Abstract
Metabolic dysfunction has reemerged as an essential hallmark of tumorigenesis, and metabolic phenotypes are increasingly being integrated into pre-clinical models of disease. The complexity of these metabolic networks requires systems-level interrogation, and metabolic flux analysis (MFA) with stable isotope tracing present a suitable conceptual framework for such systems. Here we review efforts to elucidate mechanisms through which metabolism influences tumor growth and survival, with an emphasis on applications using stable isotope tracing and MFA. Through these approaches researchers can now quantify pathway fluxes in various in vitro and in vivo contexts to provide mechanistic insights at molecular and physiological scales respectively. Knowledge and discoveries in cancer models are paving the way toward applications in other biological contexts and disease models. In turn, MFA approaches will increasingly help to uncover new therapeutic opportunities that enhance human health.
Keywords: Stable isotope tracing, metabolic flux analysis, metabolism, metabolomics, cancer, mitochondria
1. Introduction
1.1 Renewed interest in metabolism
A largely forgotten vestige of biochemistry coursework, metabolism is once again being appreciated as a driver of human disease rather than a downstream effect of some genetic or transcriptional changes. Since the advent of the genomics revolution, biomedical research has largely focused on the roles of DNA and RNA dysregulation in disease. This information has led to an international, multidisciplinary effort to catalog, sequence, and interpret large amounts of genomics data from various sources (Cancer Genome Atlas Research et al., 2013). While these efforts have generated large amounts of publically-available, highly-curated data and new insights into a range of diseases, the next steps often require researchers to look beyond the mutational or allelic status of disease-associated genes and gain a more mechanistic understanding of these changes. As such, higher level activities of the cell are now coming into focus as drivers of pathological phenotype – e.g. transcriptional and translational regulation, epigenetic states, and systems-level metabolic activities.
Indeed, recent work in cancer, metabolic syndrome, and regenerative medicine has highlighted situations where metabolic alterations precede other canonical modes of biological control (e.g. transcriptional activation), demonstrating its importance in biomedicine. Metabolism, or the biochemical reactions executed by cells, is essential for the maintenance of cellular function and the response to extrinsic and intrinsic cues. To control such a complex network, mammalian metabolism has evolved a regulatory framework and interconnectivity that ensures robust functionality. Advanced methods are required (and are now becoming available) to decipher the regulation of these processes and their dysfunction in disease settings (Cordes and Metallo, 2016).
In this review, we will establish the critical need for studying metabolism at a systems-level, introduce methodological advances that have enabled interrogation of mammalian metabolism, and highlight recent work that has utilized stable isotope tracing and MFA to better understand human disease.
1.2 Thermodynamics and topology of metabolism
Metabolic reactions can largely be broken down into three main components or functions – bioenergetics, biosynthesis, and redox balance – with each component having a unique network behavior requiring systems-level interrogation.
The thermodynamic reality of a cell is the constant need to generate energy that can be coupled to unfavorable reactions with a positive Gibbs free energy (Bennett et al., 2009; Lunt and Vander Heiden, 2011; Park et al., 2016). In practice, nutrients are imported into cells then catabolized to regenerate adenosine triphosphate (ATP), the metabolic currency of cells. Due to the high energy stored in its phosphoanhydride bonds, ATP hydrolysis is required to drive thermodynamically unfavorable reactions and allow them to proceed at sufficient rates. The use of ATP regeneration and hydrolysis in disparate metabolic pathways is a prime example of how metabolic interconnectivity facilitates life and highlights the importance of the first law of thermodynamics in understanding metabolic function (Beard et al., 2002; Beard and Qian, 2005; Nagrath et al., 2007).
This connection results in two axioms of bioenergetics: (1) If a cell is consuming large amounts of ATP, a concomitant production of ATP molecules is needed. A cell can turnover its ATP pool over six times per minute (Jacobus, 1985), and ATP levels are “nearly universally homeostatic” (Hochachka and McClelland, 1997). Therefore, the proper biological unit of measure is the ATP regeneration rate (flux) rather than a metabolite level or ratio (i.e. nucleotide pool ratios). (2) Cells have evolved enough production capacity and storage (e.g. glycolysis, oxidative phosphorylation, creatine kinase, adenylate cyclase) to meet this demand in the face of various insults (e.g. substrate deprivation, hypoxia). This results in a topological reality where many pathways are connected by ATP, resulting in highly interdependent nodes within the metabolic network. More complex cells like eukaryotes have evolved further to compartmentalize reactions to facilitate (and complicate) pathway function further.
In addition to maintenance of energetic homeostasis, an important role of metabolism is to provide biosynthetic precursors. Highly proliferative cells – such as immune cells, tumor cells, and transit-amplifying stem cells – require a doubling of cellular biomass each time they divide. In addition, all somatic cells have established rates of lipid and protein turnover/nucleotide synthesis that require constant production of biosynthetic intermediates (Lemons et al., 2010). While unique mammalian auxotrophies exist that contribute to large portions of cellular biomass (i.e. essential amino acids for protein biosynthesis), cells can choose to either synthesize or uptake macronutrients for the remaining portion of needed biomass. While the “cheapest” route for a cell would be to uptake all macromolecules, network topology might dictate the need for flux through a pathway to provide substrates for a different pathway. This interdependency results in a coupling of catabolic and anabolic reactions.
Biological (and electrical) energy flow is coordinated by the movement of electrons, and these transfers are mediated by oxidoreductases and reducing equivalents (e.g. nicotinamide adenine dinucleotide (NAD+), nicotinamide adenine dinucleotide phosphate (NADP+), and flavin adenine dinucleotide (FAD)). At a high level, cells extract electrons from reduced substrates (e.g. carbohydrates, fatty acids) and secrete oxidized byproducts (e.g. lactate, CO2). Therefore, flux through oxidative pathways consumes electron carriers and produces reducing equivalents. Cells in turn must consume electrons and regenerate electron carriers to maintain proper redox balance. For example, to maintain glycolytic rates and/or tricarboxylic acid (TCA) cycle flux, cells must constantly consume electrons via lactate dehydrogenase (LDH) or respiration to regenerate NAD+ and FAD. This point highlights one potential reason why rapidly proliferating cells exhibit high glycolytic rates (i.e. the Warburg effect). For example, diversion of glycolytic intermediates for serine biosynthesis can cause redox fluctuations or imbalances such that NAD+ is not regenerated at sufficient rates by LDH to maintain glycolytic flux. Alternate NAD+ recycling pathways such as the malate-aspartate shuttle and glycerol-phosphate shunt are active in proliferating cells but may be similarly blunted as aspartate and glycerol-3-phosphate are used for biosynthesis. By maintaining high flux through glycolysis such redox fluctuations are minimized. Redox balance in cells also extends to environmental stresses through the consumption of reducing equivalents to regenerate antioxidants (i.e. the cycling of reduced (GSH) and oxidized (GSSG) glutathione). This redox control in cells demonstrates how cells have evolved metabolic interconnections to maintain homeostasis.
2. Methods of quantifying fluxes
2.1 Need of metabolic tracing
The interconnectivity, redundancies, and cross-dependencies that exist within metabolic pathways manifest themselves in classic emergent network behavior, where changes in one node can result in far-reaching and unforeseen states. For example, altering one pathway by modulating substrate availability or through molecular and pharmacological interventions can lead to system-wide changes in metabolic pathway fluxes as cells attempt to maintain homeostasis (Hackett et al., 2016). Historically, technological limitations forced scientists to interrogate metabolism at the resolution of individual enzymes. While this approach led to the elucidation of fundamental metabolic pathways, like the TCA cycle, a critical need for systems-level analyses has now emerged.
With technological advances such as gas chromatography-coupled mass spectrometry (GC/MS), liquid chromatography-coupled mass spectrometry (LC/MS), and nuclear magnetic resonance spectroscopy (NMR), researchers now have the ability to rapidly and simultaneously quantify large numbers of metabolites in a given biological setting (Fiehn et al., 2000). These developments have been essential in driving both the rapid growth in new information about metabolic control/function and the metabolic basis of human disease (Lunt et al., 2015; Mitsuishi et al., 2012; Park et al., 2017). In addition to the inherent complexities of studying any network, mammalian metabolism has unique features and must be studied at multiple length scales (Figure 1) For example, many metabolic pathways have many redundant, compartment-specific forms that can be regulated independently (e.g. TCA cycle enzymes in the mitochondria, cytosol, and/or peroxisome), or cells can reside in local cellular communities that interact to elicit a broader function (e.g. beta cells within islets or stromal-epithelial interactions). On the other hand, diseases manifest themselves throughout the body, where dysregulated insulin secretion by the pancreas in diabetes affects distal muscle microvascular, liver, adipose tissue, and neurological functions.
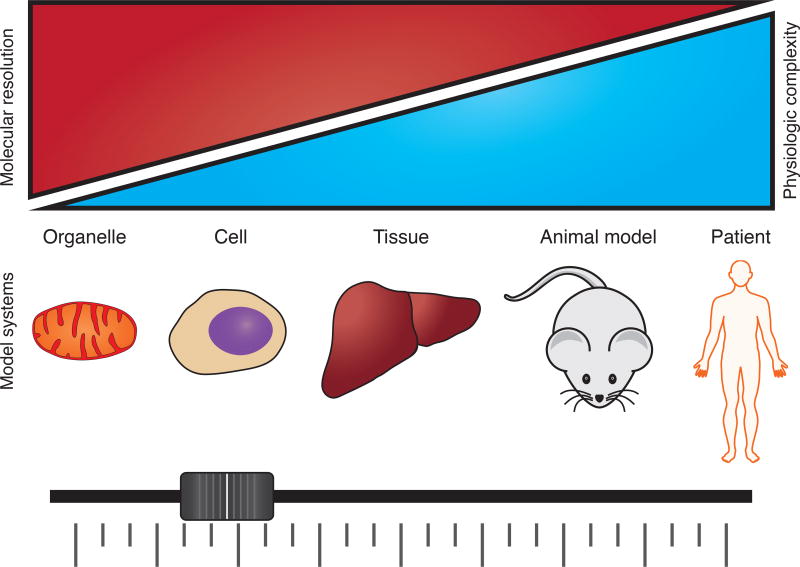
Figure 1. MFA applied to biological systems at different scales comes with a tradeoff in molecular resolution versus physiologic relevance.
Use of metabolic flux analysis is technically feasible in many systems, but measurements in more physiologically complex systems come at a cost of molecular resolution. Integration of in vivo and in vitro MFA results will be important in the future as more therapeutic targets in metabolic pathways are identified.
With these realities of network and length-scale complexity, recent work has focused on the use of systems biology to parse through and integrate all available “omics” data – genomics, transcriptomics, and metabolomics. However, sequencing data is better used for identification of novel mutations in metabolic disease (Cancer Genome Atlas Research et al., 2013; Lewis et al., 2010) and pathway activation (Lackey et al., 2013), as germline mutations and transcript level changes do not always directly map to changes in a specific metabolic pathway. Additionally, metabolomics studies have been successfully used to identify metabolic shifts and implicate potentially altered metabolic pathways (Johnson et al., 2016). However, rapid metabolomics platforms serve as a hypothesis generating methodology because one cannot necessarily infer metabolic flux alterations a priori through metabolite level changes. Since the primary driver of metabolic phenotypes is alteration of flux, stable isotope tracing and metabolic flux analysis (MFA) have emerged as critically important tools for interrogating metabolism (Hellerstein, 2004).
2.2 Stable isotope tracing
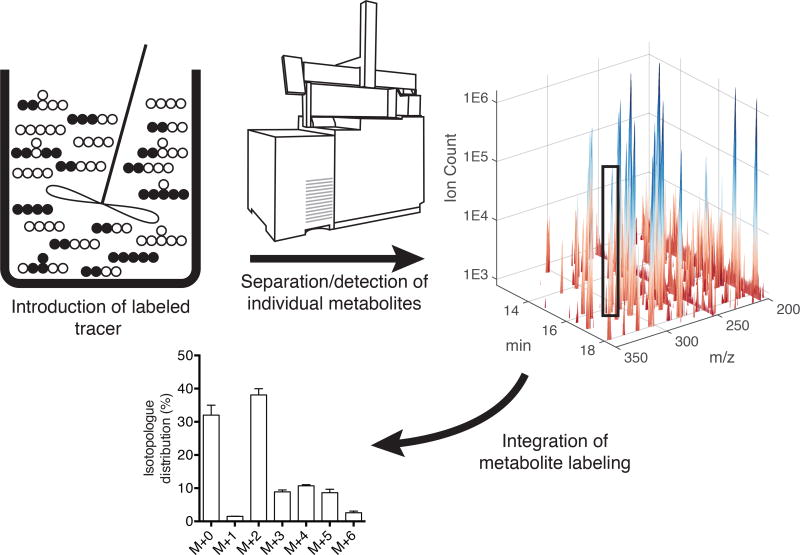
Modeling approaches have been applied to metabolic systems for some time and center around the need to conserve mass in the context of network stoichiometry and cellular needs (Stephanopoulos, 1999). These systems are often highly underdetermined, and fluxes are resolved to varying extents by the application of constraints, which may include uptake/secretion from media, transcriptomics or proteomics data, and/or isotopic labeling (Sauer, 2004). The most detailed information is often provided by the use of stable isotope tracing and metabolomics, whereby a given atom of interest is “tracked” throughout the metabolic network by culturing cells with a tracer (e.g. [1−13C]glutamine where 13C isotope is in the 1 position of the glutamine molecule). Analogous to a dye mixing through a continuously-stirred tank reactor, stable isotopes (e.g. 13C, 2H, and 15N) within a given substrate are fed to cells, tissues, or animals which then consume the “heavy” metabolite of interest and metabolize it in various downstream reactions (Figure 2). By then measuring the presence of an isotopologue–a metabolite with a different molecular weight due to the presence of the stable isotope–the fraction of an individual molecule coming from a tracer can be quantified using knowledge of atom transitions throughout the metabolic network (Figure 2).
Figure 2. Stable isotope tracing paradigm.
Isotopologue or mass isotopomer distributions (MIDs) are the central measurement in metabolic flux analysis. Stable isotope variants (i.e. 13C, 15N, 2H) of carbohydrates, fatty acids, or amino acids are introduced into a biological system of interest. The labeled atoms of interest propagate throughout the metabolic network, and the biological matrix is sampled as needed. Mass spectrometry is used to measure isotope enrichment within individual metabolite pools to determine MIDs for all compounds of interest.
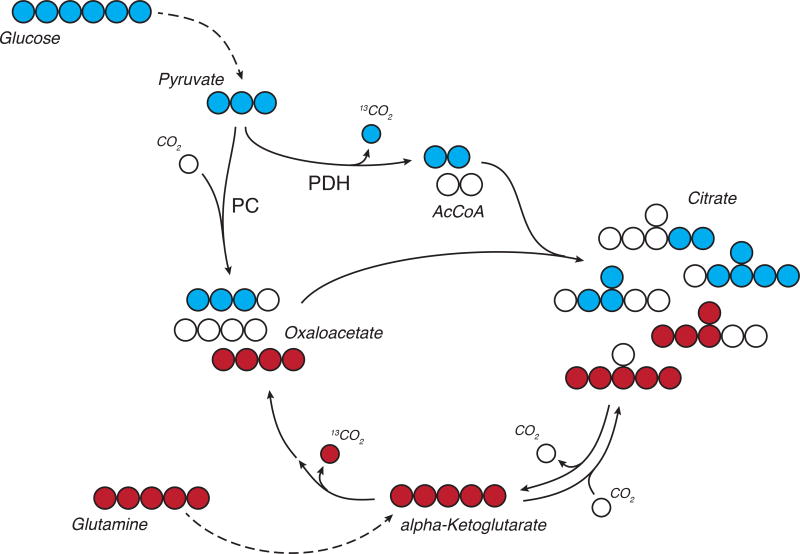
As an example, when cells metabolize [U-13C6]glucose the fully-labeled pyruvate generated from glycolysis may be oxidized and/or carboxylated in mitochondria (Figure 3). When the cell oxidizes pyruvate, the 13C carbon in the first position of pyruvate will be lost during the decarboxylation step of pyruvate dehydrogenase (PDH), yielding an M+2 labeled AcCoA. When pyruvate is metabolized by pyruvate carboxylase (PC) all three 13C atoms will be present on the resulting M+3 oxaloacetate. These metabolites condense to form citrate, resulting in a pool of labeled species with mass increments from 0 to 6, depending on the relative contribution of PC, PDH, and other pathways that produce or consume AcCoA, oxaloacetate, and citrate (Figure 3). This isotopologue or mass isotopomer distribution (MID) subsequently allows for inference of flux through certain metabolic reactions (Figure 2). In this simplified metabolite network, the ratio of the M+2 portion of the citrate pool vs the M+3 portion is a proxy of how many pyruvate molecules were catalyzed by PDH vs PC. However, data generated in real metabolic networks is more complex than that presented here due to TCA cycling and additional inputs into the citrate pool. Since many input and output fluxes influence labeling in well-connected metabolite pools, computational tools are often necessary to resolve information on fluxes for such systems (Buescher et al., 2015).
Figure 3. Tracing TCA metabolism using 13C glucose and glutamine.
In this example, labeling on citrate and other intermediates from fully labeled [U-13C6]glucose changes depending on routes used for anaplerosis and AcCoA generation. Oxidation of glucose-derived pyruvate by PDH results in M+2 citrate. Carboxylation through PC results in M+3 or M+5 citrate. [U-13C5]glutamine oxidation or reduction results in M+4 and M+5 citrate, respectively. Taken together, relative flux changes in well-connected nodes (e.g. TCA cycles) result in measureable differences in labeling. Open circles depict 12C carbon atoms, filled circles depict 13C carbon atoms.
MIDs therefore contain detailed information on relative fluxes, and these data are incorporated into models that estimate fluxes and associated confidence intervals within a given biological system (Sauer, 2006). The choice of tracer(s) will impact the specific pathways and fluxes to be resolved and should be considered carefully (Crown et al., 2012; Metallo et al., 2009). Ultimately, MFA integrates extracellular flux measurements (e.g. glucose uptake and lactate secretion), biomass composition, growth rates, and intracellular steady state labeling data to estimate intracellular fluxes (Stephanopoulos et al., 1998; Wiechert, 2001). By constraining potential flux measurements with physiological biomass demands and metabolite fluxes in and out of the system, MFA solves the inverse problem – where intracellular fluxes are estimated, theoretical labeling patterns calculated, error between theoretical and experimental data calculated, and estimated fluxes iterated through error minimization until a best fit is achieved (Zamboni, 2011). Long applied to study microbial and prokaryote metabolic networks (Sauer, 2006), advances in computational frameworks (Antoniewicz et al., 2006; Antoniewicz et al., 2007) and software packages (Quek et al., 2009; Weitzel et al., 2013; Young, 2014; Young et al., 2008; Zamboni et al., 2005) have made mammalian applications far more tractable. Exchange fluxes (i.e. the minimum of the forward and reverse flux for a given reaction) can be the most difficult to resolve (Wiechert, 2007). Compartmentation also complicates analyses and interpretation of labeling data (Zamboni, 2011) and indeed MFA can help to resolve such information in certain settings (Jiang et al., 2016a; Jiang et al., 2016b; Vacanti et al., 2014). Most MFA applications rely on the resolution of fluxes in a scaled-down, user-defined subset of the metabolic network, such as glycolysis, the oxPPP, and the TCA cycle (Sauer, 2006). Researchers have begun to apply genome-wide metabolic reaction networks in MFA studies of microbes more recently (Gopalakrishnan and Maranas, 2015; McCloskey et al., 2016).
Better resolution of intracellular fluxes can be achieved by incorporating dynamic labeling and pool size information into non-stationary MFA (NS-MFA) models. Steady-state labeling provides a relative measure of fluxes into and out of metabolic pools but requires the system to be at both metabolic and isotopic steady-state (Noh et al., 2007). Such data are often not very informative for the analysis of linear pathways (e.g. glycolysis) or exchange fluxes. NS-MFA provides an alternative computational framework for integration of labeling data, extracellular fluxes, and biomass demands (Jazmin and Young, 2013). Unlike traditional MFA which relies on algebraic solutions, the transient labeling data and pool size data are incorporated into an ODE-based model (Young et al., 2011). While increased precision is achieved by incorporation of more experimental data, more care is needed on experimental design (e.g. sampling and quenching) and more data acquisition/analysis is required (Jazmin and Young, 2013; Noh and Wiechert, 2006). This review will focus almost exclusively on steady-state MFA and basic tracing applications; however, use of NS-MFA has been reviewed extensively (Wiechert and Noh, 2013), and numerous protocols are available (Jazmin and Young, 2013; Nanchen et al., 2007; Yuan et al., 2008). This approach is increasingly being applied to mammalian systems (Fan et al., 2014; Maier et al., 2009; Munger et al., 2008).
When applied in a coordinated fashion, stable isotope tracing, metabolomics, and computational modeling can effectively resolve metabolic flux alterations in the context of both microenvironmental cues and pathophysiological alterations. In short, stable isotopes can inform on aspects of metabolism that cannot be learned through other measurements. The remainder of this review will focus on recent examples in biomedicine of how stable isotope tracing and MFA have been used to understand the metabolic mechanisms driving human disease and associated pathologies. A primary (and still emerging) area of focus is applications to cancer biology, though additional examples will be included to highlight the versatility of these approaches.
3. Cancer
3.1 Renewed appreciation of metabolic dysregulation in cancer
A desire to resolve the metabolic differences between normal tissue, tumors, and metastatic cells has re-invigorated interest in metabolic tracing and flux analysis over the last decade. Metabolism is tightly linked to the pathophysiology of a cancer cell, an observation first described by Otto Warburg in the early 20th century. He noted that rat tumors were susceptible to glucose deprivation (rather than oxygen deprivation) and exhibited higher than normal “fermentation” (glycolysis) to meet their ATP demands (Warburg et al., 1927). He later extended these observations to postulate mitochondrial dysfunction as the cause of neoplasia, since mitochondrial “poisons” are carcinogenic and cancer cells increased fermentation in response to irreversible low respiration rates (Warburg, 1956). Although at that time others (correctly) questioned whether mitochondrial dysfunction was a driver of neoplasia, in part due to radioactive isotope tracing indicating that mitochondria respiration was still active in cancer cells (Weinhouse, 1956), the phenomenon that cancer cells are highly glycolytic was widely accepted (Burk and Schade, 1956). Over time, however, the idea of metabolism as a driver of tumorigenesis largely fell to way side.
Cancer has now been reappreciated as a disease of metabolism (Hanahan and Weinberg, 2011; Pavlova and Thompson, 2016). Recent work has succeeded in reinvigorating the study of metabolism as a means to both detect and study cancer growth (Boroughs and DeBerardinis, 2015; Vander Heiden and DeBerardinis, 2017). For example, since the late 1990s, accumulation of 2-deoxy-2-[18F]fluoro-D-glucose (FDG) and subsequent imaging through positron emission tomography (PET) has been an FDA-approved method (FDG-PET) for the noninvasive detection of tumors (Farwell et al., 2014). Related approaches now aim to study consumption of other nutrients or specific metabolic rates using novel tracer compounds or hyperpolarized NMR (Chaumeil et al., 2014; Choi et al., 2012; Rodrigues et al., 2014; Venneti et al., 2015) In addition to these diagnostic approaches, significant effort is now being applied to elucidate how metabolic pathways contribute to cancer initiation and progression (Weinberg and Chandel, 2009).
Beyond the metabolic reprogramming required for proliferation, the discovery of mutations in genes encoding metabolic enzymes that directly impact tumorigenesis has been an important catalyst driving this resurgence in metabolic research (DeBerardinis et al., 2008; Gasparre et al., 2007). For example, the first widely characterized metabolic mutations were the loss of succinate dehydrogenase (SDH) and fumarate hydratase (FH), which are associated with development of paragangliomas and leiomyosarcomas, respectively (King et al., 2006). These loss-of-function mutations lead to increases in succinate or fumarate levels within tumors, which are thought to inhibit aKG-dependent dioxygenases that impact HIF1α stabilization and other biological processes (MacKenzie et al., 2007; Selak et al., 2005; Xiao et al., 2012). Metabolic modeling was used to understand how a FH-null cancer cell could operate without a functional TCA cycle, elucidating a critical dependency on heme biosynthesis (Frezza et al., 2011). More generally, these findings highlighted critical links between metabolism and tumor formation while offering potential new avenues for therapeutic intervention.
Another critical demonstration of metabolic alterations in cancer is the discovery of mutant isocitrate dehydrogenase (mtIDH) tumors. First identified via exome sequencing of gliomas (Balss et al., 2008; Yan et al., 2009), both IDH1 and IDH2 are now known to be mutated somewhat frequently in acute myeloid leukemia, low-grade gliomas, and chondrosarcomas (Dang et al., 2016). These mutations are characterized by a gain-of-function, where D-2-hydroxyglutarate (2HG) is produced at millimolar concentrations intracellularly (Parker and Metallo, 2015). Mutant IDH1 and IDH2 reduce aKG to 2HG by consuming an NADPH reducing equivalent, either in the cytosol or mitochondria (Dang et al., 2009). Similar to SDH and FH-null tumors, 2HG can disrupt aKG-dependent dioxygenase activity, in particular those regulating DNA and histone demethylation, and tumors often present with hypermethylation phenotype (Figueroa et al., 2010; Lu et al., 2012; Ma et al., 2015; Turcan et al., 2012; Xu et al., 2011). This mutation connects a fundamental node in the metabolic network with deep biological perturbations that are associated with tumor progression. Due to the highly compartment-specific and cofactor-dependent nature of this class of mutations, metabolic tracing is uniquely situated to understand the underlying metabolic features in these tumors (Izquierdo-Garcia et al., 2015). However, cells harboring such mutations exhibit only minor metabolic changes under normal physiological conditions, but under hypoxic or pharmacological redox stresses that impact mitochondrial function more tractable changes have emerged (Chan et al., 2015; Grassian et al., 2014; Tateishi et al., 2015).
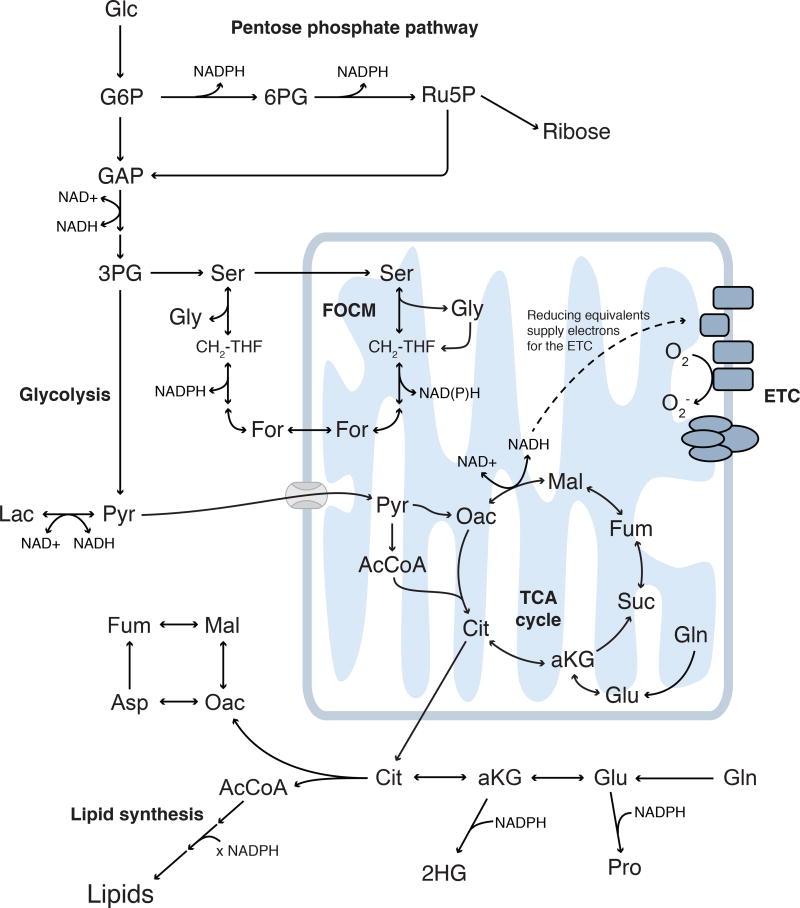
While these examples demonstrate how mutations in TCA cycle enzymes directly contribute to tumorigenesis, cancers in general hijack different metabolic pathways to fuel their proliferative needs (Figure 4). These pathways vary with environment, tissue of origin, and the genetic landscape of that cell. Therefore, a critical need exists to extend these MFA methods to understand how diverse cancers alter their metabolism to survive and what metabolic features can be therapeutically targeted.
Figure 4. Metabolic pathways dysregulated in the context of disease.
Glycolysis and the pentose phosphate pathway are fueled by glucose and generate biosynthetic intermediates, reducing equivalents, and ATP. Mitochondria are fueled by pyruvate, amino acids, and lipids, performing both anabolic and catabolic metabolism to generate energy. Serine, glycine, and folate-mediated one carbon metabolism are active in both cytosol and mitochondrial compartments. These pathways are connected orthogonally via cofactors and other disease- or tissue-specific pathways; as such, pathways beyond central carbon metabolism must be investigated in specific biological contexts.
3.2 Glutamine metabolism
Glutamine, the most abundant amino acid in plasma and culture media, is consumed by cancer cells in vitro at rates greater than any other amino acid. As such, glucose and glutamine are the most highly consumed carbon substrates in tumor cell cultures. Despite this fact, Hosios et al. recently applied 13C and 14C tracers to observe that glucose and glutamine only make up 25% of a cancer cell’s total dry weight and only around 50% of its carbon (Hosios et al., 2016). The remaining carbon was found to come generally from amino acid uptake (both essential and non-essential amino acids), highlighting the large protein component of mammalian cells and contrasting lower organisms that can derive their biomass carbon entirely from glucose (Hosios et al., 2016). These data showcase the utility of flux-based studies that trace the fate of carbon atoms within cells, as more traditional “black box” approach (i.e. only looking at metabolite secretions and uptakes) would have suggested a smaller role for amino acid carbon.
These results also demonstrate the importance of protein synthesis for cancer cell growth, which requires both carbon and nitrogen. Indeed, glutamine is first and foremost a nitrogen donor (and/or carrier) within mammals. It is a precursor to glutamate, proline, and other amino acids; in addition, it is also an obligate nitrogen donor for asparagine, nucleotides, and hexosamines. Several studies have highlighted the importance of glutamine availability in driving these processes (DeBerardinis et al., 2007; Wellen et al., 2010; Zhang et al., 2014). In fact, hexosamine biosynthetic fluxes in cultured cells are similar those measured for nucleotide (i.e., ribose) synthesis in proliferating stem cells (Badur et al., 2015). Glutathione is an antioxidant present at high concentrations within cells, and recent studies have highlighted the role of glutaminase and the xCT transporter in coordinating glutamine uptake, glutamate secretion, and cystine consumption from culture medium in cancer cells (Muir et al., 2017; Shin et al., 2017). Indeed, the high rates of glutaminolysis that occur in cultured tumor cells is at least partially attributable to the need for cystine uptake.
In the absence of glutamine, cancer cells can become on dependent on non-essential amino acid or protein uptake from stroma or the microenvironment, respectively. Tracer-based studies have described the importance of macropinocytosis and autophagy in allowing tumors to acquire proteinogenic amino acids under such nutrient-limiting conditions (Commisso et al., 2013; Davidson et al., 2017; Kamphorst et al., 2015). Alternatively, pancreatic tumor stroma use autophagy to provide alanine for cancer cell growth (Sousa et al., 2016). Yang et al. performed MFA modeling to delineate the role of cancer-associated fibroblasts (CAFs) in providing glutamine to ovarian cancer cells (Yang et al., 2016). Although it remains challenging to deconvolute labeling results and decipher cell-specific fluxes (Gebreselassie and Antoniewicz, 2015), analysis of systems containing multiple cell types will continue to grow in importance as we gain a better understanding of tumor heterogeneity and immune cell interactions.
Some tumor cells rewire their mitochondria such that alternate substrates are used to fuel TCA metabolism. For example, the mitochondrial pyruvate carrier (MPC) is often expressed at lower levels in colorectal cancer and over-expression of MPC mitigates cell growth under anchorage-independence or as xenografts (Schell et al., 2014). Notably, respiration is unchanged upon inhibition or knockdown of MPC (Divakaruni et al., 2013), suggesting mitochondria remain functional and active. MFA studies on cells with reduced MPC activity or expression have highlighted how cells compensate when pyruvate flux into mitochondria is compromised (Vacanti et al., 2014; Yang et al., 2014). Under these conditions, glutaminolysis is significantly increased to maintain anaplerotic flux and biosynthesis of amino acids (e.g. aspartate), nucleotides, and fatty acids. β-oxidation of fatty acids was increased nearly 10-fold in Mpc2 knockdown cells, and additional evidence indicated that BCAA catabolism was elevated upon MPC inhibition (Vacanti et al., 2014). These studies highlight how mitochondria adapt to MPC inhibition. While this rewiring may benefit tumor growth, therapeutic benefits in diseases such as metabolic syndrome and neurodegeneration may also emerge (Divakaruni et al., 2017; Ghosh et al., 2016; Gray et al., 2015; McCommis et al., 2015).
Oxidative stress also causes rewiring of glutamine metabolism within mitochondria. Indeed, in response to hypoxic insult pyruvate oxidation is decreased (Eales et al., 2016) and cells rely on glutamine to support proliferation (Weinberg et al., 2010). Glutaminolytic flux is increased to support oxidative TCA metabolism (Fan et al., 2013; Grassian et al., 2014; Le et al., 2012), since respiration remains active in low oxygen conditions. Thus, oxidation of aKG sustains respiration. However, NADP-dependent IDHs are reversible and have the capacity to reductively carboxylate aKG in mammals (Des Rosiers et al., 1995; Yoo et al., 2008), offering cells another pathway to generate AcCoA and reducing equivalents. Detailed tracer studies and MFA have more recently been applied to better understand how this pathway is controlled. Indeed, hypoxia reprograms TCA metabolism such that reductive carboxylation is the major route through which cells produce citrate and lipogenic AcCoA (Metallo et al., 2012; Wise et al., 2011). Similar changes occur in “pseudohypoxic” renal carcinoma cells (RCC) that are deficient in the Von Hippel-Lindau tumor suppressor (Metallo et al., 2012; Wise et al., 2011); tumors where this pathway may be therapeutically relevant. Indeed, evidence from in vivo tumor models and patient samples suggest this mode of TCA metabolism is active in VHL-deficient RCC downstream of HIFs (Gameiro et al., 2013b; Hakimi et al., 2016). This pathway also seems critical for aspartate production upstream of the pyrimidine synthesis pathway (Okazaki et al., 2017).
At the same time, mitochondrial redox stress caused by mutations in mitochondrial Complex I or III induce cells to activate the reductive carboxylation pathway, with similar changes occurring using pharmacological inhibitors of the electron transport chain (ETC) (Mullen et al., 2012). Roles for the mitochondrial nicotinamide nucleotide transhydrogenase (NNT) enzyme in driving this metabolic state have also been established (Gameiro et al., 2013a; Mullen et al., 2014). These findings have all suggesting that the cellular redox state and pyridine nucleotides influence reductive carboxylation activity. Indeed, modulation of NAD+/NADH ratio and citrate abundance are critical drivers of reductive carboxylation flux (Fendt et al., 2013). As such, activity in this pathway seems to be driven by redox stress caused by many different physiological conditions, including hypoxia, mitochondrial inhibitors, and lipid deficiency.
3.3 Redox metabolism
Reducing equivalents in the cell are transported between reactions using pyridine nucleotides, NAD+ and NADP+. These cofactors are essential for the various oxidoreductase reactions required for proper biosynthesis and redox control, with NADPH selectively required for cellular anabolism (i.e. fatty acid and proline synthesis) and antioxidant response (i.e. regeneration of GSH) (Lunt and Vander Heiden, 2011). A major contributor to cytosolic NADPH production is the oxPPP (Stincone et al., 2014), extensively studied with a variety of 13C glucose tracers (Lee et al., 1998; Metallo et al., 2009). However, these tracers cannot establish cofactor specificity and do not directly measure reducing equivalent pool.
Instead, because the transfer of electrons occurs through the transfer of a hydride anion, use of 3H (tritium) (Katz et al., 1965; Rendina et al., 1984) and 2H (deuteurium) (Ben-Yoseph et al., 1994; Ruhl et al., 2012) glucose tracers provides deep insight into cellular electron pools. Through the use of [1−2H] and [3−2H]glucose tracers, labeling of cytosolic NADPH was achieved through oxPPP enzymes, G6PD and PGD respectively (Fan et al., 2014; Lewis et al., 2014). Total cellular NADPH production flux was estimated to be ~10 nmol µL−1 hr−1 (5–20% of glucose uptake rate) by estimation of oxPPP contribution to NADPH and measurement oxPPP flux (Fan et al., 2014). Concomitant analysis of NADPH consumption (i.e. fatty acid, DNA, and proline synthesis) revealed that biosynthetic demands of NADPH was only 80% of production with the rest presumably used in redox defense (Fan et al., 2014). Hydride transfer from NADPH to lipids can also be used as an indirect measure of cytosolic NADPH labeling, such that ISA-based modeling allows estimation of tracer contributions to this metabolic pool (Lewis et al., 2014). The importance of the oxidative pentose phosphate pathway in pluripotent stem cells (greater than many cancer cells) (Zhang et al., 2016) and malic enzyme in adipocytes (Liu et al., 2016) have been elucidated using this approach.
Reducing equivalents cannot be directly transported across intracellular membranes (e.g. mitochondria) and these reactions are highly compartmentalized (Stincone et al., 2014). Instead, the cell relies on futile metabolic cycles to transport reducing equivalents into organelles (e.g. malate-aspartate shuttle) and maintain proper, compartmental redox homeostasis (LaNoue and Schoolwerth, 1979). Use of [4−2H]glucose was able to label both cytosolic and mitochondrial NADH pools, through GAPDH and malate-aspartate shuttle respectively (Lewis et al., 2014). To better elucidate compartment-specific redox metabolism, an endogenous redox reporter system was developed through low-level, ecotopic expression of mtIDH in cytosol or mitochondria (Lewis et al., 2014). Examination of labeling on 2HG found that the oxPPP contributed significantly to cytosolic NADPH but the mitochondrial NADPH pool was mostly labeled by hydride anions from NADH (Lewis et al., 2014). Taken together, these results highlight the powerful application of positional deuterium labels as donors for compartment-specific electron pools.
Somatic cells have evolved their metabolism to reside within distinct niches. Normal cells reside in close contact with the extracellular matrix (ECM). For a cancerous cell to metastasize to a distant site, the cell must depart its ECM-rich niche and survive in atypical microenvironments. Cancer cells undergoing metastasis must therefore reprogram metabolic pathways to overcome such stresses. Previous studies have shown that ECM-detachment induces increased levels of cellular reactive oxygen species (ROS) and can lead to anoikis in non-transformed cells (Schafer et al., 2009). Activation of the PI(3)K pathway in this context led to higher glucose consumption and increased cell survival after ECM detachment, due to increased oxPPP flux and which maintains β-oxidation and ATP levels (Grassian et al., 2011; Schafer et al., 2009). More recently, Piskounova et al. observed that metastatic cells increased expression of enzymes in one carbon metabolism (discussed below) and more specifically the mitochondrial NADPH producing enzyme, ALDH1L2 (Piskounova et al., 2015). These enzymes increase survival of ECM-detached cancer cells and enhance metastatic potential of tumor cells in vivo (Piskounova et al., 2015).
Stable isotope tracing has recently been used to elucidate the specific directionality of how some cellular metabolic pathways are perturbed to enable NADPH production under anchorage independent stress. Using both 13C glucose and glutamine tracers, cells grown in anchorage-independent conditions were found to oxidize less glucose and exhibited increased reductive carboxylation activity (Jiang et al., 2016b). However, unlike previous studies of reductive carboxylation, these effects were not due to any HIF-mediated changes to the cell, did not change the contribution of glutamine carbon to fatty acid synthesis, and could be reversed by simply re-attaching the cells to ECM (Jiang et al., 2016b). Instead, reductive carboxylation flux coordinated metabolic shuttling of cytosolic NADPH into the mitochondrial matrix to enhance cell survival (Jiang et al., 2016b). Furthermore, CRISPR knockouts of both IDH1 and IDH2 and [3−2H]glucose tracing confirmed that reductive carboxylation flux occurred in the cytosol but used to generate mitochondrial NADPH (Jiang et al., 2016b). This leaves a model where cells protect against increased mitochondrial oxidative stress after detachment by using the futile cycle of IDH1 and IDH2 to transport NADPH into the mitochondria and regenerate mitochondrial GSH.
3.4 Serine biosynthesis and one carbon metabolism
Serine is a critically important metabolite for proliferating cells given its role in biosynthetic and redox-associated pathways (Parker and Metallo, 2016). Indeed, phosphoglycerate dehydrogenase (PHGDH) catalyzes one of the initial steps of serine synthesis and is amplified in some breast cancers and melanomas (Locasale et al., 2011; Possemato et al., 2011). Glycine lies immediately downstream of serine and is important for cell growth due to its use in purine metabolism and glutathione synthesis (Jain et al., 2012). Serine also contributes to folate-mediated one carbon metabolism (FOCM), which lies at a critical biosynthetic node supporting nucleotide synthesis as well as methylation (Fox and Stover, 2008; Tibbetts and Appling, 2010). Intriguingly, several enzymes within these pathways are expressed at higher levels in aggressive tumors, including the mitochondrial enzyme methylene tetrahydrofolate dehydrogenase 2 (MTHFD2) (Nilsson et al., 2014). And this pathway is classically targeted in cancer and autoimmune diseases using the chemotherapeutics methotrexate or Pemetrexed (Locasale, 2013). However, even with the wealth of evidence demonstrating its importance, the specific mechanisms through which this pathway supports tumor growth and survival is still not definitively clear.
Analysis of tumor cell responses to serine and glycine deprivation has identified specific susceptibilities in cells as a function of their genotype. In particular, loss of p53 sensitized colon cancer cells to serine/glycine starvation by arresting cells in the G1 phase of the cell cycle (Maddocks et al., 2013). Additionally, p53 deficiency induced shunting of serine to glycine for glutathione synthesis to support antioxidant functions (Maddocks et al., 2013). Various other stress (often associated with redox) can modulate sensitivity to serine and/or glycine deprivation as well as the serine synthesis pathway, including metformin and hypoxia (Gravel et al., 2014; Ye et al., 2014). More recently, serine and glycine deprivation was shown to reduce tumor growth in several genetically-engineered mouse models of cancer (Maddocks et al., 2017). These results highlight the importance of serine availability for tumor growth, though the metabolic driver of this sensitization downstream of serine is not fully clear. To this end, Jain et al. applied extracellular flux analysis of metabolites consumed and secreted by the NCI-60 panel of cell lines and observed that glycine uptake correlated most tightly with cell growth rate (Jain et al., 2012). Tracing with [13C]glycine was then used to suggest that the glycine is directly used to support de novo purine synthesis rather supplying 1C units (Jain et al., 2012). However, glycine alone does not rescue cell growth in serine-deprived conditions (Labuschagne et al., 2014; Maddocks et al., 2013). Extensive tracing of serine and glycine conversion to nucleotides in HCT116 cells has indicated that glycine cannot replace serine due to the required consumption of 1C units and its impact on purine nucleotides (Labuschagne et al., 2014), suggesting that cells selectively uptake serine to generate both glycine and 1C units.
Notably, removal of dietary serine and glycine was not effective in Kras mutant tumors, presumably due to the upregulation of serine biosynthesis in tumors of this genotype (Maddocks et al., 2017). Other oncogenic pathways have also been associated with this metabolic pathway. For example, NRF2 is the master transcriptional regulator of the cellular antioxidant response and regulates expression of serine biosynthesis enzymes in non-small cell lung cancer (DeNicola et al., 2015). Through a mechanism driven by the transcription factor ATF4, NRF2 expression was found to contribute to tumorigenesis by activating serine biosynthesis and supporting FOCM and transsulfuration reactions (glutathione) (DeNicola et al., 2015). Similar mechanisms mediated through mTORC1 have also been implicated to upregulate de novo purine biosynthesis (Ben-Sahra et al., 2016). Consistent with the amplification of PHGDH in breast cancers and activation by ATF4, these pathways are important for breast cancer cell in anchorage-independent conditions and as xenografts. Taken together, these results highlight an important role for serine metabolism in tumor growth, in particular downstream of cellular stresses.
Beyond nucleotide biosynthesis, serine has an established role in supplying mitochondrial glycine/1C units through FOCM, with the former contributing to heme biosynthesis. Importantly, FOCM can supply mitochondrial reducing equivalents through 1C oxidation enzymes (e.g. MTHFD2, MTHFD2L, ALDH1L1) (Fox and Stover, 2008; Tibbetts and Appling, 2010) or glycine cleavage (Zhang et al., 2012), and flux balance analysis (FBA) modeling has suggested this pathway coordinates ATP regeneration along with glycolysis (Vazquez et al., 2011). Experimental evidence has also recently supported a role for this pathway in generating reducing equivalents in proliferating cells. Indeed, only knockdown of oxPPP and FOCM enzymes perturbed cellular redox state (Fan et al., 2014). Additionally, glycine oxidation measured with 14C tracers was found to be greater than purine synthesis rates, further suggesting a role in redox homeostasis (Fan et al., 2014). Through the use of mutant IDH2 reporters and 2H serine tracers (section 3.3), FOCM was demonstrated to contribute significantly to mitochondrial reducing equivalent pools (Lewis et al., 2014). Importantly, minimal label from serine was observed in cytosolic reporters or on palmitate, suggesting mitochondrial oxidation of 1C units MTHFD2 or MTHFD2L was the predominant route of NAD(P)H regeneration in this pathway (Lewis et al., 2014). In fact, as previously suggested by Herbig et al. (Herbig et al., 2002), most cells were found to supply cytosolic 1C units through mitochondrial FOCM flux, even to the point of secreting excess formate (Ducker et al., 2016; Meiser et al., 2016). Loss of mitochondrial FOCM enzymes made cells dependent on extracellular serine/glycine and retarded growth of xenografts, but compensatory reversal of FOCM flux was observed both in vitro and in vivo (Ducker et al., 2016). Several studies have also connected these pathways to cancer through hypoxia and “stemness” (Nilsson et al., 2014; Samanta et al., 2016; Ye et al., 2014), highlighting the need to study flux through this pathway in various microenvironments and biological contexts.
4. Emerging links between metabolism and epigenetics
Finally, recent studies have established critical links between metabolic pathways and cellular epigenetics. Canonical epigenetic “marks” (e.g. methylation, acetylation) on DNA, RNA, and proteins are all metabolic intermediates and demonstrate a powerful relationship between metabolic pathway flux and epigenetic regulation (Metallo and Vander Heiden, 2010). In this manner, altered metabolic pathway activity can influence gene expression in the context of disease (reviewed extensively for cancer in (Kinnaird et al., 2016)). Many metabolites (e.g. AcCoA, NAD+, aKG) also moonlight as substrates for the enzymatic addition and removal of epigenetic “marks” and other post-translational modifications (Su et al., 2016). In turn, numerous studies have elucidated how availability and/or localization of these metabolites can control histone acetylation (Wellen et al., 2009; Zhao et al., 2016), enzyme acetylation (Starai et al., 2002; Wang et al., 2010), and histone/nucleotide methylation (see section 3.1. discussion on aKG-dependent dioxygenases). For example, modulation of acetyl-CoA synthetase expression within the hippocampus decreased availability of AcCoA for histone acetylation and impaired long-term spatial memory (Mews et al., 2017). While these studies have effectively demonstrated the causal link between metabolism and epigenetics, how dysregulation of distal metabolic pathways can modulate epigenetics remains poorly understood in many contexts. The widely studied epigenetic signature, methylation, connects amino acid metabolism (methionine and serine) to nucleotides through transfer of methyl groups (Gut and Verdin, 2013) and provides one such example of distal metabolic reprogramming of epigenetics.
While methionine is considered the primary methylation donor through S-adenosyl methionine (SAM) pools (Cantoni, 1953), generation of 1C units from serine and remethylation of homocysteine provides an alternate source that links glycolytic flux to methylation (Lane and Fan, 2015). Indeed in vivo tracer analysis of whole-body SAM pools confirmed that methionine was the primary donor of methyl groups (~70% of methyl group flux), though 1C units needed for re-methylation came solely from serine (estimated to be ~3% of total serine flux) (Davis et al., 2004). Loss of the nutrient sensor LKB1 increased serine biosynthesis and cycling that led to enhanced DNA methylation and retrotransposon silencing in a mouse-model of pancreatic cancer (Kottakis et al., 2016). In addition, methionine deprivation reversibly altered histone methylation (e.g. H3K4me3) and expression of 1C–consuming enzymes to presumably reduce SAM consumption (Mentch et al., 2015). On the other hand, in vitro analysis of cellular DNA and RNA found that serine provided 1C units for methylation only under methionine starvation conditions (Maddocks et al., 2016). Serine, however, was found to support methylation through purine synthesis (i.e. ATP) for SAM production (Maddocks et al., 2016). These findings highlight yet another set of pathways and biological functions through which serine influences cancer biology.
5. Observations from in vivo studies
Many of the studies discussed above applied MFA and related approaches to cancer cells cultured in vitro. Clearly, in vivo models better recapitulate the physiologic conditions of human tumors; as such, increasing efforts have focused on in vivo tracing methods to characterize metabolism in these settings. However, the design, execution, and interpretation of such studies is complicated by a number of different factors: 1) administration of tracer may impact metabolism and downstream signaling, which is particularly important for glucose and insulin signaling, 2) labeling is quickly scrambled due to cross-tissue metabolic activity, and 3) multiple cell types exist within each tissue (e.g. epithelia, stroma). As such, comprehensive models that incorporate isotope labeling data from in vivo studies may have limited impact. On the other hand, analyses that focus on specific pathways/reactions have yielded insights into the metabolism of tumors in vivo.
As noted above, glutamine’s role as a major substrate fueling TCA metabolism in cancer cells is well documented (DeBerardinis and Cheng, 2010). The concept of decreased glucose oxidation and increased glutamine anaplerosis has largely been studied using in vitro models (DeBerardinis et al., 2007; Gameiro et al., 2013b; Metallo et al., 2012). More recently, in vivo studies have challenged the concept that glutamine rather than glucose is the predominant supporting the TCA cycle in tumors. While some early studies highlighted a potential role for glutamine synthase (GS) in supporting breast cancer growth (Kung et al., 2011), more definitive evidence of GS supporting tumor growth has come from in vivo tracing studies. Using 15N tracing, Tardito et al. observed that glioblastoma cell lines sustained growth in physiological and glutamine-free conditions through the amination of glutamate to provide glutamine for purine synthesis (Tardito et al., 2015). Subsequently, infusion of mice (or human patients) with either 13C glucose or 13C glutamine followed by enrichment analysis demonstrated that both GBM xenografts and contralateral primary brain tissues synthesized glutamine de novo from glucose, with little evidence of high glutaminolysis activity (Tardito et al., 2015).
If glutamine is not required for anaplerosis, what pathways take its place in vivo? To this end, early studies on the mitochondrial metabolism of non-small-cell lung cancer (NSCLC) tumors noted increased PC activity in human tumor biopsies that were infused with 13C glucose prior to surgery and analyzed by NMR (Sellers et al., 2015). These results and additional studies with tumor slices incubated with 13C glucose and glutamine tracers demonstrated that pyruvate carboxylase (PC) was an important anaplerotic path used by tumor cells to support mitochondrial metabolism (Sellers et al., 2015). Subsequently, knockdown of PC in lung cancer cell lines severely limited biosynthesis and growth of these cells as xenografts (Sellers et al., 2015). Similar results were previously noted in cell-based tracer studies using glutamine-free conditions (Cheng et al., 2011). Indeed, PC may emerge as a more critical enzyme in tumors with defective TCA metabolism. For example, PC flux is critical for supporting aspartate synthesis in cultured SDH-null cells (Cardaci et al., 2015; Lussey-Lepoutre et al., 2015). However how these rare tumors metabolize glucose and glutamine in vivo is not well characterized.
Detailed tracer studies in genetically engineered mouse models (GEMMs) of lung cancer as well as human patients have further supported the importance of glucose oxidation and anaplerosis in tumors (Davidson et al., 2016; Hensley et al., 2016; Sellers et al., 2015). Infusion of glucose tracers and enrichment analysis in three independent NSCLC GEMMs demonstrated that all tumor types exhibited increased glucose oxidation through PDH and PC as compared to adjacent normal lung tissue (Davidson et al., 2016). However, when cells derived from the GEMMs are placed in vitro, there is an increased reliance on glutamine, and Gls1 knockout cell lines could not be expanded in culture (Davidson et al., 2016). Conversely, knockout cell lines of both Pdh1a and Pcx in GEMM cells were successfully isolated in vitro but could not form xenografts (Davidson et al., 2016). The importance of mitochondrial glucose metabolism has also been demonstrated in human lung tumors (Hensley et al., 2016). After first examining tumor characteristics such as grade, stage, stromal fraction, mutation status, and perfusion, Hensley et al. infused 13C glucose before quantifying isotope enrichment within tumor biopsies and normal lung tissue (Hensley et al., 2016). Interestingly, lactate labeling suggested catabolism of lactate itself contributed significantly more to tumors, and this was confirmed using a 13C lactate tracer in syngenic mouse xenograft models (Hensley et al., 2016). Finally, by correlating enrichment results with tumor perfusion data, a model where highly perfused tumors consumed more alternative fuels from the circulation (e.g. lactate) and less perfused tumors more exclusively used glucose as a primary fuel source was constructed (Hensley et al., 2016). These findings challenge Warburg’s notion of defective mitochondria and the concept that tumors preferentially use glycolysis which pervades the literature.
Beyond mitochondrial metabolism, the routes through which tumors acquire lipids are also of great interest to the research community. Fatty acids can be taken up through the circulation or synthesized de novo. In fact, some cancer cell populations upregulate expression of CD36, a fatty acid scavenger that is also important for the survival of metastatic cells (Hale et al., 2014; Nath et al., 2015; Pascual et al., 2017). While some cell types preferentially consume lipids from their environment (Yao et al., 2016; Zhang et al., 2016), many tumors upregulate fatty acid biosynthetic machinery (Brusselmans et al., 2005; Hatzivassiliou et al., 2005; Milgraum et al., 1997). In human cancers, aggressiveness is correlated with upregulation of fatty acid synthesis machinery (FASN) but different cell types show varying sensitivity to FASN inhibition (Benjamin et al., 2015). While sensitivity to FASN inhibition could not be explained by the relative rate of palmitate synthesis, application of 13C glucose and lipidomics to quantify synthesis of intact lipids indicated that FASN inhibitor sensitivity correlated with the synthesis of signaling lipids (Benjamin et al., 2015). However, lipids are significantly more abundant in the body compared to cell culture media, so questions remained about the importance of de novo lipogenesis for tumor progression. To this end, fatty acid synthesis is readily quantified in vivo using 2H2O, as deuterons are incorporated into fatty acids through numerous pathways (Previs et al., 2014). Administration of 2H2O to tumor-bearing mice indicated that tumor lipids contained large fractions of newly synthesized fatty acids (Svensson et al., 2016). Similar results were obtained in both xenografts and GEMMs, which are better vascularized and likely to have adequate circulating lipids available (Svensson et al., 2016). Furthermore, treatment of these animal models with an AcCoA carboxylase inhibitor impeded growth and synergized with co-treatment carboplatin (Svensson et al., 2016). Taken together, while cell culture-based experiments will continue to be important for defining metabolic processes at the cellular and sub-cellular levels, these studies highlight the importance and utility of analyzing tumors in their physiologic microenvironment.
6. Conclusion
Metabolomics, stable isotope tracing, and metabolic flux analysis are powerful platform technologies that facilitate the study of human disease. Through careful design and execution of MFA experiments, researchers now have the ability to interrogate metabolic fluxes in a variety of biological contexts. Simplified systems provide molecular-level resolution but lack physiological relevance; in vivo models and patient studies have more clinical significance but provide less mechanistic insight (Figure 1). A wealth of new knowledge into the metabolic basis of tumorigenesis and cancer cell proliferation has now emerged over the past decade. Since each tissue and disease state involves distinct metabolic pathways, application of MFA to various biological systems offers a path that will be rich in new discoveries. For example, with a well-described role of metabolism (Vacanti and Metallo, 2013), MFA is increasingly being applied to study unique features of hPSCs and their regenerative medicine applications (Gu et al., 2016; Zhang et al., 2016). Established metabolic pathways are now being observed to have distinct functions in certain tissues or cell types (Green et al., 2016; Mayers et al., 2016), and new pathways are being discovered that modulate immune cell function (Cordes et al., 2016; Lampropoulou et al., 2016). In all these situations, elucidation of metabolic fluxes will be essential to fully appreciate the mechanisms through which metabolism contributes to human disease.
Acknowledgments
We thank Mari Gartner and members of the Metallo Lab for their helpful feedback and apologize to those researchers whose work we were unable to cite. This work was supported by the California Institute of Regenerative Medicine (RB5-07356), an NSF CAREER Award (1454425), NIH grant (R01-CA188652), and a Camile and Henry Dreyfus Teacher-Scholar Award (all to C.M.M.). M.G.B. is supported by a NSF Graduate Research Fellowship (DGE-1144086).
Abbreviations
- BCAA
branched-chain amino acid
- ETC
electron transport chain
- FOCM
folate-mediated one carbon metabolism
- HIFs
hypoxia-induced factors
- ISA
isotopomer spectral analysis
- ODE
ordinary differential equation
- oxPPP
oxidative pentose phosphate pathway
- 2HG
2-hydroxyglutarate
- 3PG
3-phosphoglycerate
- 6PG
6-phosphogluconate
- AcCoA
acetyl coenzyme A
- aKG
alpha-ketoglutarate
- Asp
aspartate
- CH2-THF
5,10-methylenetetrahydrofolate
- Cit
citrate
- For
formate
- Fum
fumarate
- G6P
glucose 6-phosphate
- GAP
glyceraldehyde 3-phosphate
- Glc
glucose
- Glu
glutamate
- Gln
glutamine
- Gly
glycine
- Lac
lactate
- Mal
malate
- Oac
oxaloacetate
- Pro
praline
- Pyr
pyruvate
- Ru5P
ribulose 5-phosphate
- Ser
serine
- Suc
succinate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoniewicz MR, Kelleher JK, Stephanopoulos G. Determination of confidence intervals of metabolic fluxes estimated from stable isotope measurements. Metab Eng. 2006;8:324–37. doi: 10.1016/j.ymben.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Antoniewicz MR, Kelleher JK, Stephanopoulos G. Elementary metabolite units (EMU): a novel framework for modeling isotopic distributions. Metab Eng. 2007;9:68–86. doi: 10.1016/j.ymben.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badur MG, Zhang H, Metallo CM. Enzymatic passaging of human embryonic stem cells alters central carbon metabolism and glycan abundance. Biotechnol J. 2015;10:1600–11. doi: 10.1002/biot.201400749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balss J, Meyer J, Mueller W, Korshunov A, Hartmann C, von Deimling A. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116:597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- Beard DA, Liang SD, Qian H. Energy balance for analysis of complex metabolic networks. Biophys J. 2002;83:79–86. doi: 10.1016/S0006-3495(02)75150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard DA, Qian H. Thermodynamic-based computational profiling of cellular regulatory control in hepatocyte metabolism. Am J Physiol Endocrinol Metab. 2005;288:E633–44. doi: 10.1152/ajpendo.00239.2004. [DOI] [PubMed] [Google Scholar]
- Ben-Sahra I, Hoxhaj G, Ricoult SJ, Asara JM, Manning BD. mTORC1 induces purine synthesis through control of the mitochondrial tetrahydrofolate cycle. Science. 2016;351:728–33. doi: 10.1126/science.aad0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yoseph O, Kingsley PB, Ross BD. Metabolic loss of deuterium from isotopically labeled glucose. Magn Reson Med. 1994;32:405–9. doi: 10.1002/mrm.1910320317. [DOI] [PubMed] [Google Scholar]
- Benjamin DI, Li DS, Lowe W, Heuer T, Kemble G, Nomura DK. Diacylglycerol Metabolism and Signaling Is a Driving Force Underlying FASN Inhibitor Sensitivity in Cancer Cells. ACS Chem Biol. 2015;10:1616–23. doi: 10.1021/acschembio.5b00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Kimball EH, Gao M, Osterhout R, Van Dien SJ, Rabinowitz JD. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat Chem Biol. 2009;5:593–9. doi: 10.1038/nchembio.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351–9. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusselmans K, De Schrijver E, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the acetyl-CoA-carboxylase-alpha gene induces growth inhibition and apoptosis of prostate cancer cells. Cancer Res. 2005;65:6719–25. doi: 10.1158/0008-5472.CAN-05-0571. [DOI] [PubMed] [Google Scholar]
- Buescher JM, Antoniewicz MR, Boros LG, Burgess SC, Brunengraber H, Clish CB, DeBerardinis RJ, Feron O, Frezza C, Ghesquiere B, Gottlieb E, Hiller K, Jones RG, Kamphorst JJ, Kibbey RG, Kimmelman AC, Locasale JW, Lunt SY, Maddocks OD, Malloy C, Metallo CM, Meuillet EJ, Munger J, Noh K, Rabinowitz JD, Ralser M, Sauer U, Stephanopoulos G, St-Pierre J, Tennant DA, Wittmann C, Vander Heiden MG, Vazquez A, Vousden K, Young JD, Zamboni N, Fendt SM. A roadmap for interpreting (13)C metabolite labeling patterns from cells. Curr Opin Biotechnol. 2015;34:189–201. doi: 10.1016/j.copbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk D, Schade AL. On respiratory impairment in cancer cells. Science. 1956;124:270–2. [PubMed] [Google Scholar]
- Cancer Genome Atlas Research, N. Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C, Stuart JM. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45:1113–20. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantoni GL. S-Adenosylmethionine - a New Intermediate Formed Enzymatically from L-Methionine and Adenosinetriphosphate. Journal of Biological Chemistry. 1953;204:403–416. [PubMed] [Google Scholar]
- Cardaci S, Zheng L, MacKay G, van den Broek NJ, MacKenzie ED, Nixon C, Stevenson D, Tumanov S, Bulusu V, Kamphorst JJ, Vazquez A, Fleming S, Schiavi F, Kalna G, Blyth K, Strathdee D, Gottlieb E. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat Cell Biol. 2015;17:1317–26. doi: 10.1038/ncb3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong WJ, Zhao F, Medeiros BC, Tyvoll DA, Majeti R. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med. 2015;21:178–84. doi: 10.1038/nm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumeil MM, Larson PE, Woods SM, Cai L, Eriksson P, Robinson AE, Lupo JM, Vigneron DB, Nelson SJ, Pieper RO, Phillips JJ, Ronen SM. Hyperpolarized [1–13C] glutamate: a metabolic imaging biomarker of IDH1 mutational status in glioma. Cancer Res. 2014;74:4247–57. doi: 10.1158/0008-5472.CAN-14-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Sudderth J, Yang C, Mullen AR, Jin ES, Mates JM, DeBerardinis RJ. Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc Natl Acad Sci U S A. 2011;108:8674–9. doi: 10.1073/pnas.1016627108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, Yang XL, Mashimo T, Raisanen JM, Marin-Valencia I, Pascual JM, Madden CJ, Mickey BE, Malloy CR, Bachoo RM, Maher EA. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18:624–9. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–7. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes T, Metallo CM. Tracing insights into human metabolism using chemical engineering approaches. Curr Opin Chem Eng. 2016;14:72–81. doi: 10.1016/j.coche.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes T, Wallace M, Michelucci A, Divakaruni AS, Sapcariu SC, Sousa C, Koseki H, Cabrales P, Murphy AN, Hiller K, Metallo CM. Immunoresponsive Gene 1 and Itaconate Inhibit Succinate Dehydrogenase to Modulate Intracellular Succinate Levels. J Biol Chem. 2016;291:14274–84. doi: 10.1074/jbc.M115.685792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crown SB, Ahn WS, Antoniewicz MR. Rational design of (1)(3)C-labeling experiments for metabolic flux analysis in mammalian cells. BMC Syst Biol. 2012;6:43. doi: 10.1186/1752-0509-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–44. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang L, Yen K, Attar EC. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol. 2016;27:599–608. doi: 10.1093/annonc/mdw013. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Jonas O, Keibler MA, Hou HW, Luengo A, Mayers JR, Wyckoff J, Del Rosario AM, Whitman M, Chin CR, Condon KJ, Lammers A, Kellersberger KA, Stall BK, Stephanopoulos G, Bar-Sagi D, Han J, Rabinowitz JD, Cima MJ, Langer R, Vander Heiden MG. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat Med. 2017;23:235–241. doi: 10.1038/nm.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson SM, Papagiannakopoulos T, Olenchock BA, Heyman JE, Keibler MA, Luengo A, Bauer MR, Jha AK, O’Brien JP, Pierce KA, Gui DY, Sullivan LB, Wasylenko TM, Subbaraj L, Chin CR, Stephanopolous G, Mott BT, Jacks T, Clish CB, Vander Heiden MG. Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer. Cell Metab. 2016;23:517–28. doi: 10.1016/j.cmet.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SR, Stacpoole PW, Williamson J, Kick LS, Quinlivan EP, Coats BS, Shane B, Bailey LB, Gregory JF., 3rd Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am J Physiol Endocrinol Metab. 2004;286:E272–9. doi: 10.1152/ajpendo.00351.2003. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Cheng T. Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–24. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci U S A. 2007;104:19345–50. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Chen PH, Mullarky E, Sudderth JA, Hu Z, Wu D, Tang H, Xie Y, Asara JM, Huffman KE, Wistuba II, Minna JD, DeBerardinis RJ, Cantley LC. NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat Genet. 2015;47:1475–81. doi: 10.1038/ng.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Rosiers C, Di Donato L, Comte B, Laplante A, Marcoux C, David F, Fernandez CA, Brunengraber H. Isotopomer analysis of citric acid cycle and gluconeogenesis in rat liver. Reversibility of isocitrate dehydrogenase and involvement of ATP-citrate lyase in gluconeogenesis. J Biol Chem. 1995;270:10027–36. doi: 10.1074/jbc.270.17.10027. [DOI] [PubMed] [Google Scholar]
- Divakaruni AS, Wallace M, Buren C, Martyniuk K, Andreyev AY, Li E, Fields JA, Cordes T, Reynolds IJ, Bloodgood BL, Raymond LA, Metallo CM, Murphy AN. Inhibition of the mitochondrial pyruvate carrier protects from excitotoxic neuronal death. J Cell Biol. 2017;216:1091–1105. doi: 10.1083/jcb.201612067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divakaruni AS, Wiley SE, Rogers GW, Andreyev AY, Petrosyan S, Loviscach M, Wall EA, Yadava N, Heuck AP, Ferrick DA, Henry RR, McDonald WG, Colca JR, Simon MI, Ciaraldi TP, Murphy AN. Thiazolidinediones are acute, specific inhibitors of the mitochondrial pyruvate carrier. Proc Natl Acad Sci U S A. 2013;110:5422–7. doi: 10.1073/pnas.1303360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducker GS, Chen L, Morscher RJ, Ghergurovich JM, Esposito M, Teng X, Kang Y, Rabinowitz JD. Reversal of Cytosolic One-Carbon Flux Compensates for Loss of the Mitochondrial Folate Pathway. Cell Metab. 2016;23:1140–53. doi: 10.1016/j.cmet.2016.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eales KL, Hollinshead KE, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis. 2016;5:e190. doi: 10.1038/oncsis.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T, Rabinowitz JD. Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol Syst Biol. 2013;9:712. doi: 10.1038/msb.2013.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Ye J, Kamphorst JJ, Shlomi T, Thompson CB, Rabinowitz JD. Quantitative flux analysis reveals folate-dependent NADPH production. Nature. 2014;510:298–302. doi: 10.1038/nature13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell MD, Pryma DA, Mankoff DA. PET/CT imaging in cancer: current applications and future directions. Cancer. 2014;120:3433–45. doi: 10.1002/cncr.28860. [DOI] [PubMed] [Google Scholar]
- Fendt SM, Bell EL, Keibler MA, Olenchock BA, Mayers JR, Wasylenko TM, Vokes NI, Guarente L, Vander Heiden MG, Stephanopoulos G. Reductive glutamine metabolism is a function of the alpha-ketoglutarate to citrate ratio in cells. Nat Commun. 2013;4:2236. doi: 10.1038/ncomms3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiehn O, Kopka J, Dormann P, Altmann T, Trethewey RN, Willmitzer L. Metabolite profiling for plant functional genomics. Nat Biotechnol. 2000;18:1157–61. doi: 10.1038/81137. [DOI] [PubMed] [Google Scholar]
- Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, Li Y, Bhagwat N, Vasanthakumar A, Fernandez HF, Tallman MS, Sun Z, Wolniak K, Peeters JK, Liu W, Choe SE, Fantin VR, Paietta E, Lowenberg B, Licht JD, Godley LA, Delwel R, Valk PJ, Thompson CB, Levine RL, Melnick A. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–67. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JT, Stover PJ. Folate-mediated one-carbon metabolism. Vitam Horm. 2008;79:1–44. doi: 10.1016/S0083-6729(08)00401-9. [DOI] [PubMed] [Google Scholar]
- Frezza C, Zheng L, Folger O, Rajagopalan KN, MacKenzie ED, Jerby L, Micaroni M, Chaneton B, Adam J, Hedley A, Kalna G, Tomlinson IP, Pollard PJ, Watson DG, Deberardinis RJ, Shlomi T, Ruppin E, Gottlieb E. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature. 2011;477:225–8. doi: 10.1038/nature10363. [DOI] [PubMed] [Google Scholar]
- Gameiro PA, Laviolette LA, Kelleher JK, Iliopoulos O, Stephanopoulos G. Cofactor balance by nicotinamide nucleotide transhydrogenase (NNT) coordinates reductive carboxylation and glucose catabolism in the tricarboxylic acid (TCA) cycle. J Biol Chem. 2013a;288:12967–77. doi: 10.1074/jbc.M112.396796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameiro PA, Yang J, Metelo AM, Perez-Carro R, Baker R, Wang Z, Arreola A, Rathmell WK, Olumi A, Lopez-Larrubia P, Stephanopoulos G, Iliopoulos O. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab. 2013b;17:372–85. doi: 10.1016/j.cmet.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparre G, Porcelli AM, Bonora E, Pennisi LF, Toller M, Iommarini L, Ghelli A, Moretti M, Betts CM, Martinelli GN, Ceroni AR, Curcio F, Carelli V, Rugolo M, Tallini G, Romeo G. Disruptive mitochondrial DNA mutations in complex I subunits are markers of oncocytic phenotype in thyroid tumors. Proc Natl Acad Sci U S A. 2007;104:9001–6. doi: 10.1073/pnas.0703056104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreselassie NA, Antoniewicz MR. (13)C-metabolic flux analysis of co-cultures: A novel approach. Metab Eng. 2015;31:132–9. doi: 10.1016/j.ymben.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Tyson T, George S, Hildebrandt EN, Steiner JA, Madaj Z, Schulz E, Machiela E, McDonald WG, Escobar Galvis ML, Kordower JH, Van Raamsdonk JM, Colca JR, Brundin P. Mitochondrial pyruvate carrier regulates autophagy, inflammation, and neurodegeneration in experimental models of Parkinson’s disease. Sci Transl Med. 2016;8:368ra174. doi: 10.1126/scitranslmed.aag2210. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S, Maranas CD. 13C metabolic flux analysis at a genome-scale. Metab Eng. 2015;32:12–22. doi: 10.1016/j.ymben.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Grassian AR, Metallo CM, Coloff JL, Stephanopoulos G, Brugge JS. Erk regulation of pyruvate dehydrogenase flux through PDK4 modulates cell proliferation. Genes Dev. 2011;25:1716–33. doi: 10.1101/gad.16771811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassian AR, Parker SJ, Davidson SM, Divakaruni AS, Green CR, Zhang X, Slocum KL, Pu M, Lin F, Vickers C, Joud-Caldwell C, Chung F, Yin H, Handly ED, Straub C, Growney JD, Vander Heiden MG, Murphy AN, Pagliarini R, Metallo CM. IDH1 mutations alter citric acid cycle metabolism and increase dependence on oxidative mitochondrial metabolism. Cancer Res. 2014;74:3317–31. doi: 10.1158/0008-5472.CAN-14-0772-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel SP, Hulea L, Toban N, Birman E, Blouin MJ, Zakikhani M, Zhao Y, Topisirovic I, St-Pierre J, Pollak M. Serine deprivation enhances antineoplastic activity of biguanides. Cancer Res. 2014;74:7521–33. doi: 10.1158/0008-5472.CAN-14-2643-T. [DOI] [PubMed] [Google Scholar]
- Gray LR, Sultana MR, Rauckhorst AJ, Oonthonpan L, Tompkins SC, Sharma A, Fu X, Miao R, Pewa AD, Brown KS, Lane EE, Dohlman A, Zepeda-Orozco D, Xie J, Rutter J, Norris AW, Cox JE, Burgess SC, Potthoff MJ, Taylor EB. Hepatic Mitochondrial Pyruvate Carrier 1 Is Required for Efficient Regulation of Gluconeogenesis and Whole-Body Glucose Homeostasis. Cell Metab. 2015;22:669–81. doi: 10.1016/j.cmet.2015.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CR, Wallace M, Divakaruni AS, Phillips SA, Murphy AN, Ciaraldi TP, Metallo CM. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol. 2016;12:15–21. doi: 10.1038/nchembio.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu W, Gaeta X, Sahakyan A, Chan AB, Hong CS, Kim R, Braas D, Plath K, Lowry WE, Christofk HR. Glycolytic Metabolism Plays a Functional Role in Regulating Human Pluripotent Stem Cell State. Cell Stem Cell. 2016;19:476–490. doi: 10.1016/j.stem.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut P, Verdin E. The nexus of chromatin regulation and intermediary metabolism. Nature. 2013;502:489–98. doi: 10.1038/nature12752. [DOI] [PubMed] [Google Scholar]
- Hackett SR, Zanotelli VR, Xu W, Goya J, Park JO, Perlman DH, Gibney PA, Botstein D, Storey JD, Rabinowitz JD. Systems-level analysis of mechanisms regulating yeast metabolic flux. Science. 2016:354. doi: 10.1126/science.aaf2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi AA, Reznik E, Lee CH, Creighton CJ, Brannon AR, Luna A, Aksoy BA, Liu EM, Shen R, Lee W, Chen Y, Stirdivant SM, Russo P, Chen YB, Tickoo SK, Reuter VE, Cheng EH, Sander C, Hsieh JJ. An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell. 2016;29:104–16. doi: 10.1016/j.ccell.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale JS, Otvos B, Sinyuk M, Alvarado AG, Hitomi M, Stoltz K, Wu Q, Flavahan W, Levison B, Johansen ML, Schmitt D, Neltner JM, Huang P, Ren B, Sloan AE, Silverstein RL, Gladson CL, DiDonato JA, Brown JM, McIntyre T, Hazen SL, Horbinski C, Rich JN, Lathia JD. Cancer stem cell-specific scavenger receptor CD36 drives glioblastoma progression. Stem Cells. 2014;32:1746–58. doi: 10.1002/stem.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Zhao F, Bauer DE, Andreadis C, Shaw AN, Dhanak D, Hingorani SR, Tuveson DA, Thompson CB. ATP citrate lyase inhibition can suppress tumor cell growth. Cancer Cell. 2005;8:311–21. doi: 10.1016/j.ccr.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Hellerstein MK. New stable isotope-mass spectrometric techniques for measuring fluxes through intact metabolic pathways in mammalian systems: introduction of moving pictures into functional genomics and biochemical phenotyping. Metab Eng. 2004;6:85–100. doi: 10.1016/j.ymben.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, Jiang L, Ko B, Skelton R, Loudat L, Wodzak M, Klimko C, McMillan E, Butt Y, Ni M, Oliver D, Torrealba J, Malloy CR, Kernstine K, Lenkinski RE, DeBerardinis RJ. Metabolic Heterogeneity in Human Lung Tumors. Cell. 2016;164:681–94. doi: 10.1016/j.cell.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbig K, Chiang EP, Lee LR, Hills J, Shane B, Stover PJ. Cytoplasmic serine hydroxymethyltransferase mediates competition between folate-dependent deoxyribonucleotide and S-adenosylmethionine biosyntheses. J Biol Chem. 2002;277:38381–9. doi: 10.1074/jbc.M205000200. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, McClelland GB. Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J Exp Biol. 1997;200:381–6. doi: 10.1242/jeb.200.2.381. [DOI] [PubMed] [Google Scholar]
- Hosios AM, Hecht VC, Danai LV, Johnson MO, Rathmell JC, Steinhauser ML, Manalis SR, Vander Heiden MG. Amino Acids Rather than Glucose Account for the Majority of Cell Mass in Proliferating Mammalian Cells. Dev Cell. 2016;36:540–9. doi: 10.1016/j.devcel.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo-Garcia JL, Viswanath P, Eriksson P, Cai L, Radoul M, Chaumeil MM, Blough M, Luchman HA, Weiss S, Cairncross JG, Phillips JJ, Pieper RO, Ronen SM. IDH1 Mutation Induces Reprogramming of Pyruvate Metabolism. Cancer Res. 2015;75:2999–3009. doi: 10.1158/0008-5472.CAN-15-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus WE. Respiratory control and the integration of heart high-energy phosphate metabolism by mitochondrial creatine kinase. Annu Rev Physiol. 1985;47:707–25. doi: 10.1146/annurev.ph.47.030185.003423. [DOI] [PubMed] [Google Scholar]
- Jain M, Nilsson R, Sharma S, Madhusudhan N, Kitami T, Souza AL, Kafri R, Kirschner MW, Clish CB, Mootha VK. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–4. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazmin LJ, Young JD. Isotopically nonstationary 13C metabolic flux analysis. Methods Mol Biol. 2013;985:367–90. doi: 10.1007/978-1-62703-299-5_18. [DOI] [PubMed] [Google Scholar]
- Jiang L, Boufersaoui A, Yang C, Ko B, Rakheja D, Guevara G, Hu Z, DeBerardinis RJ. Quantitative metabolic flux analysis reveals an unconventional pathway of fatty acid synthesis in cancer cells deficient for the mitochondrial citrate transport protein. Metab Eng. 2016a doi: 10.1016/j.ymben.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Shestov AA, Swain P, Yang C, Parker SJ, Wang QA, Terada LS, Adams ND, McCabe MT, Pietrak B, Schmidt S, Metallo CM, Dranka BP, Schwartz B, DeBerardinis RJ. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature. 2016b;532:255–8. doi: 10.1038/nature17393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016 doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphorst JJ, Nofal M, Commisso C, Hackett SR, Lu W, Grabocka E, Vander Heiden MG, Miller G, Drebin JA, Bar-Sagi D, Thompson CB, Rabinowitz JD. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 2015;75:544–53. doi: 10.1158/0008-5472.CAN-14-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J, Rognstad R, Kemp RG. Isotope Discrimination Effects in the Metabolism of Tritiated Glucose. J Biol Chem. 1965;240:PC1484–6. [PubMed] [Google Scholar]
- King A, Selak MA, Gottlieb E. Succinate dehydrogenase and fumarate hydratase: linking mitochondrial dysfunction and cancer. Oncogene. 2006;25:4675–82. doi: 10.1038/sj.onc.1209594. [DOI] [PubMed] [Google Scholar]
- Kinnaird A, Zhao S, Wellen KE, Michelakis ED. Metabolic control of epigenetics in cancer. Nat Rev Cancer. 2016;16:694–707. doi: 10.1038/nrc.2016.82. [DOI] [PubMed] [Google Scholar]
- Kottakis F, Nicolay BN, Roumane A, Karnik R, Gu H, Nagle JM, Boukhali M, Hayward MC, Li YY, Chen T, Liesa M, Hammerman PS, Wong KK, Hayes DN, Shirihai OS, Dyson NJ, Haas W, Meissner A, Bardeesy N. LKB1 loss links serine metabolism to DNA methylation and tumorigenesis. Nature. 2016;539:390–395. doi: 10.1038/nature20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung HN, Marks JR, Chi JT. Glutamine synthetase is a genetic determinant of cell type-specific glutamine independence in breast epithelia. PLoS Genet. 2011;7:e1002229. doi: 10.1371/journal.pgen.1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labuschagne CF, van den Broek NJ, Mackay GM, Vousden KH, Maddocks OD. Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Rep. 2014;7:1248–58. doi: 10.1016/j.celrep.2014.04.045. [DOI] [PubMed] [Google Scholar]
- Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, Karpe F, Humphreys S, Bedinger DH, Dunn TN, Thomas AP, Oort PJ, Kieffer DA, Amin R, Bettaieb A, Haj FG, Permana P, Anthony TG, Adams SH. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity. Am J Physiol Endocrinol Metab. 2013;304:E1175–87. doi: 10.1152/ajpendo.00630.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SC, Griss T, Weinheimer CJ, Khader S, Randolph GJ, Pearce EJ, Jones RG, Diwan A, Diamond MS, Artyomov MN. Itaconate Links Inhibition of Succinate Dehydrogenase with Macrophage Metabolic Remodeling and Regulation of Inflammation. Cell Metab. 2016;24:158–66. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane AN, Fan TW. Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res. 2015;43:2466–85. doi: 10.1093/nar/gkv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaNoue KF, Schoolwerth AC. Metabolite transport in mitochondria. Annu Rev Biochem. 1979;48:871–922. doi: 10.1146/annurev.bi.48.070179.004255. [DOI] [PubMed] [Google Scholar]
- Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, Zimmerman LJ, Liebler DC, Slebos RJ, Lorkiewicz PK, Higashi RM, Fan TW, Dang CV. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 2012;15:110–21. doi: 10.1016/j.cmet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WN, Boros LG, Puigjaner J, Bassilian S, Lim S, Cascante M. Mass isotopomer study of the nonoxidative pathways of the pentose cycle with [1,2–13C2]glucose. Am J Physiol. 1998;274:E843–51. doi: 10.1152/ajpendo.1998.274.5.E843. [DOI] [PubMed] [Google Scholar]
- Lemons JM, Feng XJ, Bennett BD, Legesse-Miller A, Johnson EL, Raitman I, Pollina EA, Rabitz HA, Rabinowitz JD, Coller HA. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol. 2010;8:e1000514. doi: 10.1371/journal.pbio.1000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CA, Parker SJ, Fiske BP, McCloskey D, Gui DY, Green CR, Vokes NI, Feist AM, Vander Heiden MG, Metallo CM. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol Cell. 2014;55:253–63. doi: 10.1016/j.molcel.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis NE, Schramm G, Bordbar A, Schellenberger J, Andersen MP, Cheng JK, Patel N, Yee A, Lewis RA, Eils R, Konig R, Palsson BO. Large-scale in silico modeling of metabolic interactions between cell types in the human brain. Nat Biotechnol. 2010;28:1279–85. doi: 10.1038/nbt.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Shah S, Fan J, Park JO, Wellen KE, Rabinowitz JD. Malic enzyme tracers reveal hypoxia-induced switch in adipocyte NADPH pathway usage. Nat Chem Biol. 2016;12:345–52. doi: 10.1038/nchembio.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat Rev Cancer. 2013;13:572–83. doi: 10.1038/nrc3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H, Sasaki AT, Anastasiou D, Mullarky E, Vokes NI, Sasaki M, Beroukhim R, Stephanopoulos G, Ligon AH, Meyerson M, Richardson AL, Chin L, Wagner G, Asara JM, Brugge JS, Cantley LC, Vander Heiden MG. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–74. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Ward PS, Kapoor GS, Rohle D, Turcan S, Abdel-Wahab O, Edwards CR, Khanin R, Figueroa ME, Melnick A, Wellen KE, O’Rourke DM, Berger SL, Chan TA, Levine RL, Mellinghoff IK, Thompson CB. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature. 2012;483:474–8. doi: 10.1038/nature10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt SY, Muralidhar V, Hosios AM, Israelsen WJ, Gui DY, Newhouse L, Ogrodzinski M, Hecht V, Xu K, Acevedo PN, Hollern DP, Bellinger G, Dayton TL, Christen S, Elia I, Dinh AT, Stephanopoulos G, Manalis SR, Yaffe MB, Andrechek ER, Fendt SM, Vander Heiden MG. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol Cell. 2015;57:95–107. doi: 10.1016/j.molcel.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–64. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- Lussey-Lepoutre C, Hollinshead KE, Ludwig C, Menara M, Morin A, Castro-Vega LJ, Parker SJ, Janin M, Martinelli C, Ottolenghi C, Metallo C, Gimenez-Roqueplo AP, Favier J, Tennant DA. Loss of succinate dehydrogenase activity results in dependency on pyruvate carboxylation for cellular anabolism. Nat Commun. 2015;6:8784. doi: 10.1038/ncomms9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Jiang B, Deng W, Gu ZK, Wu FZ, Li T, Xia Y, Yang H, Ye D, Xiong Y, Guan KL. D-2-hydroxyglutarate is essential for maintaining oncogenic property of mutant IDH-containing cancer cells but dispensable for cell growth. Oncotarget. 2015;6:8606–20. doi: 10.18632/oncotarget.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie ED, Selak MA, Tennant DA, Payne LJ, Crosby S, Frederiksen CM, Watson DG, Gottlieb E. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–9. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks OD, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E, Vousden KH. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542–6. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks OD, Labuschagne CF, Adams PD, Vousden KH. Serine Metabolism Supports the Methionine Cycle and DNA/RNA Methylation through De Novo ATP Synthesis in Cancer Cells. Mol Cell. 2016;61:210–21. doi: 10.1016/j.molcel.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddocks ODK, Athineos D, Cheung EC, Lee P, Zhang T, van den Broek NJF, Mackay GM, Labuschagne CF, Gay D, Kruiswijk F, Blagih J, Vincent DF, Campbell KJ, Ceteci F, Sansom OJ, Blyth K, Vousden KH. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature. 2017;544:372–376. doi: 10.1038/nature22056. [DOI] [PubMed] [Google Scholar]
- Maier K, Hofmann U, Bauer A, Niebel A, Vacun G, Reuss M, Mauch K. Quantification of statin effects on hepatic cholesterol synthesis by transient (13)C-flux analysis. Metab Eng. 2009;11:292–309. doi: 10.1016/j.ymben.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Mayers JR, Torrence ME, Danai LV, Papagiannakopoulos T, Davidson SM, Bauer MR, Lau AN, Ji BW, Dixit PD, Hosios AM, Muir A, Chin CR, Freinkman E, Jacks T, Wolpin BM, Vitkup D, Vander Heiden MG. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science. 2016;353:1161–5. doi: 10.1126/science.aaf5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey D, Young JD, Xu S, Palsson BO, Feist AM. Modeling Method for Increased Precision and Scope of Directly Measurable Fluxes at a Genome-Scale. Anal Chem. 2016;88:3844–52. doi: 10.1021/acs.analchem.5b04914. [DOI] [PubMed] [Google Scholar]
- McCommis KS, Chen Z, Fu X, McDonald WG, Colca JR, Kletzien RF, Burgess SC, Finck BN. Loss of Mitochondrial Pyruvate Carrier 2 in the Liver Leads to Defects in Gluconeogenesis and Compensation via Pyruvate-Alanine Cycling. Cell Metab. 2015;22:682–94. doi: 10.1016/j.cmet.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser J, Tumanov S, Maddocks O, Labuschagne CF, Athineos D, Van Den Broek N, Mackay GM, Gottlieb E, Blyth K, Vousden K, Kamphorst JJ, Vazquez A. Serine one-carbon catabolism with formate overflow. Sci Adv. 2016;2:e1601273. doi: 10.1126/sciadv.1601273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentch SJ, Mehrmohamadi M, Huang L, Liu X, Gupta D, Mattocks D, Gomez Padilla P, Ables G, Bamman MM, Thalacker-Mercer AE, Nichenametla SN, Locasale JW. Histone Methylation Dynamics and Gene Regulation Occur through the Sensing of One-Carbon Metabolism. Cell Metab. 2015;22:861–73. doi: 10.1016/j.cmet.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, Vander Heiden MG, Iliopoulos O, Stephanopoulos G. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2012;481:380–4. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Vander Heiden MG. Metabolism strikes back: metabolic flux regulates cell signaling. Genes Dev. 2010;24:2717–22. doi: 10.1101/gad.2010510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metallo CM, Walther JL, Stephanopoulos G. Evaluation of 13C isotopic tracers for metabolic flux analysis in mammalian cells. J Biotechnol. 2009;144:167–74. doi: 10.1016/j.jbiotec.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mews P, Donahue G, Drake AM, Luczak V, Abel T, Berger SL. Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature. 2017;546:381–386. doi: 10.1038/nature22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res. 1997;3:2115–20. [PubMed] [Google Scholar]
- Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Muir A, Danai LV, Gui DY, Waingarten CY, Lewis CA, Vander Heiden MG. Environmental cystine drives glutamine anaplerosis and sensitizes cancer cells to glutaminase inhibition. Elife. 2017:6. doi: 10.7554/eLife.27713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AR, Hu Z, Shi X, Jiang L, Boroughs LK, Kovacs Z, Boriack R, Rakheja D, Sullivan LB, Linehan WM, Chandel NS, DeBerardinis RJ. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep. 2014;7:1679–90. doi: 10.1016/j.celrep.2014.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2012;481:385–8. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger J, Bennett BD, Parikh A, Feng XJ, McArdle J, Rabitz HA, Shenk T, Rabinowitz JD. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat Biotechnol. 2008;26:1179–86. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagrath D, Avila-Elchiver M, Berthiaume F, Tilles AW, Messac A, Yarmush ML. Integrated energy and flux balance based multiobjective framework for large-scale metabolic networks. Ann Biomed Eng. 2007;35:863–85. doi: 10.1007/s10439-007-9283-0. [DOI] [PubMed] [Google Scholar]
- Nanchen A, Fuhrer T, Sauer U. Determination of metabolic flux ratios from 13C–experiments and gas chromatography-mass spectrometry data: protocol and principles. Methods Mol Biol. 2007;358:177–97. doi: 10.1007/978-1-59745-244-1_11. [DOI] [PubMed] [Google Scholar]
- Nath A, Li I, Roberts LR, Chan C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep. 2015;5:14752. doi: 10.1038/srep14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson R, Jain M, Madhusudhan N, Sheppard NG, Strittmatter L, Kampf C, Huang J, Asplund A, Mootha VK. Metabolic enzyme expression highlights a key role for MTHFD2 and the mitochondrial folate pathway in cancer. Nat Commun. 2014;5:3128. doi: 10.1038/ncomms4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh K, Gronke K, Luo B, Takors R, Oldiges M, Wiechert W. Metabolic flux analysis at ultra short time scale: isotopically non-stationary 13C labeling experiments. J Biotechnol. 2007;129:249–67. doi: 10.1016/j.jbiotec.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Noh K, Wiechert W. Experimental design principles for isotopically instationary 13C labeling experiments. Biotechnol Bioeng. 2006;94:234–51. doi: 10.1002/bit.20803. [DOI] [PubMed] [Google Scholar]
- Okazaki A, Gameiro PA, Christodoulou D, Laviollette L, Schneider M, Chaves F, Stemmer-Rachamimov A, Yazinski SA, Lee R, Stephanopoulos G, Zou L, Iliopoulos O. Glutaminase and poly(ADP-ribose) polymerase inhibitors suppress pyrimidine synthesis and VHL-deficient renal cancers. J Clin Invest. 2017;127:1631–1645. doi: 10.1172/JCI87800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JO, Rubin SA, Xu YF, Amador-Noguez D, Fan J, Shlomi T, Rabinowitz JD. Metabolite concentrations, fluxes and free energies imply efficient enzyme usage. Nat Chem Biol. 2016;12:482–9. doi: 10.1038/nchembio.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TJ, Reznick J, Peterson BL, Blass G, Omerbasic D, Bennett NC, Kuich P, Zasada C, Browe BM, Hamann W, Applegate DT, Radke MH, Kosten T, Lutermann H, Gavaghan V, Eigenbrod O, Begay V, Amoroso VG, Govind V, Minshall RD, Smith ESJ, Larson J, Gotthardt M, Kempa S, Lewin GR. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science. 2017;356:307–311. doi: 10.1126/science.aab3896. [DOI] [PubMed] [Google Scholar]
- Parker SJ, Metallo CM. Metabolic consequences of oncogenic IDH mutations. Pharmacol Ther. 2015;152:54–62. doi: 10.1016/j.pharmthera.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker SJ, Metallo CM. Chasing One-Carbon Units to Understand the Role of Serine in Epigenetics. Mol Cell. 2016;61:185–6. doi: 10.1016/j.molcel.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Pascual G, Avgustinova A, Mejetta S, Martin M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, Bescos C, Di Croce L, Benitah SA. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41–45. doi: 10.1038/nature20791. [DOI] [PubMed] [Google Scholar]
- Pavlova NN, Thompson CB. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, Leitch AM, Johnson TM, DeBerardinis RJ, Morrison SJ. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–91. doi: 10.1038/nature15726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, Chen WW, Barrett FG, Stransky N, Tsun ZY, Cowley GS, Barretina J, Kalaany NY, Hsu PP, Ottina K, Chan AM, Yuan B, Garraway LA, Root DE, Mino-Kenudson M, Brachtel EF, Driggers EM, Sabatini DM. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–50. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previs SF, McLaren DG, Wang SP, Stout SJ, Zhou H, Herath K, Shah V, Miller PL, Wilsie L, Castro-Perez J, Johns DG, Cleary MA, Roddy TP. New methodologies for studying lipid synthesis and turnover: looking backwards to enable moving forwards. Biochim Biophys Acta. 2014;1842:402–13. doi: 10.1016/j.bbadis.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Quek LE, Wittmann C, Nielsen LK, Kromer JO. OpenFLUX: efficient modelling software for 13C–based metabolic flux analysis. Microb Cell Fact. 2009;8:25. doi: 10.1186/1475-2859-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendina AR, Hermes JD, Cleland WW. Use of multiple isotope effects to study the mechanism of 6-phosphogluconate dehydrogenase. BioChemistry. 1984;23:6257–62. doi: 10.1021/bi00320a056. [DOI] [PubMed] [Google Scholar]
- Rodrigues TB, Serrao EM, Kennedy BW, Hu DE, Kettunen MI, Brindle KM. Magnetic resonance imaging of tumor glycolysis using hyperpolarized 13C–labeled glucose. Nat Med. 2014;20:93–7. doi: 10.1038/nm.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhl M, Le Coq D, Aymerich S, Sauer U. 13C–flux analysis reveals NADPH-balancing transhydrogenation cycles in stationary phase of nitrogen-starving Bacillus subtilis. J Biol Chem. 2012;287:27959–70. doi: 10.1074/jbc.M112.366492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta D, Park Y, Andrabi SA, Shelton LM, Gilkes DM, Semenza GL. PHGDH Expression Is Required for Mitochondrial Redox Homeostasis, Breast Cancer Stem Cell Maintenance, and Lung Metastasis. Cancer Res. 2016;76:4430–42. doi: 10.1158/0008-5472.CAN-16-0530. [DOI] [PubMed] [Google Scholar]
- Sauer U. High-throughput phenomics: experimental methods for mapping fluxomes. Curr Opin Biotechnol. 2004;15:58–63. doi: 10.1016/j.copbio.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Sauer U. Metabolic networks in motion: 13C–based flux analysis. Mol Syst Biol. 2006;2:62. doi: 10.1038/msb4100109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–13. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell JC, Olson KA, Jiang L, Hawkins AJ, Van Vranken JG, Xie J, Egnatchik RA, Earl EG, DeBerardinis RJ, Rutter J. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell. 2014;56:400–13. doi: 10.1016/j.molcel.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Sellers K, Fox MP, Bousamra M, 2nd, Slone SP, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande R, Lane AN, Fan TW. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest. 2015;125:687–98. doi: 10.1172/JCI72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CS, Mishra P, Watrous JD, Carelli V, D’Aurelio M, Jain M, Chan DC. The glutamate/cystine xCT antiporter antagonizes glutamine metabolism and reduces nutrient flexibility. Nat Commun. 2017;8:15074. doi: 10.1038/ncomms15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa CM, Biancur DE, Wang X, Halbrook CJ, Sherman MH, Zhang L, Kremer D, Hwang RF, Witkiewicz AK, Ying H, Asara JM, Evans RM, Cantley LC, Lyssiotis CA, Kimmelman AC. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature. 2016;536:479–83. doi: 10.1038/nature19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 2002;298:2390–2. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]
- Stephanopoulos G. Metabolic fluxes and metabolic engineering. Metab Eng. 1999;1:1–11. doi: 10.1006/mben.1998.0101. [DOI] [PubMed] [Google Scholar]
- Stephanopoulos G, Aristidou AA, Nielsen JH. Metabolic engineering: principles and methodologies. San Diego: Academic Press; 1998. [Google Scholar]
- Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, Olin-Sandoval V, Gruning N, Kruger A, Tauqeer Alam M, Keller MA, Breitenbach M, Brindle KM, Rabinowitz JD, Ralser M. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 2014 doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Wellen KE, Rabinowitz JD. Metabolic control of methylation and acetylation. Curr Opin Chem Biol. 2016;30:52–60. doi: 10.1016/j.cbpa.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson RU, Parker SJ, Eichner LJ, Kolar MJ, Wallace M, Brun SN, Lombardo PS, Van Nostrand JL, Hutchins A, Vera L, Gerken L, Greenwood J, Bhat S, Harriman G, Westlin WF, Harwood HJ, Jr, Saghatelian A, Kapeller R, Metallo CM, Shaw RJ. Inhibition of acetyl-CoA carboxylase suppresses fatty acid synthesis and tumor growth of non-small-cell lung cancer in preclinical models. Nat Med. 2016;22:1108–1119. doi: 10.1038/nm.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardito S, Oudin A, Ahmed SU, Fack F, Keunen O, Zheng L, Miletic H, Sakariassen PO, Weinstock A, Wagner A, Lindsay SL, Hock AK, Barnett SC, Ruppin E, Morkve SH, Lund-Johansen M, Chalmers AJ, Bjerkvig R, Niclou SP, Gottlieb E. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat Cell Biol. 2015;17:1556–68. doi: 10.1038/ncb3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi K, Wakimoto H, Iafrate AJ, Tanaka S, Loebel F, Lelic N, Wiederschain D, Bedel O, Deng G, Zhang B, He T, Shi X, Gerszten RE, Zhang Y, Yeh JR, Curry WT, Zhao D, Sundaram S, Nigim F, Koerner MV, Ho Q, Fisher DE, Roider EM, Kemeny LV, Samuels Y, Flaherty KT, Batchelor TT, Chi AS, Cahill DP. Extreme Vulnerability of IDH1 Mutant Cancers to NAD+ Depletion. Cancer Cell. 2015;28:773–84. doi: 10.1016/j.ccell.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbetts AS, Appling DR. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu Rev Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- Turcan S, Rohle D, Goenka A, Walsh LA, Fang F, Yilmaz E, Campos C, Fabius AW, Lu C, Ward PS, Thompson CB, Kaufman A, Guryanova O, Levine R, Heguy A, Viale A, Morris LG, Huse JT, Mellinghoff IK, Chan TA. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature. 2012;483:479–83. doi: 10.1038/nature10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacanti NM, Divakaruni AS, Green CR, Parker SJ, Henry RR, Ciaraldi TP, Murphy AN, Metallo CM. Regulation of substrate utilization by the mitochondrial pyruvate carrier. Mol Cell. 2014;56:425–35. doi: 10.1016/j.molcel.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacanti NM, Metallo CM. Exploring metabolic pathways that contribute to the stem cell phenotype. Biochim Biophys Acta. 2013;1830:2361–9. doi: 10.1016/j.bbagen.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, DeBerardinis RJ. Understanding the Intersections between Metabolism and Cancer Biology. Cell. 2017;168:657–669. doi: 10.1016/j.cell.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez A, Markert EK, Oltvai ZN. Serine biosynthesis with one carbon catabolism and the glycine cleavage system represents a novel pathway for ATP generation. PLoS One. 2011;6:e25881. doi: 10.1371/journal.pone.0025881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Dunphy MP, Zhang H, Pitter KL, Zanzonico P, Campos C, Carlin SD, La Rocca G, Lyashchenko S, Ploessl K, Rohle D, Omuro AM, Cross JR, Brennan CW, Weber WA, Holland EC, Mellinghoff IK, Kung HF, Lewis JS, Thompson CB. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci Transl Med. 2015;7:274ra17. doi: 10.1126/scitranslmed.aaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning ZB, Zeng R, Xiong Y, Guan KL, Zhao S, Zhao GP. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010;327:1004–7. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. J Gen Physiol. 1927;8:519–30. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg F, Chandel NS. Mitochondrial metabolism and cancer. Ann N Y Acad Sci. 2009;1177:66–73. doi: 10.1111/j.1749-6632.2009.05039.x. [DOI] [PubMed] [Google Scholar]
- Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010;107:8788–93. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhouse S. On respiratory impairment in cancer cells. Science. 1956;124:267–9. doi: 10.1126/science.124.3215.267. [DOI] [PubMed] [Google Scholar]
- Weitzel M, Noh K, Dalman T, Niedenfuhr S, Stute B, Wiechert W. 13CFLUX2--high-performance software suite for (13)C-metabolic flux analysis. Bioinformatics. 2013;29:143–5. doi: 10.1093/bioinformatics/bts646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–80. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW, Rabinowitz JD, Coller HA, Thompson CB. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 2010;24:2784–99. doi: 10.1101/gad.1985910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechert W. 13C metabolic flux analysis. Metab Eng. 2001;3:195–206. doi: 10.1006/mben.2001.0187. [DOI] [PubMed] [Google Scholar]
- Wiechert W. The thermodynamic meaning of metabolic exchange fluxes. Biophys J. 2007;93:2255–64. doi: 10.1529/biophysj.106.099895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechert W, Noh K. Isotopically non-stationary metabolic flux analysis: complex yet highly informative. Curr Opin Biotechnol. 2013;24:979–86. doi: 10.1016/j.copbio.2013.03.024. [DOI] [PubMed] [Google Scholar]
- Wise DR, Ward PS, Shay JE, Cross JR, Gruber JJ, Sachdeva UM, Platt JM, DeMatteo RG, Simon MC, Thompson CB. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc Natl Acad Sci U S A. 2011;108:19611–6. doi: 10.1073/pnas.1117773108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, Liu L, Liu Y, Yang C, Xu Y, Zhao S, Ye D, Xiong Y, Guan KL. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326–38. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, Liu LX, Jiang WQ, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM, Xiong Y. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–73. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Ko B, Hensley CT, Jiang L, Wasti AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche M, Rutter J, Merritt ME, DeBerardinis RJ. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell. 2014;56:414–24. doi: 10.1016/j.molcel.2014.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Achreja A, Yeung TL, Mangala LS, Jiang D, Han C, Baddour J, Marini JC, Ni J, Nakahara R, Wahlig S, Chiba L, Kim SH, Morse J, Pradeep S, Nagaraja AS, Haemmerle M, Kyunghee N, Derichsweiler M, Plackemeier T, Mercado-Uribe I, Lopez-Berestein G, Moss T, Ram PT, Liu J, Lu X, Mok SC, Sood AK, Nagrath D. Targeting Stromal Glutamine Synthetase in Tumors Disrupts Tumor Microenvironment-Regulated Cancer Cell Growth. Cell Metab. 2016;24:685–700. doi: 10.1016/j.cmet.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao CH, Fowle-Grider R, Mahieu NG, Liu GY, Chen YJ, Wang R, Singh M, Potter GS, Gross RW, Schaefer J, Johnson SL, Patti GJ. Exogenous Fatty Acids Are the Preferred Source of Membrane Lipids in Proliferating Fibroblasts. Cell Chem Biol. 2016;23:483–93. doi: 10.1016/j.chembiol.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Fan J, Venneti S, Wan YW, Pawel BR, Zhang J, Finley LW, Lu C, Lindsten T, Cross JR, Qing G, Liu Z, Simon MC, Rabinowitz JD, Thompson CB. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov. 2014;4:1406–17. doi: 10.1158/2159-8290.CD-14-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo H, Antoniewicz MR, Stephanopoulos G, Kelleher JK. Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line. J Biol Chem. 2008;283:20621–7. doi: 10.1074/jbc.M706494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD. INCA: a computational platform for isotopically non-stationary metabolic flux analysis. Bioinformatics. 2014;30:1333–5. doi: 10.1093/bioinformatics/btu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD, Shastri AA, Stephanopoulos G, Morgan JA. Mapping photoautotrophic metabolism with isotopically nonstationary (13)C flux analysis. Metab Eng. 2011;13:656–65. doi: 10.1016/j.ymben.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JD, Walther JL, Antoniewicz MR, Yoo H, Stephanopoulos G. An elementary metabolite unit (EMU) based method of isotopically nonstationary flux analysis. Biotechnol Bioeng. 2008;99:686–99. doi: 10.1002/bit.21632. [DOI] [PubMed] [Google Scholar]
- Yuan J, Bennett BD, Rabinowitz JD. Kinetic flux profiling for quantitation of cellular metabolic fluxes. Nat Protoc. 2008;3:1328–40. doi: 10.1038/nprot.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni N. 13C metabolic flux analysis in complex systems. Curr Opin Biotechnol. 2011;22:103–8. doi: 10.1016/j.copbio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Zamboni N, Fischer E, Sauer U. FiatFlux--a software for metabolic flux analysis from 13C–glucose experiments. BMC Bioinformatics. 2005;6:209. doi: 10.1186/1471-2105-6-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Badur MG, Divakaruni AS, Parker SJ, Jager C, Hiller K, Murphy AN, Metallo CM. Distinct Metabolic States Can Support Self-Renewal and Lipogenesis in Human Pluripotent Stem Cells under Different Culture Conditions. Cell Rep. 2016;16:1536–47. doi: 10.1016/j.celrep.2016.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fan J, Venneti S, Cross JR, Takagi T, Bhinder B, Djaballah H, Kanai M, Cheng EH, Judkins AR, Pawel B, Baggs J, Cherry S, Rabinowitz JD, Thompson CB. Asparagine plays a critical role in regulating cellular adaptation to glutamine depletion. Mol Cell. 2014;56:205–18. doi: 10.1016/j.molcel.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, Bhakoo KK, Jayapal SR, Nichane M, Yu Q, Ahmed DA, Tan C, Sing WP, Tam J, Thirugananam A, Noghabi MS, Pang YH, Ang HS, Mitchell W, Robson P, Kaldis P, Soo RA, Swarup S, Lim EH, Lim B. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148:259–72. doi: 10.1016/j.cell.2011.11.050. [DOI] [PubMed] [Google Scholar]
- Zhao S, Torres A, Henry RA, Trefely S, Wallace M, Lee JV, Carrer A, Sengupta A, Campbell SL, Kuo YM, Frey AJ, Meurs N, Viola JM, Blair IA, Weljie AM, Metallo CM, Snyder NW, Andrews AJ, Wellen KE. ATP-Citrate Lyase Controls a Glucose-to-Acetate Metabolic Switch. Cell Rep. 2016;17:1037–1052. doi: 10.1016/j.celrep.2016.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]