ECHO) program with a focus on the ECHO priority areas: upper and lower airway disease; pediatric obesity; neurodevelopment; positive child health; and pre-, peri-, and post-natal outcomes. Its purpose is to provide an opportunity for children in IDeA states to participate in clinical trials and to build clinical trial research capacity in the IDeA states.
Assessment of Research Capacity at ISPCTN Sites
The ISPCTN comprises 17 clinical site awardees representing 17 IDeA states and a Data Coordinating and Operations Center (DCOC) (Figure 1). Several of the site awardees include more than one practice site within the state. Most of the sites have a high proportion of rural and medically underserved children, including several states with significant native and tribal populations. Additionally, the sites include a wide array of research experience and infrastructure among the clinical trial teams and institutions. To capture this diversity, the DCOC developed and administered a site assessment survey to evaluate the following: the clinical experience of the principal investigators, co-investigators, and study staff; availability of other research staff; recruitment methods that had been used in other studies; availability of imaging, laboratory, and pharmacy facilities and neonatal intensive care units (NICUs); and the use of electronic medical records. As the 17 clinical site awardees include 24 separate institutions, surveys were completed for all 24 institutions. At the majority of the institutions (71%), at least one investigator is currently participating in a multicenter clinical trial network.
Figure 1.
Map of the IDeA States Pediatric Clinical Trials Network (17 sites and DCOC)
The survey indicated that there is widespread availability of on-site imaging facilities: ultrasound (100%), x-ray (96%), magnetic resonance imaging (92%), and computed tomography (88%). Twenty-two institutions (92%) have a dedicated refrigerator available for on-site specimen storage and a refrigerated centrifuge, and 20 institutions (93%) have a research pharmacy on site. There are 19 sites (79%) with NICUs.
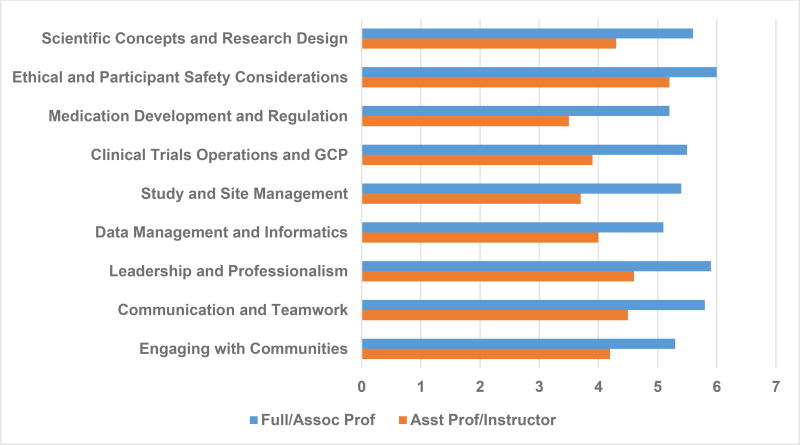
To evaluate the training needs of the clinical investigators, a needs assessment survey was developed and administered. The survey was based on evaluating the core competencies for clinical trials established by the Joint Task Force for Clinical Trial Competency [24, 25]. Core competencies were evaluated in the following categories: scientific concepts and research design; ethical and participant safety considerations; medication development and regulation; clinical trials operations and good clinical practice; study and site management; data management and informatics; leadership and professionalism; and communication and teamwork. A question was added to address the issue of engaging with communities, given the significant proportion of underrepresented children that will potentially be involved in the Network. Respondents were also asked to provide information on their academic rank, percent of protected research time, funding source(s) of clinical trial activities, tasks they have performed for a clinical trial, and current and desired roles with respect to conducting clinical trials. Following the Hennessy-Hicks model [26], respondents were asked to answer the following questions for each core competency with a number from 1–7: how important is this activity to the successful performance of your desired role in clinical trials (1 signifies “not very important” and 7 signifies “very important”); and how well do you perform that activity today (1 signifies “not well” and 7 signifies “very well”).
Self-reported performance scores in all areas were higher among senior faculty members compared to their junior counterparts (Figure 2). Average scores for all areas and academic levels were below 6.0, suggesting that universal training in all areas of competency in clinical trials would be beneficial.
Figure 2.
Performance Scores for Clinical Trial Competencies
Capacity Building and Professional Development
To provide an introduction to clinical trials, the DCOC developed six learning modules on the subject: “Overview of Clinical Trials”; “Design of Phase I Clinical Trials”; “Design of Phase II Clinical Trials”; “Randomization and Masking”; “Design of Phase III and IV Clinical Trials”; and “How Clinical Trials Differ from Cohort and Case-control Studies.” Each module consisted of a 15-minute slide presentation with a voiceover followed by a 5-minute question and answer podcast. These modules were released weekly over the summer of 2017. A total of 37 individuals viewed the modules. The evaluation survey administered at the conclusion of the series showed that 78% of respondents felt that the modules increased their knowledge and 65% felt that it would increase their research activities.
Individual sites also engaged in local professional development and capacity building activities. These activities included mentoring programs for clinicians, faculty, study coordinators, and health science students who were new to clinical trials; introductory research design workshops; online seminars regarding research regulatory concerns; and community engagement strategies to enhance collaborations with tribal and native populations. Since most Network sites are in rural areas, future capacity building needs will also address engagement of rural sites and non-academic health care providers.
The individual site evaluations of educational content as well as experiences in and results of dissemination of the locally developed content will be assessed by the DCOC to identify key lessons for engaging pediatricians across the IDeA states in faculty development activities. These evaluations will inform the content of future professional development offerings, which will draw on available resources and will include the development of materials focused on the needs of the IDeA states.
Clinical Trials
In the first year of Network activities, two clinical trials goals were pursued. First, the Network identified an existing trial that could provide an opportunity for early engagement of the Network sites in clinical trial activities. The Network’s initial clinical trial activity is to enroll study participants into an existing clinical trial conducted by the NIH-funded Pediatric Trial Network, operated out of the Duke Clinical Research Institute (DCRI): Pharmacokinetics of Understudied Drugs Administered to Children, known as POPS. Recently, the POPS study has developed population pharmacokinetic models for dosing of clindamycin in children [27, 28] and for ampicillin in neonates [29]. In this trial, children who are being treated with certain medications as part of routine medical care are asked to provide blood or another body fluid specimen for pharmacokinetic analysis at the DCRI. This trial offers the opportunity for significant capacity building and testing as Network sites are asked to obtain Institutional Review Board approval for a clinical trial, participate in site qualification and site initiation visits, and identify a team of investigators responsible for performing the trial at each institution. In addition to contributing to the study of these medications in children, POPS provides the opportunity for many investigators and research team members with little clinical research experience to apply the principles of clinical research introduced in the faculty development activities to a pediatric clinical trial and serves as an important capacity building activity for the Network. The process will guide sites to learn about local resources, identify and fill gaps in research capacity, and practice workflows common to clinical research, such as patient identification, informed consent, and data management. Participating sites from the Network will begin enrollment in the first quarter of 2018.
The second clinical trial goal was the development of new pediatric clinical research studies. Working groups of Network investigators and other subject matter experts in each of the ECHO outcomes priorities collaborated to develop and refine several ideas for pediatric clinical trials that were then evaluated by the Network based on scientific merit, feasibility, and relevance to the mission of ECHO. Two studies have been prioritized and are currently being prepared for evaluation by the NIH ECHO Protocol Review Committee.
The first study is designed to address the high rates of obesity-related asthma and the potential role of vitamin D as a treatment for obesity-related asthma in children. Vitamin D has been suggested as a beneficial therapy for asthma in some populations, but it is difficult to reach therapeutic levels of vitamin D in obese children. This trial will first identify optimal vitamin D dosing in obese children and then assess the impact of vitamin D on asthma symptoms and severity. The second study will measure the effectiveness of a clinic-based behavioral intervention delivered via electronic tablets or via newsletter to address diet and physical activity in obese children with the goal of reducing BMI in children and their families. This trial will engage rural and underrepresented minorities in a cluster-randomized design, demonstrating the unique value of this Network in reaching high-risk but understudied populations. We anticipate initiation of these two trials in 2018.
The ISPCTN is currently collaborating with the ECHO Coordinating Center at DCRI and the Neonatal Research Network to develop a clinical trial to address the management of infants with neonatal opioid withdrawal syndrome. To initiate the process, the Network surveyed NICUs and delivery sites to determine the current pharmacologic and non-pharmacologic approaches to management of these infants to provide background information for the clinical trial.
Conclusion
In its first year, the ISPCTN has made significant strides in its goals of increasing research capacity and access to clinical trials in the IDeA states. The Network has successfully implemented a web-based research skills training series to meet the needs of busy clinicians and identified several research projects that will improve our ability to care for children. Future studies will integrate findings and resources from the other ECHO components, as well as ongoing efforts to evaluate and implement other innovative pediatric research programs. The ISPCTN is uniquely poised to advance our understanding of optimal strategies for establishing clinical research programs in new sites and in training health care professionals to participate in research in ways that are effective, practical, and sustainable. The ISPCTN and the ECHO program will continue to pursue both short- and long-term gains as we improve our current understanding of child health and disease and build an infrastructure to support ongoing, cutting-edge pediatric research.
Key Points.
The IDeA States Pediatric Clinical Trials Network (ISPCTN) provides an opportunity for rural and underserved regions to participate in clinical trials.
The ISPCTN is engaged in capacity building by offering professional development opportunities and through participation in clinical trials conducted by other NIH-supported networks.
The ISPCTN has proposed two novel clinical trials with interventions for children who are overweight or obese.
Acknowledgments
The authors would like to thank the principal investigators of the IDeA States Pediatric Clinical Trials Network for their contributions to this project.
Financial Support and Sponsorship
Clinical Sites, Locations, and Principal Investigators of the IDeA States Pediatric Clinical Trials Network: Alaska Native Tribal Health Consortium, Anchorage, Alaska (Rosalyn Singleton, NIH award 8UG1OD024944-02); Arkansas Children’s Research Institute, Little Rock, Arkansas (Gregory Kearns and Laura James, NIH award 8UG1OD024945-02); Dartmouth College, Hanover, New Hampshire (Paul Palumbo, NIH award 8UG1OD024946-02); Louisiana State University Pennington Biomedical Research Center, Baton Rouge, Louisiana (Daniel Hsia, NIH award UG1OD024959-02); Nemours Alfred I. duPont Hospital for Children, Wilmington, Delaware (Judith Ross, NIH award 8UG1OD024958-02); Rhode Island Hospital, Providence, Rhode Island (Phyllis Dennery, Thomas Chun, and Abbot Laptook, NIH award 8UG1OD024951-02); University of Hawaii at Manoa, Honolulu, Hawaii (Bruce Shiramizu, NIH award 8UG1OD024948-02); University of Kansas Medical Center, Kansas City, Kansas (Russell McCulloh, NIH award 8UG1OD024943-02); University of Louisville, Louisville, Kentucky (Janice Sullivan and Sara Watson, NIH award 8UG1OD024954-02); University of Mississippi Medical Center, Jackson, Mississippi (Robert Annett and Marc Majure, NIH award 8UG1OD024942-02); University of Montana, Missoula, Montana (Paul Smith, NIH award 8UG1OD024952-02); University of Nebraska Medical Center, Omaha, Nebraska (Jessica Snowden, NIH award 8UG1OD024953-02); University of New Mexico, Albuquerque, New Mexico (Hengameh Raissy and Alberta Kong, NIH award 8UG1OD024947-02); University of Oklahoma, Oklahoma City, Oklahoma (Paul M. Darden, NIH award 8UG1OD024950-02); University of South Carolina, Columbia, South Carolina (Christine Turley, Lisa Knight, and Andrew Atz, NIH award 8UG1OD024956-02); University of Vermont, Burlington, Vermont (Kelly Cowan, NIH award 8UG1OD024955-02); and West Virginia University, Morgantown, West Virginia (J. Philip Saul, Lesley Cottrell, and Lee Pyles, NIH award 8UG1OD024949-02).
Data Coordinating and Operations Center: University of Arkansas for Medical Sciences, Little Rock, Arkansas (Jeannette Lee, NIH award 8U24OD024957-02)
Steering Committee Chair: Jill Joseph, University of California at Davis, Davis, California
Funding for this work was provided by the National Institutes of Health.
Footnotes
Conflicts of Interest
None
References
- 1.Bolen S, Tilburt J, Baffi C, et al. Defining "success" in recruitment of underrepresented populations to cancer clinical trials: moving toward a more consistent approach. Cancer. 2006;106(6):1197–204. doi: 10.1002/cncr.21745. [DOI] [PubMed] [Google Scholar]
- 2.Baquet CR, Henderson K, Commiskey P, Morrow JN. Clinical trials: the art of enrollment. Semin Oncol Nurs. 2008;24(4):262–9. doi: 10.1016/j.soncn.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burchard EG, Oh SS, Foreman MG, Celedon JC. Moving toward true inclusion of racial/ethnic minorities in federally funded studies. A key step for achieving respiratory health equality in the United States. Am J Respir Crit Care Med. 2015;191(5):514–21. doi: 10.1164/rccm.201410-1944PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slora EJ, Harris DL, Bocian AB, Wasserman RC. Pediatric clinical research networks: current status, common challenges, and potential solutions. Pediatrics. 2010;126(4):740–5. doi: 10.1542/peds.2009-3586. [DOI] [PubMed] [Google Scholar]
- 5.Schilsky RL, McIntyre OR, Holland JF, Frei E., 3rd A concise history of the cancer and leukemia group B. Clin Cancer Res. 2006;12(11 Pt 2):3553s–5s. doi: 10.1158/1078-0432.CCR-06-9000. [DOI] [PubMed] [Google Scholar]
- 6.Wasserman R, Bocian A, Harris D, Slora E. Limited capacity in US pediatric drug trials: qualitative analysis of expert interviews. Paediatr Drugs. 2011;13(2):119–24. doi: 10.2165/11584240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Choong K, Duffett M, Cook DJ, Randolph AG. The impact of clinical trials conducted by research networks in pediatric critical care. Pediatr Crit Care Med. 2016;17(9):837–44. doi: 10.1097/PCC.0000000000000835. [DOI] [PubMed] [Google Scholar]
- 8.Bakken S, Lantigua RA, Busacca LV, Bigger JT. Barriers, enablers, and incentives for research participation: a report from the Ambulatory Care Research Network (ACRN) J Am Board Fam Med. 2009;22(4):436–45. doi: 10.3122/jabfm.2009.04.090017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourgeois FT, Murthy S, Pinto C, et al. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics. 2012;130(2):285–92. doi: 10.1542/peds.2012-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen E, Uleryk E, Jasuja M, Parkin PC. An absence of pediatric randomized controlled trials in general medical journals, 1985–2004. J Clin Epidemiol. 2007;60(2):118–23. doi: 10.1016/j.jclinepi.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Thomson D, Hartling L, Cohen E, et al. Controlled trials in children: quantity, methodological quality and descriptive characteristics of pediatric controlled trials published 1948–2006. PLoS One. 2010;5(9) doi: 10.1371/journal.pone.0013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Castaldi C, Silverstein M, Bauchner H. Child versus adult research: the gap in high-quality study design. Pediatrics. 2008;122(1):52–7. doi: 10.1542/peds.2007-2849. [DOI] [PubMed] [Google Scholar]
- 13.Bourgeois FT, Olson KL, Ioannidis JP, Mandl KD. Association between pediatric clinical trials and global burden of disease. Pediatrics. 2014;133(1):78–87. doi: 10.1542/peds.2013-2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt C. Many pediatric studies are a waste of time [Internet] Scientific American. 2017 Available from: https://www.scientificamerican.com/article/many-pediatric-studies-are-a-waste-of-time/
- 15.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008;112(2):228–42. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 16*.KIDS COUNT Data Center and Data Book [Internet] The Annie E. Casey Foundation. 2017 Available from: http://www.aecf.org/work/kids-count/kids-count-data-center/. Supported by the Annie E. Casey Foundation, the KIDS COUNT Data Center website and the KIDS COUNT Data Book provide national and local data on child health and well-being. In particular, the KIDS COUNT Data Center website offers regular updates with data snapshots and policy reports demonstrating trends both nationwide and by state. This website is of special interest to researchers because of its invaluable information on child health and relevant social determinants of health.
- 17.Rural-Urban Continuum Codes [Internet] United States Department of Agriculture Economic Research Service. 2013 Available from: https://www.ers.usda.gov/data-products/rural-urban-continuum-codes.aspx.
- 18.Hillemeier MM, Lynch J, Harper S, et al. Relative or absolute standards for child poverty: a state-level analysis of infant and child mortality. Am J Public Health. 2003;93(4):652–7. doi: 10.2105/ajph.93.4.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dovey S, Weitzman M, Fryer G, et al. The ecology of medical care for children in the United States. Pediatrics. 2003;111(5 Pt 1):1024–9. doi: 10.1542/peds.111.5.1024. [DOI] [PubMed] [Google Scholar]
- 20.Cohen E, Goldman RD, Ragone A, et al. Child vs adult randomized controlled trials in specialist journals: a citation analysis of trends, 1985–2005. Arch Pediatr Adolesc Med. 2010;164(3):283–8. doi: 10.1001/archpediatrics.2009.291. [DOI] [PubMed] [Google Scholar]
- 21.AsthmaNet [Internet] The Pennsylvania State University. 2010 Available from: http://asthmanetresearch.org/
- 22.Bergeron CD, Foster C, Friedman DB, et al. Clinical trial recruitment in rural South Carolina: a comparison of investigators' perceptions and potential participant eligibility. Rural Remote Health. 2013;13(4):2567. [PubMed] [Google Scholar]
- 23.Lim CS, Follansbee-Junger KW, Crawford MS, Janicke DM. Treatment outcome research in rural pediatric populations: the challenge of recruitment. J Pediatr Psychol. 2011;36(6):696–707. doi: 10.1093/jpepsy/jsr018. [DOI] [PubMed] [Google Scholar]
- 24*.Calvin-Naylor NA, Jones CT, Wartak MM, et al. Education and training of clinical and translational study investigators and research coordinators: A competency-based approach. J Clin Transl Sci. 2017;1(1):16–25. doi: 10.1017/cts.2016.2. This paper outlines the development of a standard set of core competencies for clinical researchers, including PIs and research coordinators. This training framework is designed to increase the safety and efficiency of resarch. This paper is an excellent resource for assessing clinical trial competencies among researchers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonstein SA, Seltzer J, Li R, et al. Moving from compliance to competency: a harmonized core competency framework for the clinical research professional. Clinical Researcher. 2014 [Google Scholar]
- 26.Hicks C, Hennessy D. Hennessy-Hicks Training Needs Analysis Questionnaire and Manual [Internet] University of Birmingham. Available from: http://www.who.int/workforcealliance/knowledge/HennessyHicks_trainingneedstool.pdf.
- 27.Smith MJ, Gonzalez D, Goldman JL, et al. Pharmacokinetics of Clindamycin in Obese and Nonobese Children. Antimicrob Agents Chemother. 2017;61(4) doi: 10.1128/AAC.02014-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez D, Melloni C, Yogev R, et al. Use of opportunistic clinical data and a population pharmacokinetic model to support dosing of clindamycin for premature infants to adolescents. Clin Pharmacol Ther. 2014;96(4):429–37. doi: 10.1038/clpt.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tremoulet A, Le J, Poindexter B, et al. Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design. Antimicrob Agents Chemother. 2014;58(6):3013–20. doi: 10.1128/AAC.02374-13. [DOI] [PMC free article] [PubMed] [Google Scholar]